Large-Scale Microcarrier Culture of Chinese Perch Brain Cell for Viral Vaccine Production in a Stirred Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microcarrier Preparation
2.2. Optimization of Suspension Culture Parameters
2.2.1. Determination of the Appropriate Stirring Method
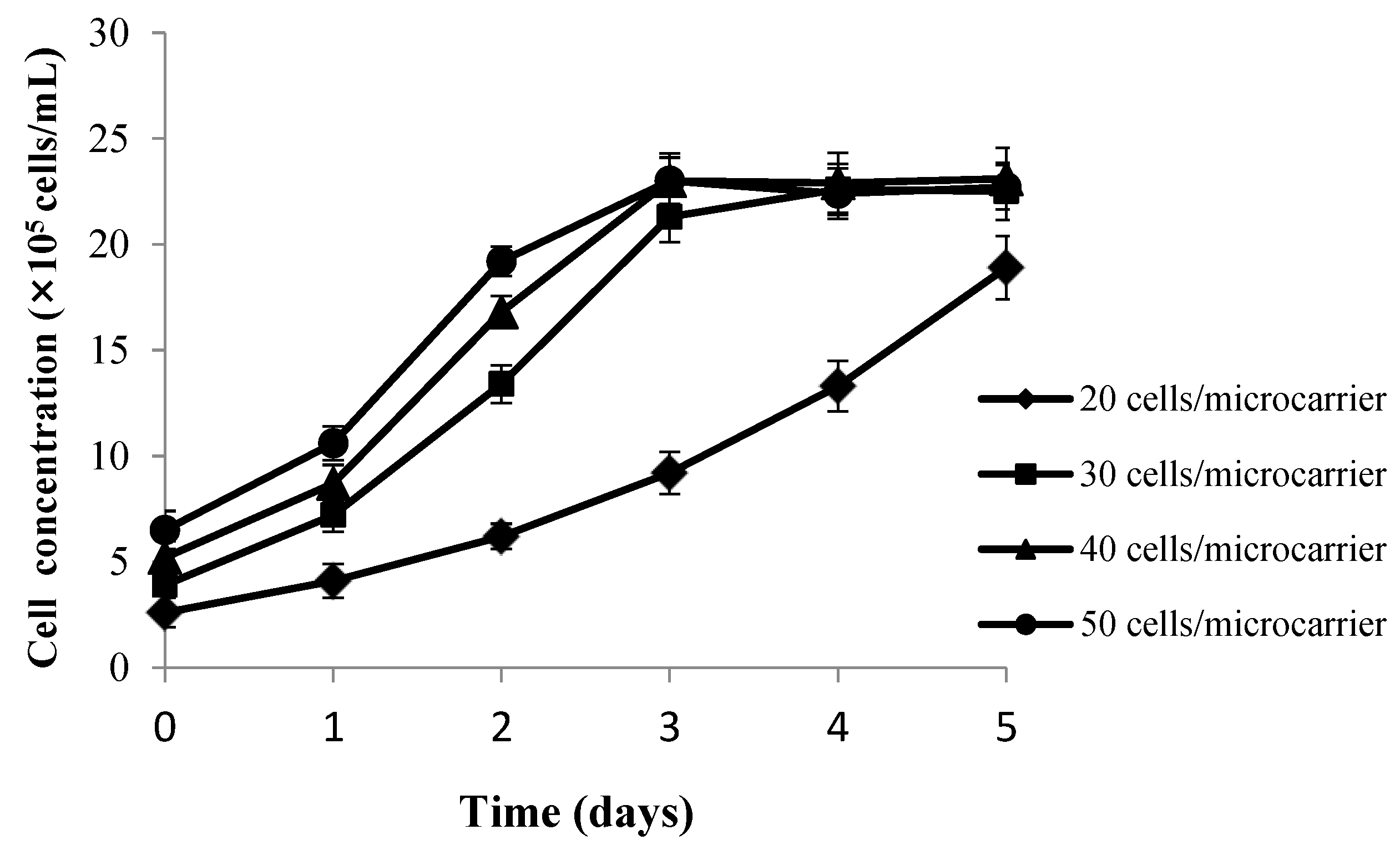
2.2.2. Determination of the Optimal Inoculated Cell Density
2.2.3. Determination of the Optimal Microcarrier Concentration
2.2.4. Determination of the Appropriate Cell Amplification Ratio in Different Culture Systems
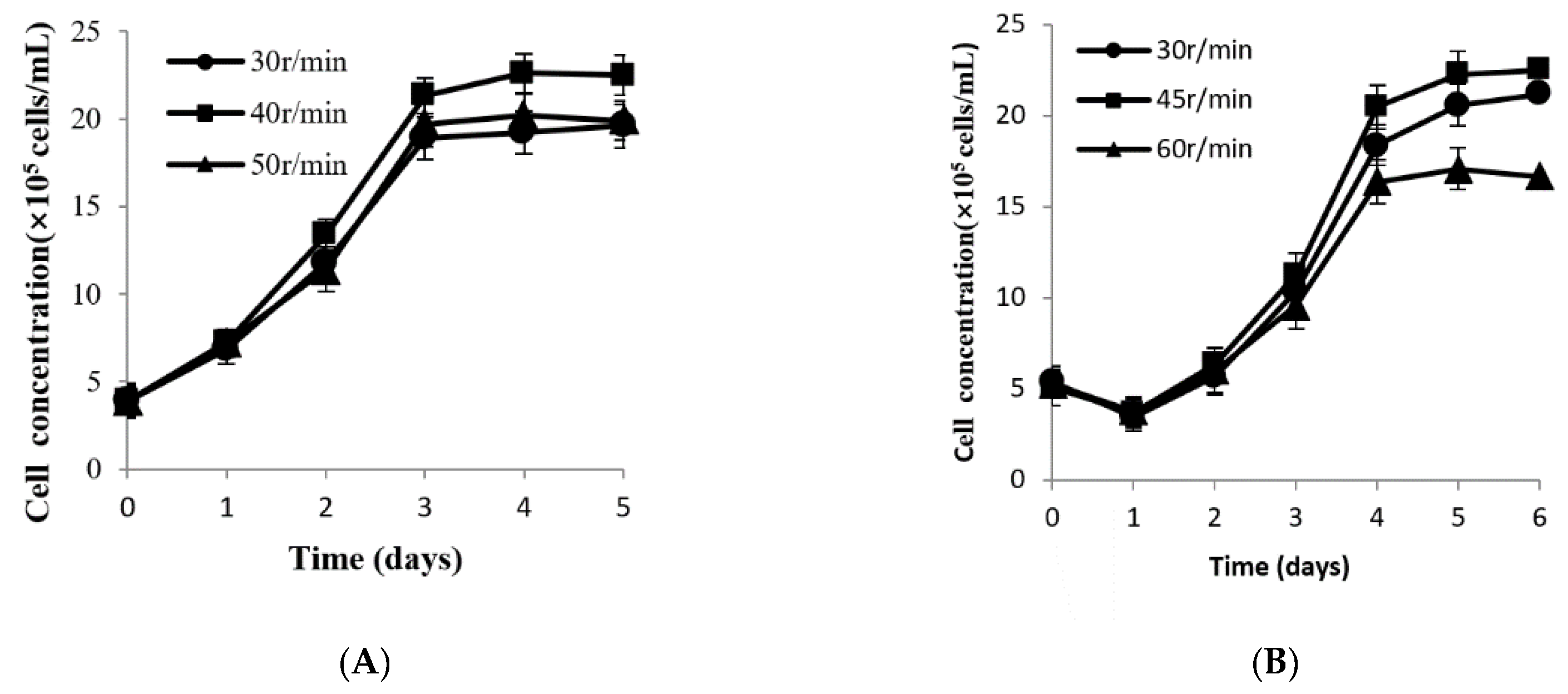
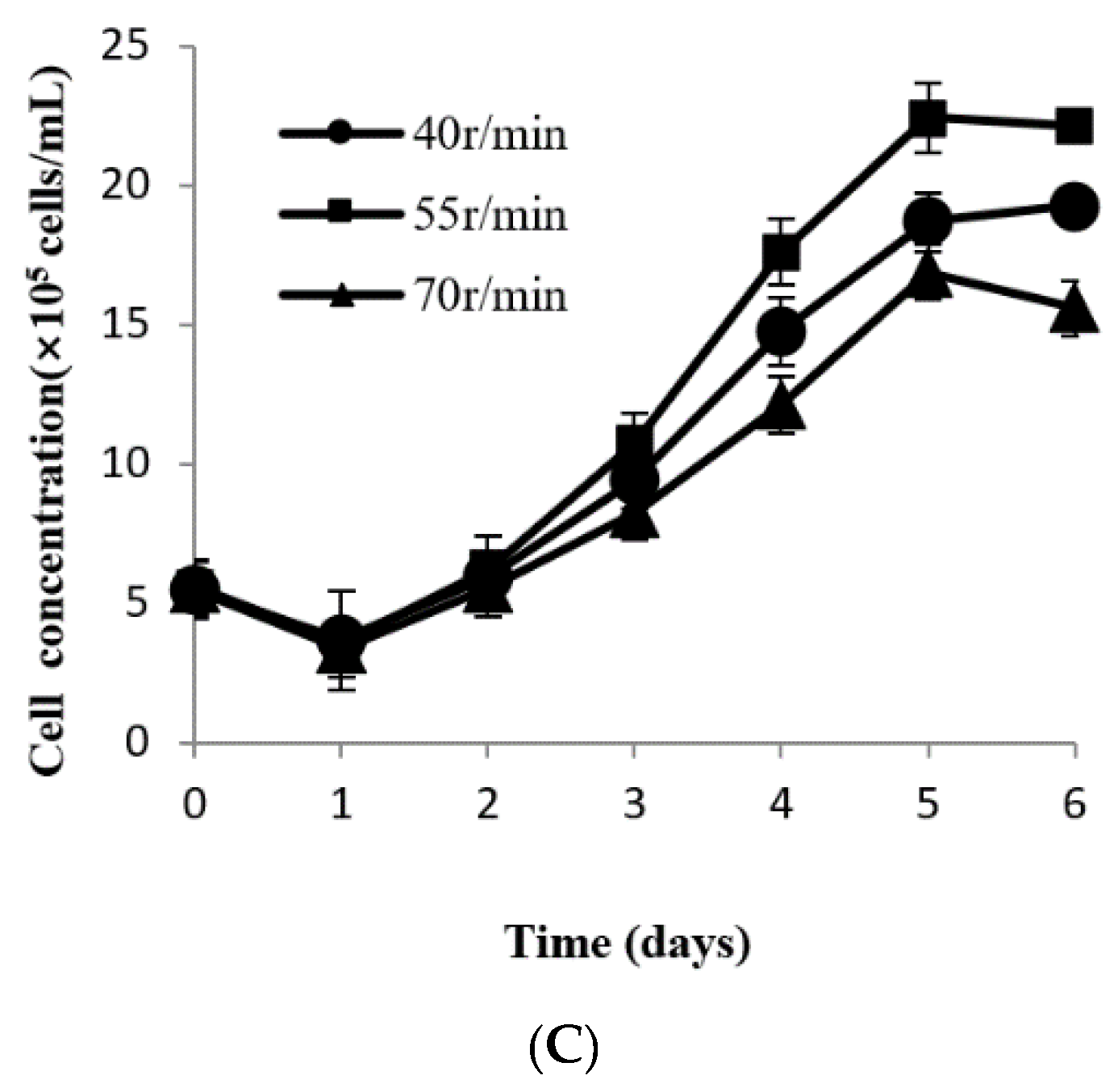
2.2.5. Determination of the Optimal Agitation Rate in Different Culture Systems
2.3. Virus Sensitivity
2.4. Statistical Analysis
3. Results
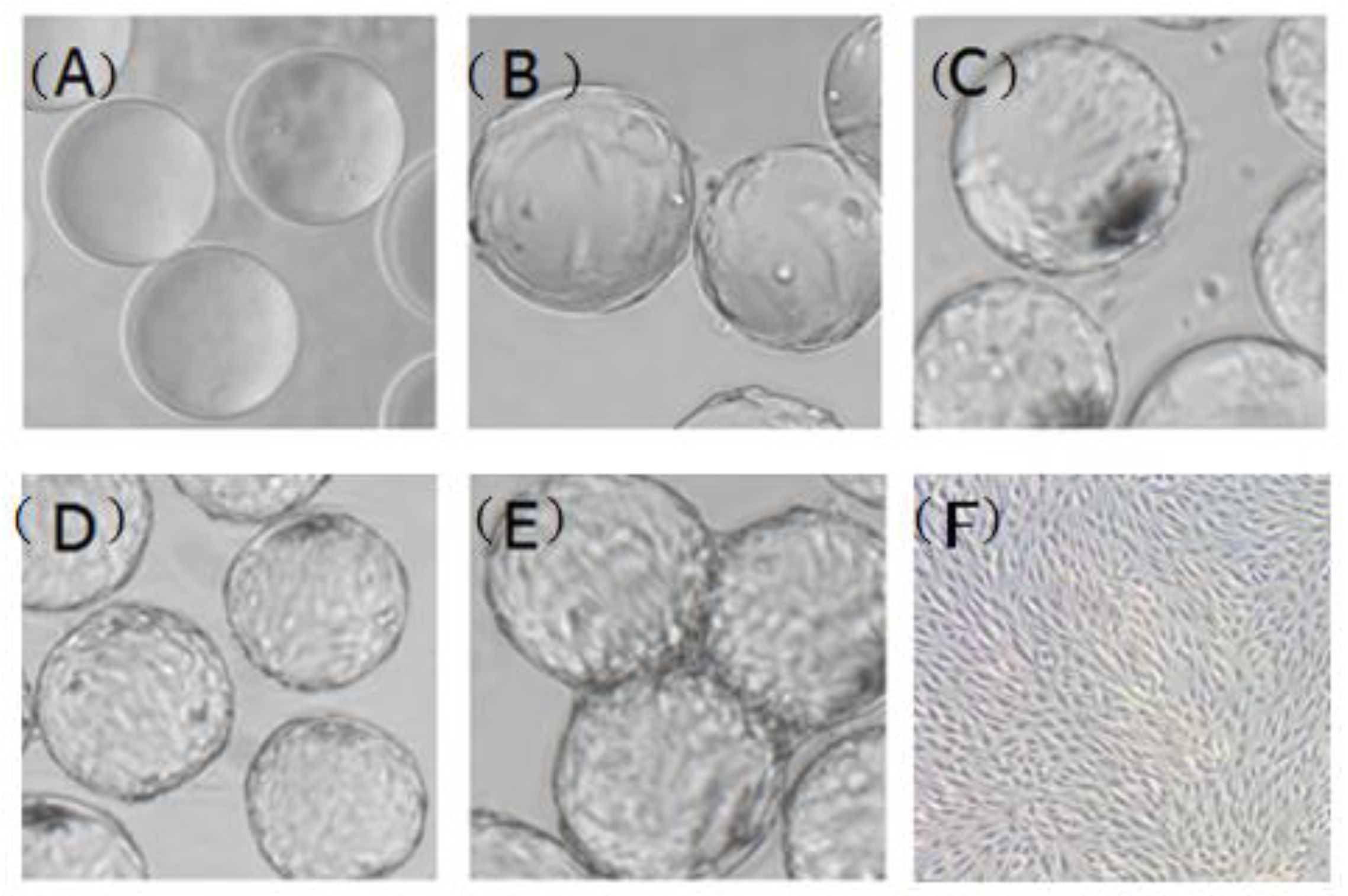
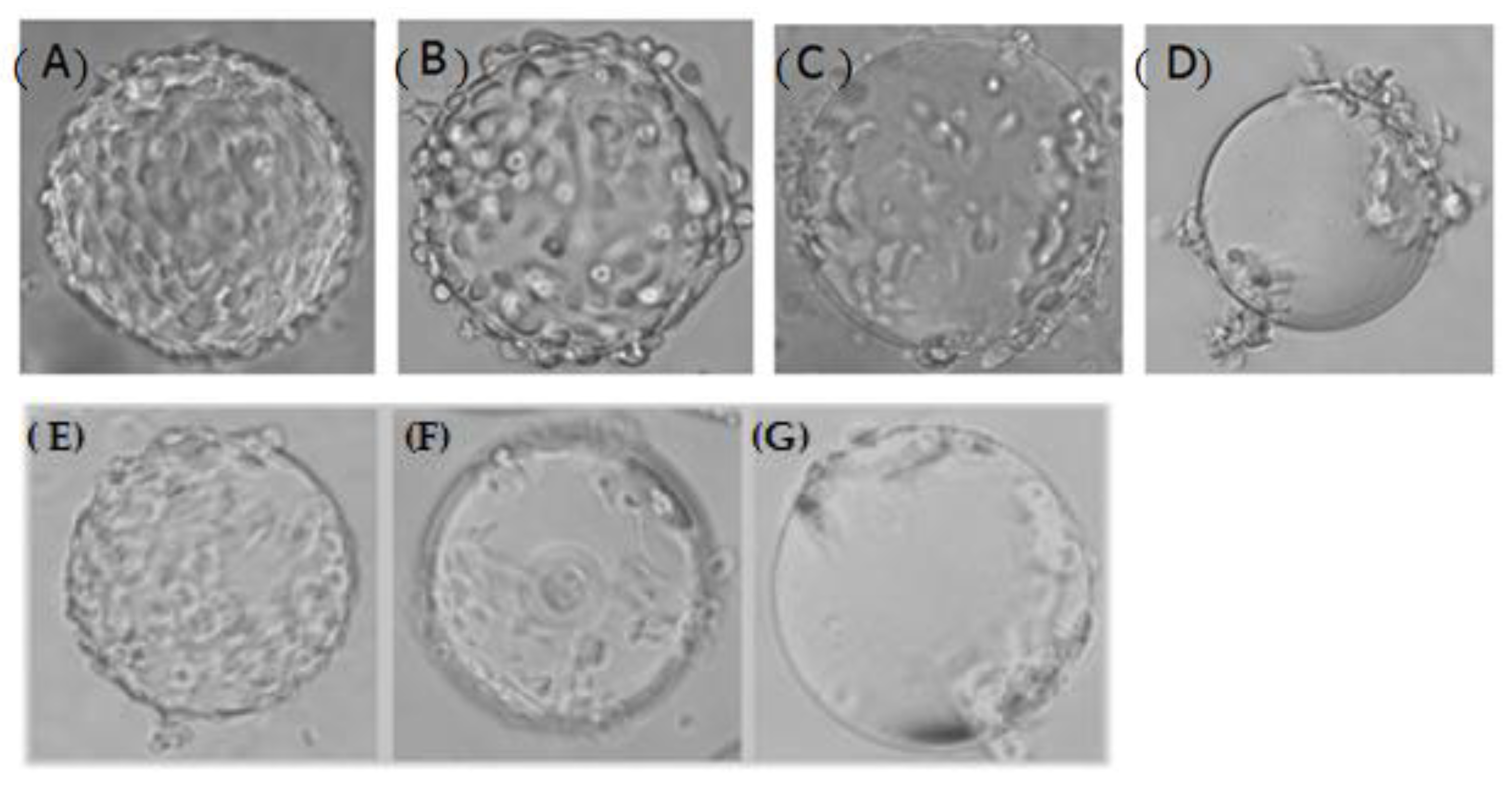
3.1. Stirring Method at the Attachment Stage of CPB Cells
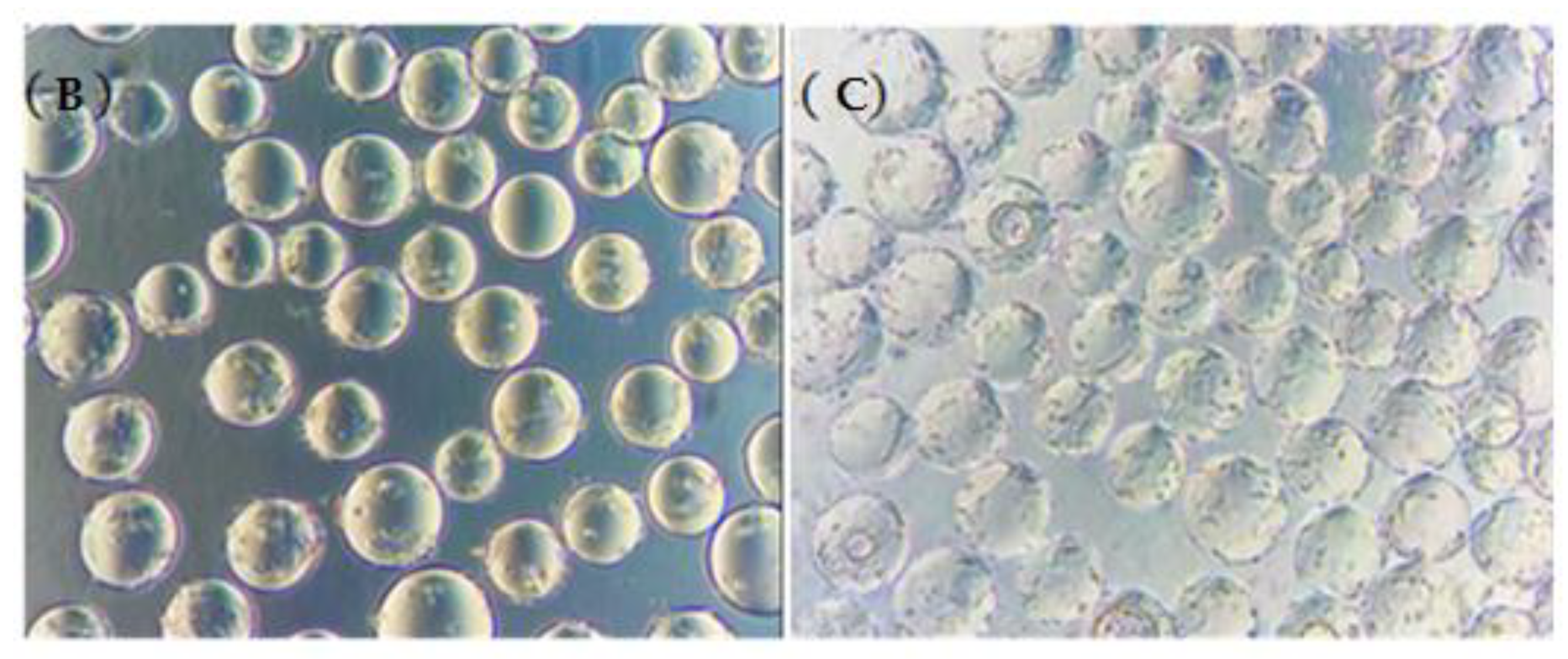
3.2. Determination of the Optimal Inoculated Cell Density
3.3. Microcarrier Concentration
3.4. Determination of the Appropriate Cell Expansion Ratio between Different Culture Systems
3.5. Determination of Appropriate Agitation Rate in Different Culture Systems
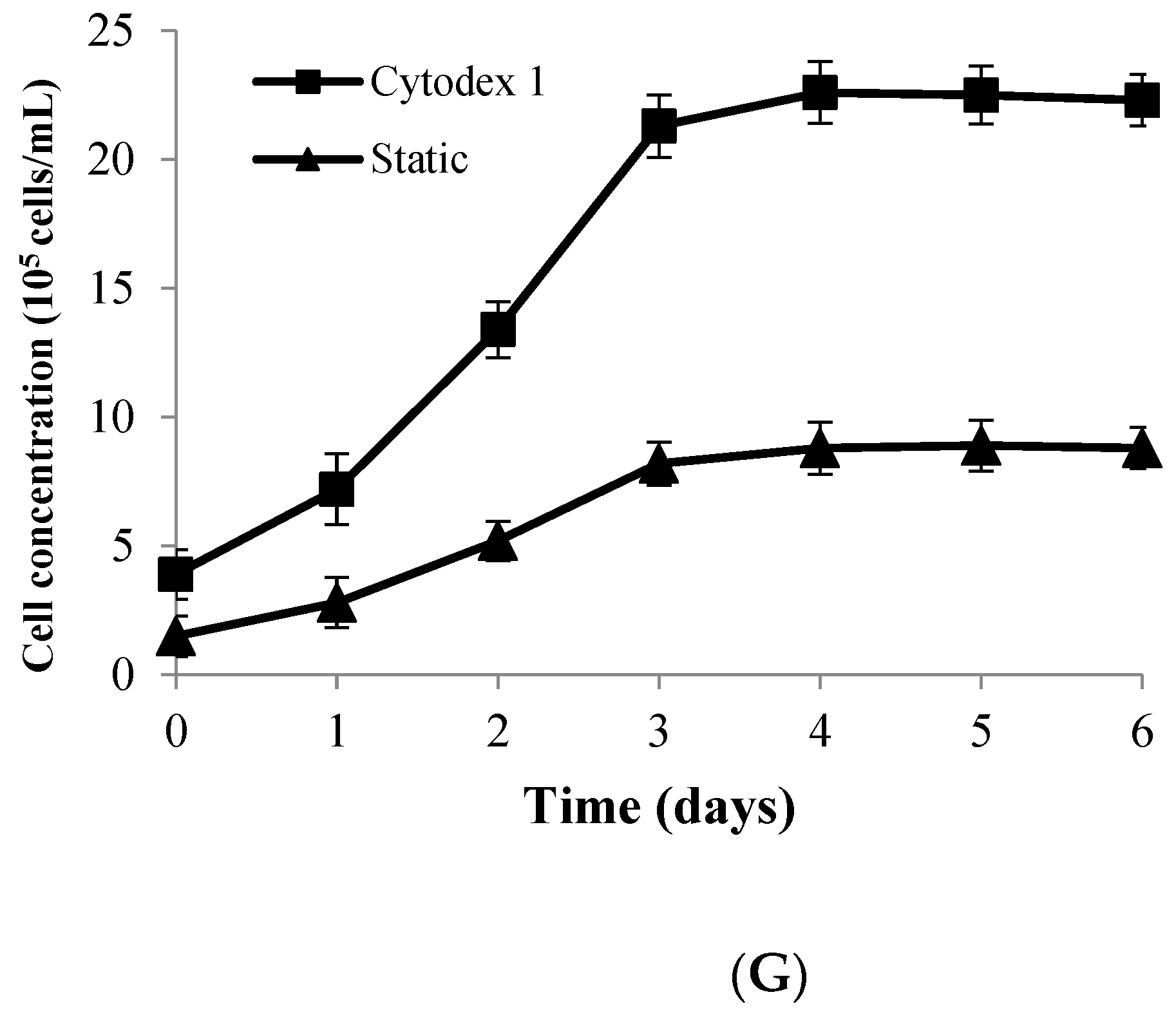
3.6. Comparison of Suspension Culture and Traditional Culture Process
3.7. Virus Sensitivity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.G.; Wang, S.P.; Zeng, K.; Huang, Z.J.; Chan, S.M. Systemic disease caused by an iridovirus-like agent in cultured mandarinfish, Siniperca chuatsi (basilewsky), in China. J. Fish Dis. 2000, 23, 219–222. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z. Three different viruses observed from the tissues of diseased mandarin fish Siniperca chuatsi. Chin. Sci. Bull. 1999, 5, 55–59. [Google Scholar]
- Fu, X.; Lin, Q.; Liang, H.; Liu, L.; Huang, Z.; Li, N.; Su, J. The biological features and genetic diversity of novel fish rhabdovirus isolates in China. Arch. Virol. 2017, 34, 579–587. [Google Scholar] [CrossRef]
- Dong, C.; Weng, S.; Shi, X.; Xu, X.; Nan, S.; He, J. Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus Res. 2008, 135, 273–281. [Google Scholar] [CrossRef]
- Fu, X.; Li, N.; Lai, Y.; Luo, X.; Wang, Y.; Shi, C.; Huang, Z.; Wu, S.; Su, J. A novel fish cell line derived from the brain of Chinese perch Siniperca chuatsi: Development and characterization. J. Fish Biol. 2015, 86, 32–45. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, X.; Zhang, Y. Chitosan and anisodamine improve the immune efficacy of inactivated infectious spleen and kidney necrosis virus vaccine in Siniperca chuatsi. Fish Shellfish Immunol. 2019, 89, 52–60. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Lin, Q.; Liu, L.; Liang, H.; Huang, Z.; Fu, X. An avirulent Micropterus salmoides rhabdovirus vaccine candidate protects Chinese perch against rhabdovirus infection. Fish Shellfish Immunol. 2018, 77, 474–480. [Google Scholar]
- Luo, X.; Fu, X.; Lin, Q.; Liu, L.; Niu, Y.; Liang, H.; Li, N. Study on the virus seed and seed baches of the mandarin fish ISKNV and SCRV bivalent inactivated vaccine. J. Northwest A F Univ. (Nat. Sci. Ed.) 2022, 50, 1–9, preprint. [Google Scholar] [CrossRef]
- Van Wezel, A.L. Growth of cell strains and primary cells on microcarriers in homogeneous culture. Nature 1967, 216, 64. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.Y.; Weng, T.C.; Tseng, Y.F.; Chen, Y.S.; Wu, C.H.; Hsiao, S.; Chou, A.H.; Chao, H.J.; Gu, A.; Wu, S.C.; et al. Microcarrier-based MDCK cell culture system for the production of influenza H5N1 vaccines. Vaccine 2008, 26, 5736–5740. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Lu, Z.; Wang, L.; Huo, Z.; Cui, J.; Zheng, T.; Dai, Q.; Chen, C.; Qin, M.; Chen, M.; et al. Rapid production of a H9N2 influenza vaccine from MDCK cells for protecting chicken against influenza virus infection. Appl. Microbiol. Biot. 2015, 99, 2999–3013. [Google Scholar] [CrossRef]
- Tree, J.A.; Richardson, C.; Fooks, A.R.; Clegg, J.C.; Looby, D. Comparison of large-scale mammalian cell culture systems with egg culture for the production of influenza virus a vaccine strains. Vaccine 2001, 19, 3444–3450. [Google Scholar] [CrossRef]
- Yang, J.; Guertin, P.; Jia, G.; Lv, Z.; Ju, D. Large-scale microcarrier culture of HEK293T cells and Vero cells in single-use bioreactors. AMB Exp. 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.; Fenge, C.; Rupprecht, J.; Tappe, A.; Greller, G.; Alves, P.; Garrondo, M.; Roldão, A. Process intensification for Peste des Petites Ruminants virus vaccine production. Vaccine 2019, 37, 7041–7051. [Google Scholar] [CrossRef]
- Shen, C.; Guilbault, C.; Li, X.; Elahi, S.M.; Gilbert, R. Development of suspension adapted Vero cell culture process technology for production of viral vaccines. Vaccine 2019, 37, 6996–7002. [Google Scholar] [CrossRef]
- Kiesslich, S.; Kim, G.N.; Shen, C.F.; Kang, C.Y.; Kamen, A.A. Bioreactor production of rVSV-based vectors in Vero cell suspension cultures. Biotechnol. Bioeng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fujii, G.; Kurashina, Y.; Terao, Y.; Azuma, T.; Morikawa, A.; Kodeki, K.; Takahara, O.; Takemura, K. Suspension culture in a t-flask with acoustic flow induced by ultrasonic irradiation. Ultrason. Sonochem. 2021, 73, 105488. [Google Scholar] [CrossRef]
- Auniņš, J.G.; Bader, B.; Caola, A.; Griffiths, J.; Katz, M.; Licari, P.; Ram, K.; Ranucci, C.S.; Zhou, W. Fluid mechanics, cell distribution and environment in cellcube bioreactors. Biotechnol. Prog. 2003, 19, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Alfred, R.; Radford, J.; Fan, J.; Boon, K.; Krawetz, R.; Rancourt, D.; Kallos, M.S. Efficient suspension bioreactor expansion of murine embryonic stem cells on microcarriers in serum-free medium. Biotechnol. Prog. 2011, 27, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, K.; Majoul, S.; Rourou, S.; Kallel, H. Development of a measles vaccine production process in MRC-5 cells grown on Cytodex1 microcarriers and in a stirred bioreactor. Appl. Microbiol. Biotechnol. 2012, 93, 1031–1040. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Tan, W.; Zhou, Y.; Ping, H. Attachment kinetics of Vero cells onto CT-3 microcarriers. J. Biosci. Bioeng. 2000, 90, 32–36. [Google Scholar] [CrossRef]
- Rourou, S.; Ark, A.; Velden, T.; Kallel, H. A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions. Vaccine 2007, 25, 3879–3889. [Google Scholar] [CrossRef] [PubMed]
- Maartens, J.H.; De-Juan-Pardo, E.; Wunner, F.M.; Simula, A.; Voelcker, N.H.; Barry, S.C.; Hutmacher, D.W. Challenges and opportunities in the manufacture and expansion of cells for therapy. Expert Opin. Biol. Ther. 2017, 17, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.; Freire, M.S.; Schulze, E.A.; Gaspar, L.P.; Castilho, L.R. Production of yellow fever virus in microcarrier-based Vero cell cultures. Vaccine 2009, 27, 6420–6423. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.L. Growth of fish cell lines on microcarriers. Appl. Environ. Microbiol. 1980, 39, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Buck, C.D.; Loh, P.C. Growth of brown bullhead (BB) and other fish cell lines on microcarriers and the production of channel catfish virus (CCV). J. Virol. Methods 1985, 10, 171–184. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Jie, M.A.; Zhou, Y.; Fan, Y.; Zeng, L. Studies on the technology for large-scale cultivation of gibel carp brain cells and cyprinid herpesvirus 2 by microcarrier. J. Ocean Univ. China 2017, 26, 784–792. [Google Scholar]
- Jia, L.L.; Zhou, Y.; Ma, J.; Fan, Y.; Liu, X.; Zeng, L. Technologies for large-scale cultivation of giant salamander cells and iridovirus by the Cytodex 3 microcarrier. J. Fish. Sci. China 2018, 25, 211–219. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, A.; Li, Y.; Lai, H.; Li, H.; Luo, Q.; Jin, S.; Chen, R. Suspension culture of Marek’s disease virus and evaluation of its immunological effects. Avian Pathol. 2019, 48, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Forestell, S.P.; Kalogerakis, N.; Behie, L.A.; Gerson, D.F. Development of the optimal inoculation conditions for microcarrier cultures. Biotechnol. Bioeng. 1992, 39, 305–313. [Google Scholar] [CrossRef]
- Clark, J.M.; Hirstenstein, H.; Gebb, C. Critical parameters in the microcarrier culture of animal cells. Dev. Biol. Stand. 1980, 46, 117–124. [Google Scholar]
- Takahashi, I.; Sato, K.; Mera, H.; Wakitani, S.; Takagi, M. Effects of agitation rate on aggregation during beads-to-beads subcultivation of microcarrier culture of human mesenchymal stem cells. Cytotechnology 2016, 69, 503–509. [Google Scholar] [CrossRef]
- Nilsson, K. Microcarrier cell culture. Biotech. Genet. Eng. Rev. 1989, 6, 403. [Google Scholar] [CrossRef]
- Brindley, D.; Moorthy, K.; Lee, J.H.; Mason, C.; Kim, H.W.; Wall, I. Bioprocess forces and their impact on cell behavior: Implications for bone regeneration therapy. J. Tissue Eng. 2011, 2011, 620247. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tsai, A.C.; Liu, Y. Biomanufacturing of human mesenchymal stem cells in cell therapy: Influence of microenvironment on scalable expansion in bioreactors. Biochem. Eng. J. 2015, 108, 44–50. [Google Scholar] [CrossRef]
- Bock, A.; Sann, H.; Schulze-Horsel, J.; Genzel, Y.; Reichl, U.; Mohler, L. Growth behaviour of number distributed adherent MDCK cells for optimization in large scale microcarrier cultures. Biotechnol. Prog. 2009, 25, 1717–1731. [Google Scholar]
- Goh, K.P.; Zhang, Z.Y.; Chen, K.L.; Reuveny, S.; Choolani, M.; Chan, J.; Oh, K.W. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores. Open Access 2013, 2, 84–97. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Pohl, S.; Pörtner, R.; Wallrapp, C.; Czermak, P. Expansion and harvesting of hMSC-TERT. Open Biomed. Eng. J. 2007, 1, 38–46. [Google Scholar] [CrossRef]
- Roberts, E.L.; Dang, T.; Lepage, S.; Alizadeh, A.H.; Walsh, T.; Koch, T.G.; Kallos, M.S. Improved expansion of equine cord blood derived mesenchymal stromal cells by using microcarriers in stirred suspension bioreactors. J. Biol. Eng. 2019, 13, 25. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, S.; Wang, Z.; Feng, E.; Leng, X.; Wang, H.; Li, Y.; Sun, M. Research on the scale-up technique of microcarrier suspension culture and digestion of marc-145 cells. Heilongjiang Anim. Sci. Vet. Med. 2019, 13, 16–20. [Google Scholar]
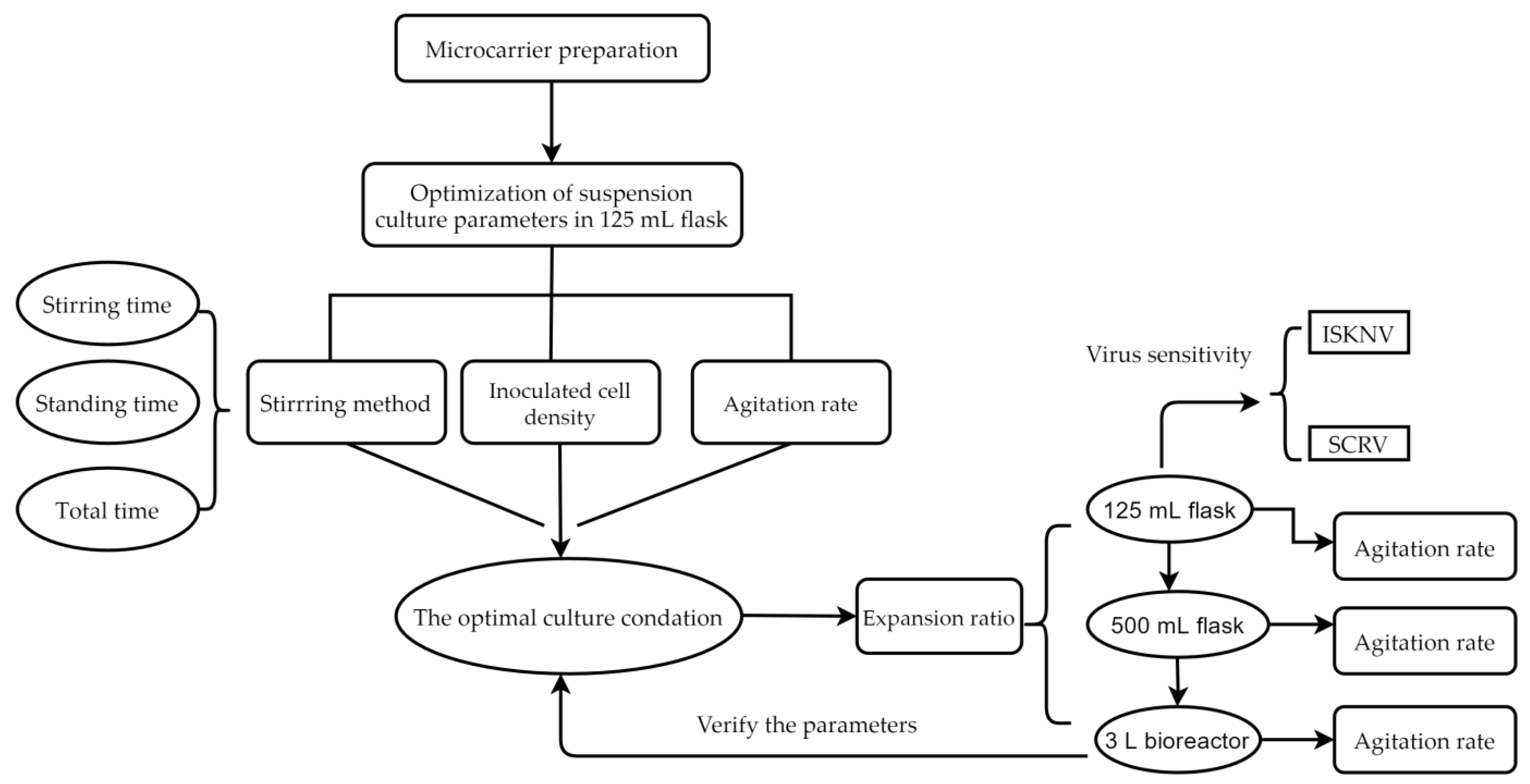

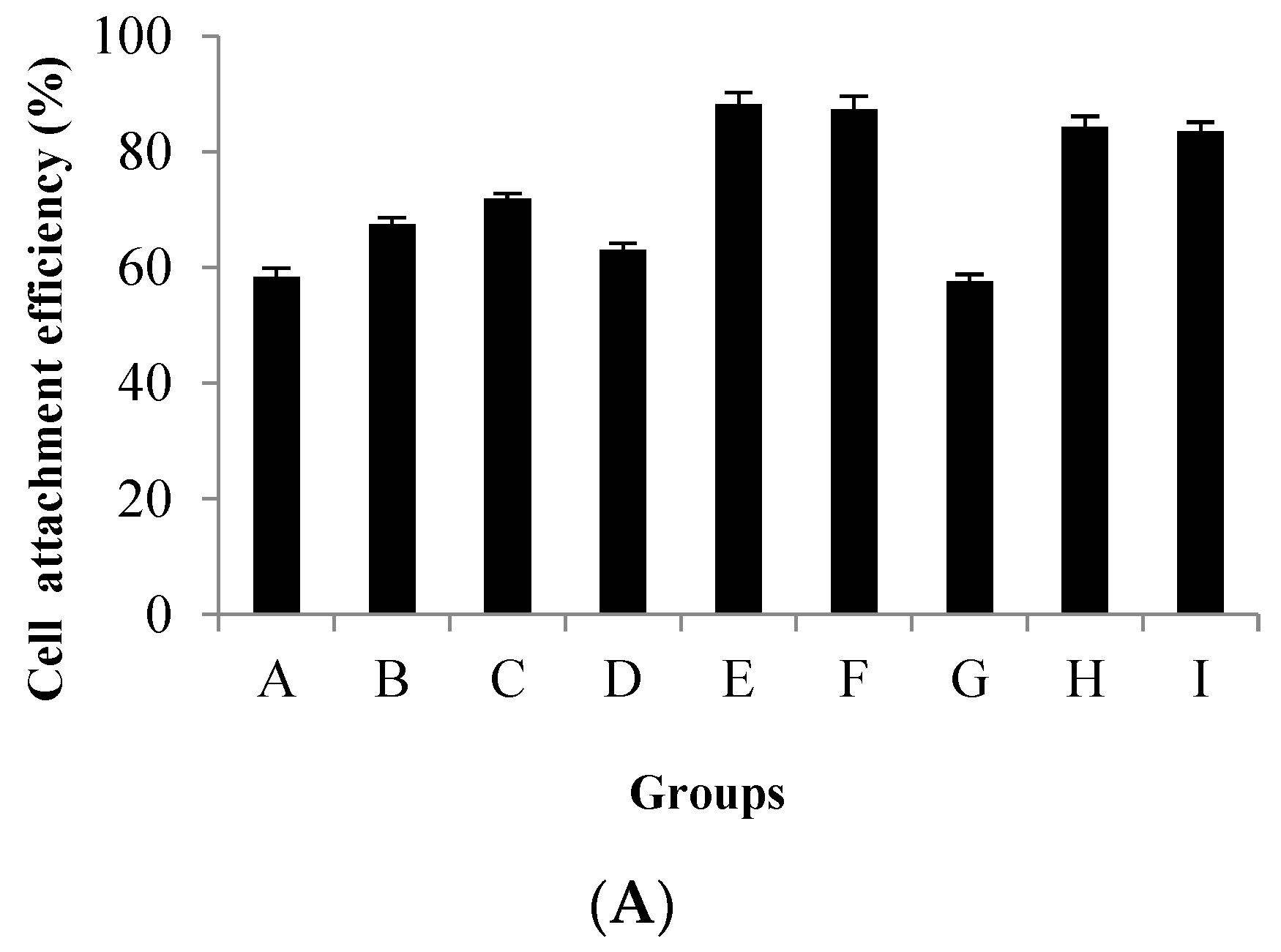


| Agitation Conditions | A | B | C | D | E | F | G | H | I |
|---|---|---|---|---|---|---|---|---|---|
| Stirring time/min | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Standing time/min | 27 | 27 | 27 | 42 | 42 | 57 | 57 | 57 | 57 |
| Total time/min | 120 | 300 | 480 | 120 | 300 | 480 | 120 | 300 | 480 |
| Microcarrier Concentration (g/L) | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| 1 Cell expanding fold | 5.91 ± 0.03 | 5.85 ± 0.05 | 5.18 ± 0.01 | 4.45 ± 0.09 |
| 2 Specific growth rate | 0.58 ± 0.03 | 0.57 ± 0.01 | 0.55 ± 0.03 | 0.50 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Niu, Y.; Fu, X.; Lin, Q.; Liang, H.; Liu, L.; Li, N. Large-Scale Microcarrier Culture of Chinese Perch Brain Cell for Viral Vaccine Production in a Stirred Bioreactor. Vaccines 2021, 9, 1003. https://doi.org/10.3390/vaccines9091003
Luo X, Niu Y, Fu X, Lin Q, Liang H, Liu L, Li N. Large-Scale Microcarrier Culture of Chinese Perch Brain Cell for Viral Vaccine Production in a Stirred Bioreactor. Vaccines. 2021; 9(9):1003. https://doi.org/10.3390/vaccines9091003
Chicago/Turabian StyleLuo, Xia, Yinjie Niu, Xiaozhe Fu, Qiang Lin, Hongru Liang, Lihui Liu, and Ningqiu Li. 2021. "Large-Scale Microcarrier Culture of Chinese Perch Brain Cell for Viral Vaccine Production in a Stirred Bioreactor" Vaccines 9, no. 9: 1003. https://doi.org/10.3390/vaccines9091003
APA StyleLuo, X., Niu, Y., Fu, X., Lin, Q., Liang, H., Liu, L., & Li, N. (2021). Large-Scale Microcarrier Culture of Chinese Perch Brain Cell for Viral Vaccine Production in a Stirred Bioreactor. Vaccines, 9(9), 1003. https://doi.org/10.3390/vaccines9091003





