Efficient Maternal to Neonate Transfer of Neutralizing Antibodies after SARS-CoV-2 Vaccination with BNT162b2: A Case-Report and Discussion of the Literature
Abstract
:1. Introduction
2. Case Description and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., 3rd; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef]
- Ellington, S.; Strid, P.; Tong, V.T.; Woodworth, K.; Galang, R.R.; Zambrano, L.D.; Nahabedian, J.; Anderson, K.; Gilboa, S.M. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status–United States, January 22–June 7, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 769–775. [Google Scholar] [CrossRef]
- Riley, L.E. mRNA COVID-19 Vaccines in Pregnant Women. N. Engl. J. Med. 2021, 384, 2342–2343. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Graham, A.L. Potential Maternal and Infant Outcomes from (Wuhan) Coronavirus 2019-nCoV Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses 2020, 12, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettirosso, E.; Giles, M.; Cole, S.; Rees, M. COVID-19 and pregnancy: A review of clinical characteristics, obstetric outcomes and vertical transmission. Aust. New Zealand J. Obstet. Gynaecol. 2020, 60, 640–659. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Maykin, M.M.; Heuser, C.; Feltovich, H. Pregnant people deserve the protection offered by SARS-CoV-2 vaccines. Vaccine 2021, 39, 171–172. [Google Scholar] [CrossRef]
- Craig, A.M.; Hughes, B.L.; Swamy, G.K. Coronavirus disease 2019 vaccines in pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 3, 100295. [Google Scholar] [CrossRef] [PubMed]
- Bookstein Peretz, S.; Regev, N.; Novick, L.; Nachshol, M.; Goffer, E.; Ben-David, A.; Asraf, K.; Doolman, R.; Sapir, E.; Regev Yochay, G.; et al. Short-term outcome of pregnant women vaccinated by BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obs. Gynecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.G.; Grinceviciene, S.; Haldre, K.; Lonnee-Hoffmann, R.; Donders, F.; Tsiakalos, A.; Adriaanse, A.; Martinez de Oliveira, J.; Ault, K.; Mendling, W.; et al. ISIDOG Consensus Guidelines on COVID-19 Vaccination for Women before, during and after Pregnancy. J. Clin. Med. 2021, 10, 2902. [Google Scholar] [CrossRef]
- Ory, S.; Veiga, A.; Horton, M.; Gianaroli, L. Joint IFFS/ESHRE statement on COVID-19 vaccination for pregnant women and those considering pregnancy. Hum. Reprod. Open 2021, 2021, hoab016. [Google Scholar] [CrossRef] [PubMed]
- Society for Maternal-Fetal Medicine. Society for Maternal-Fetal Medicine (SMFM) Statement: SARS-CoV-2 Vaccination in Pregnancy. Available online: https://s3.amazonaws.com/cdn.smfm.org/media/2591/SMFM_Vaccine_Statement_12-1-20_(final).pdf (accessed on 21 July 2021).
- Razzaghi, H.; Kahn, K.E.; Black, C.L.; Lindley, M.C.; Jatlaoui, T.C.; Fiebelkorn, A.P.; Havers, F.P.; D’Angelo, D.V.; Cheung, A.; Ruther, N.A.; et al. Influenza and Tdap Vaccination Coverage Among Pregnant Women–United States, April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1391–1397. [Google Scholar] [CrossRef]
- Levy, A.T.; Singh, S.; Riley, L.E.; Prabhu, M. Acceptance of COVID-19 vaccination in pregnancy: A survey study. Am. J. Obstet. Gynecol. MFM 2021, 3, 100399. [Google Scholar] [CrossRef]
- Gerretsen, H.E.; Sande, C.J. Development of respiratory syncytial virus (RSV) vaccines for infants. J. Infect. 2017, 74 (Suppl. 1), S143–S146. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Uyeki, T.M. Effects of influenza on pregnant women and infants. Am. J. Obstet. Gynecol. 2012, 207, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef] [Green Version]
- Mithal, L.B.; Machut, K.Z.; Muller, W.J.; Kociolek, L.K. SARS-CoV-2 Infection in Infants Less than 90 Days Old. J. Pediatr. 2020, 224, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Whitaker, M.; O’Halloran, A.; Kambhampati, A.; Chai, S.J.; Reingold, A.; Armistead, I.; Kawasaki, B.; Meek, J.; Yousey-Hindes, K.; et al. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, March 1–July 25, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Richtmann, R.; Torloni, M.R.; Oyamada Otani, A.R.; Levi, J.E.; Crema Tobara, M.; de Almeida Silva, C.; Dias, L.; Miglioli-Galvao, L.; Martins Silva, P.; Macoto Kondo, M. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: A case series. Case Rep. Womens Health 2020, 27, e00243. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, E.; Jang, M.; Burd, I. COVID-19 in pregnancy: Placental and neonatal involvement. Am. J. Reprod. Immunol. 2020, 84, e13306. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. COVID-19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatr. Infect. Dis. J. 2020, 39, 469–477. [Google Scholar] [CrossRef]
- Gurol-Urganci, I.; Jardine, J.E.; Carroll, F.; Draycott, T.; Dunn, G.; Fremeaux, A.; Harris, T.; Hawdon, J.; Morris, E.; Muller, P.; et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: National cohort study. Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [Green Version]
- Beharier, O.; Plitman Mayo, R.; Raz, T.; Nahum Sacks, K.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021, 184, 628–642.e610. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obste.t Gynecol 2021. [Google Scholar] [CrossRef] [PubMed]
- Zdanowski, W.; Wasniewski, T. Evaluation of SARS-CoV-2 Spike Protein Antibody Titers in Cord Blood after COVID-19 Vaccination during Pregnancy in Polish Healthcare Workers: Preliminary Results. Vaccines 2021, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Bayart, J.L.; Mullier, F.; Dogne, J.M.; Closset, M.; Douxfils, J. Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2). Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Favresse, J.; Bayart, J.L.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogne, J.M.; et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Bayart, J.L.; Morimont, L.; Closset, M.; Wieers, G.; Roy, T.; Gerin, V.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Ausselet, N.; et al. Confounding Factors Influencing the Kinetics and Magnitude of Serological Response Following Administration of BNT162b2. Microorganisms 2021, 9, 1340. [Google Scholar] [CrossRef]
- Favresse, J.; Eucher, C.; Elsen, M.; Tre-Hardy, M.; Dogne, J.M.; Douxfils, J. Clinical Performance of the Elecsys Electrochemiluminescent Immunoassay for the Detection of SARS-CoV-2 Total Antibodies. Clin. Chem. 2020, 66, 1104–1106. [Google Scholar] [CrossRef]
- Favresse, J.; Gillot, C.; Di Chiaro, L.; Eucher, C.; Elsen, M.; Van Eeckhoudt, S.; David, C.; Morimont, L.; Dogné, J.-M.; Douxfils, J. Neutralizing Antibodies in COVID-19 Patients and Vaccine Recipients after Two Doses of BNT162b2. Viruses 2021, 13, 1364. [Google Scholar] [CrossRef]
- Atyeo, C.; Deriso, E.A.; Davis, C.; Bordt, E.A.; Deguzman, R.M.; Shook, L.L.; Yonker, L.M.; Fasano, A.; Akinwunmi, B.; Lauffenburger, D.A.; et al. COVID-19 mRNA vaccines drive differential Fc-functional profiles in pregnant, lactating, and non-pregnant women. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mithal, L.B.; Otero, S.; Shanes, E.D.; Goldstein, J.A.; Miller, E.S. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am. J. Obstet. Gynecol. 2021, 2225, 192–194. [Google Scholar] [CrossRef]
- Gill, L.; Jones, C.W. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibodies in Neonatal Cord Blood After Vaccination in Pregnancy. Obstet. Gynecol. 2021, 137, 894–896. [Google Scholar] [CrossRef]
- Munoz, F.M.; Bond, N.H.; Maccato, M.; Pinell, P.; Hammill, H.A.; Swamy, G.K.; Walter, E.B.; Jackson, L.A.; Englund, J.A.; Edwards, M.S.; et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: A randomized clinical trial. JAMA 2014, 311, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.R.; Fong, Y.; Li, S.H.; Yang, F.; Jennewein, M.F.; Weiner, J.A.; Harrell, E.A.; Mangold, J.F.; Goswami, R.; Seage, G.R., 3rd; et al. Fc Characteristics Mediate Selective Placental Transfer of IgG in HIV-Infected Women. Cell 2019, 178, 190–201.e11. [Google Scholar] [CrossRef]
- Firan, M.; Bawdon, R.; Radu, C.; Ober, R.J.; Eaken, D.; Antohe, F.; Ghetie, V.; Ward, E.S. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int. Immunol. 2001, 13, 993–1002. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennewein, M.F.; Goldfarb, I.; Dolatshahi, S.; Cosgrove, C.; Noelette, F.J.; Krykbaeva, M.; Das, J.; Sarkar, A.; Gorman, M.J.; Fischinger, S.; et al. Fc Glycan-Mediated Regulation of Placental Antibody Transfer. Cell 2019, 178, 202–215.e214. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.T.; Maamary, J.; Tan, G.S.; Bournazos, S.; Davis, C.W.; Krammer, F.; Schlesinger, S.J.; Palese, P.; Ahmed, R.; Ravetch, J.V. Anti-HA Glycoforms Drive B Cell Affinity Selection and Determine Influenza Vaccine Efficacy. Cell 2015, 162, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Toner, L.E.; Gelber, S.E.; Pena, J.A.; Fox, N.S.; Rebarber, A. A Case Report to Assess Passive Immunity in a COVID Positive Pregnant Patient. Am. J. Perinatol. 2020, 37, 1280–1282. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in Infants Born to Mothers with COVID-19 Pneumonia. JAMA 2020, 323, 1848–1849. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Gonzalez, J.; Edwards, K.; Mallajosyula, V.; Buzzanco, A.S.; Sherwood, R.; Buffone, C.; Kathale, N.; Providenza, S.; Xie, M.M.; et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat. Immunol. 2021, 22, 67–73. [Google Scholar] [CrossRef]
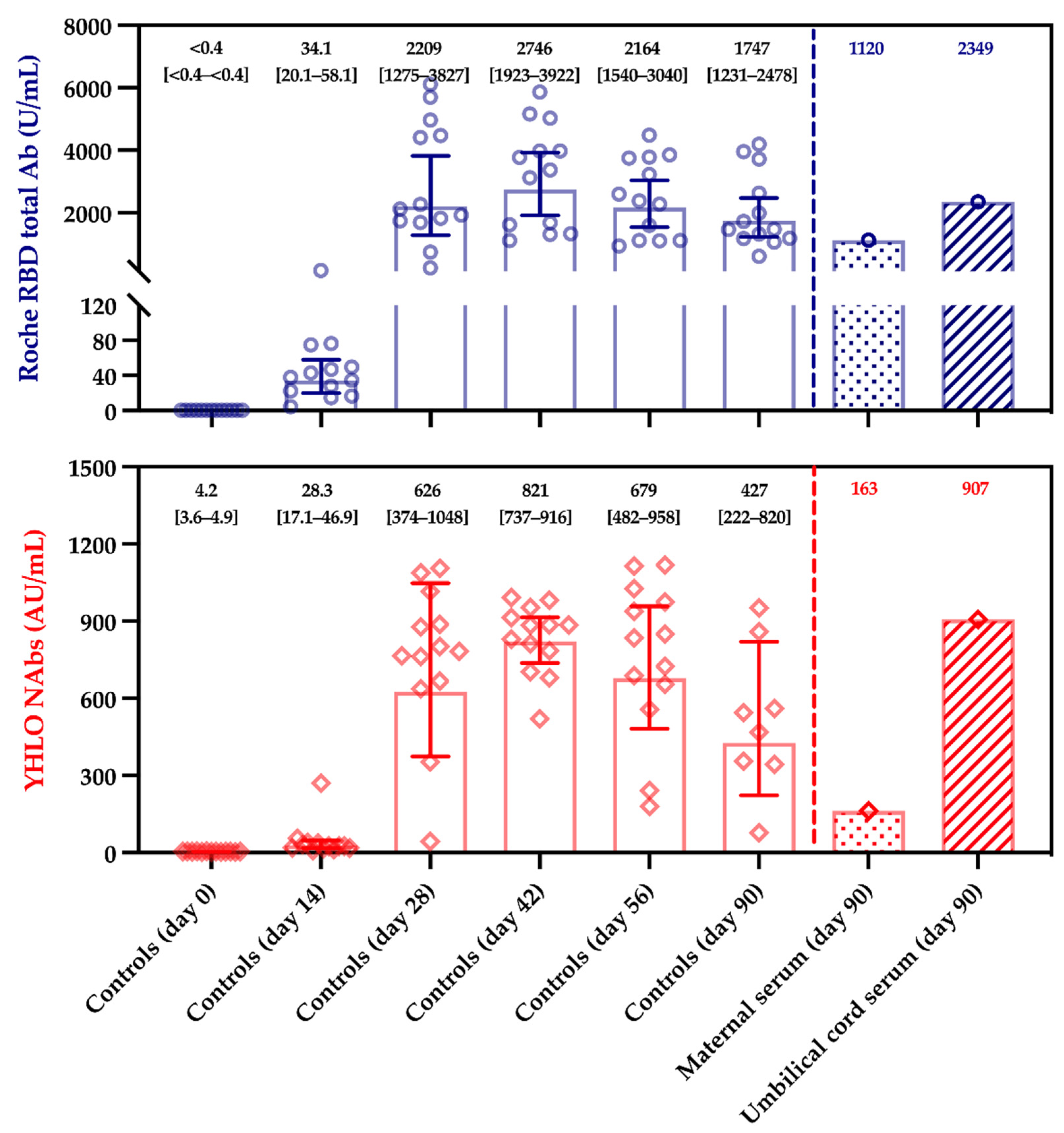
| Serum Samples | Anti-N Titer (U/mL) | Anti-S Titer (U/mL) | sVNT (AU/mL) | pVNT (Dilution Factor) |
|---|---|---|---|---|
| Maternal | <0.165 (negative) | 1120 | 162.9 | 1/40 (positive) |
| Umbilical cord blood | <0.165 (negative) | 2349 | 906.4 | 1/60 (positive) |
| Maternal to fetal transfer ratio † | / | 2.10 | 5.56 | 1.50 ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douxfils, J.; Gillot, C.; De Gottal, É.; Vandervinne, S.; Bayart, J.-L.; Dogné, J.-M.; Favresse, J. Efficient Maternal to Neonate Transfer of Neutralizing Antibodies after SARS-CoV-2 Vaccination with BNT162b2: A Case-Report and Discussion of the Literature. Vaccines 2021, 9, 907. https://doi.org/10.3390/vaccines9080907
Douxfils J, Gillot C, De Gottal É, Vandervinne S, Bayart J-L, Dogné J-M, Favresse J. Efficient Maternal to Neonate Transfer of Neutralizing Antibodies after SARS-CoV-2 Vaccination with BNT162b2: A Case-Report and Discussion of the Literature. Vaccines. 2021; 9(8):907. https://doi.org/10.3390/vaccines9080907
Chicago/Turabian StyleDouxfils, Jonathan, Constant Gillot, Émilie De Gottal, Stéphanie Vandervinne, Jean-Louis Bayart, Jean-Michel Dogné, and Julien Favresse. 2021. "Efficient Maternal to Neonate Transfer of Neutralizing Antibodies after SARS-CoV-2 Vaccination with BNT162b2: A Case-Report and Discussion of the Literature" Vaccines 9, no. 8: 907. https://doi.org/10.3390/vaccines9080907
APA StyleDouxfils, J., Gillot, C., De Gottal, É., Vandervinne, S., Bayart, J.-L., Dogné, J.-M., & Favresse, J. (2021). Efficient Maternal to Neonate Transfer of Neutralizing Antibodies after SARS-CoV-2 Vaccination with BNT162b2: A Case-Report and Discussion of the Literature. Vaccines, 9(8), 907. https://doi.org/10.3390/vaccines9080907







