Abstract
Rickettsioses are febrile, potentially lethal infectious diseases that are a serious health threat, especially in poor income countries. The causative agents are small obligate intracellular bacteria, rickettsiae. Rickettsial infections are emerging worldwide with increasing incidence and geographic distribution. Nonetheless, these infections are clearly underdiagnosed because methods of diagnosis are still limited and often not available. Another problem is that the bacteria respond to only a few antibiotics, so delayed or wrong antibiotic treatment often leads to a more severe outcome of the disease. In addition to that, the development of antibiotic resistance is a serious threat because alternative antibiotics are missing. For these reasons, prophylactic vaccines against rickettsiae are urgently needed. In the past years, knowledge about protective immunity against rickettsiae and immunogenic determinants has been increasing and provides a basis for vaccine development against these bacterial pathogens. This review provides an overview of experimental vaccination approaches against rickettsial infections and perspectives on vaccination strategies.
1. Introduction
Rickettsiae are small obligate intracellular bacteria of the family of Rickettsiacea that cause febrile potentially fatal diseases in humans. The family of Rickettsiacea consists of two genera, Rickettsia and Orientia. Orientia (O.) tsutsugamushi has long been considered the only member of the latter. Meanwhile, two additional species have been recently identified (candidatus O. chuto [1] and candidatus O. chiloensis [2]). The genus Rickettsia is further divided into four major groups of species according to phylogenetic relationship and way of transmission. The majority of rickettsial species belong to the spotted fever group (SFG). More than 20 species of this group have been identified so far. Prominent representatives of this group are R. rickettsii, the causative agent of Rocky Mountain spotted fever (RMSF), and R. conorii that causes Mediterranean spotted fever (MSF). The second group is the typhus group (TG) of rickettsiae that has two members, R. prowazekii, the causative agent of epidemic typhus, which was and is a serious threat in times of war, and R. typhi that causes endemic or murine typhus. The third group of pathogenic rickettsiae is the transitional group (R. felis, R. akari, R. australis), while the fourth group, the ancestral group (R. bellii and R. canadensis), is non-pathogenic.
Transmission of rickettsiae to vertebrates generally occurs through arthropods that carry the bacteria as endosymbionts in the gut epithelium. Once infected, the arthropod stays infected for life and transmits the bacteria transovarially and transstadially to the next generation. R. prowazekii is transmitted from human to human via the body louse (Pediculus humanus), while all other so-far-known rickettsial species are transmitted to humans via ectoparasites from rodents, predominantly from rats and mice. Rodents serve as a natural reservoir and play a key role in the distribution of rickettsial infections. It is, therefore, not surprising that rickettsial infections generally occur worldwide, which is especially true for TG rickettsiae while other rickettsial species appear endemic in certain areas of the world (Table 1). All SFG rickettsiae are transmitted by ticks (genera Dermacentor, Rhipicephalus, Amblyomma, Hyalomma, or Ixodes), while mites are the vectors for the transmission of orientia species (Leptotrombidium deliense) and R. akari (house mouse mite Liponyssoides sanguineus). The only so-far-known rickettsial species that are transmitted by fleas are R. typhi (predominantly the rat flea Xenopsylla cheopis) and R. felis, the causative agent of cat-flea typhus (the cat flea Ctenocephalides felis) [3].

Table 1.
Family of Rickettsiaceae, rickettsial diseases and distribution. The table gives an overview of so-far-identified members of the family of Rickettsiaceae. SFG: spotted fever group, TG: typhus group, SF: spotted fever.
In the case of TG rickettsiae, transmission occurs via deposit of feces from an arthropod that has been infected by ingesting blood from an infected rodent. The bacteria are then scratched into the wound. SFG rickettsiae can also be transmitted via the bite of an infected tick. After entering the vertebrate skin, rickettsiae first infect phagocytic cells and then spread into endothelial cells (ECs) that form the inner wall of the blood vessels and represent the dominant target cells [4,5]. Rickettsiae replicate free in the cytosol of infected ECs and are released by different mechanisms. SFG rickettsiae can induce focal lysis of the cellular membrane to be released and spread to adjacent cells [6,7]. TG rickettsiae multiply until lysis or burst of the cell [7], and orientia performs a kind of budding similar to viruses [8]. Free bacteria then infect adjacent ECs leading to local blood leakages and inflammatory reactions that can become visible as an eschar at the site of entry in the infection with some SFG rickettsiae (e.g., R. conorii but not R. rickettsii), orientia, and R. prowazekii but not R. typhi. The bacteria further systemically spread throughout the body via the bloodstream and can enter nearly all organs, where they also infect other cells, predominantly monocytes/macrophages (MΦ) [4,5,9]. These cells are considered to function as a niche for bacterial replication and to play a central role as a vehicle for dissemination [10,11,12]. Disseminated rickettsial infections cause a characteristic skin rash in approximately 60% of the patients, which is due to local blood leakages and inflammatory reactions.
The disease that is caused by the infection with different rickettsial species appears quite similar but with different severity. Patients usually develop high fever and suffer from headaches and abdominal pain. Because of the systemic distribution of the bacteria in the body, the infections can lead to multiple organ pathology, including pneumonia, meningoencephalitis, nephritis, myocarditis, hepatic damage, and other complications that can be fatal. The highest mortality is observed for the infection with R. rickettsii (>20% without antibiotic treatment and nowadays 1–7% [13]) followed by epidemic typhus caused by R. prowazekii that usually appears under poor hygienic conditions and lack of medical care (15–30%) [14,15]. Due to the relatively high mortality, R. rickettsii and R. prowazekii are classified as potential biological weapons.
Disease surveillance data are rare because diagnosis is still problematic. In a recent study from the Chinese Center for Disease Control, it was described that the incidence of orientia infections increased from 0.09/100,000 in 2006 to 1.6/100,000 in 2016 and that the disease that was originally endemic to southern China expanded to all provinces [16]. In the U.S., an increase in spotted fever rickettsioses is observed, and also endemic typhus caused by R. typhi appears with steadily increasing incidence predominantly in Southern California, Texas, and Hawaii [17,18,19]. The Centers for Disease Control (CDC) recorded 738 cases of endemic typhus in Texas in 2018 compared to 222 in 2013 and 157 in 2008 (https://www.dshs.texas.gov/IDCU/disease/murine_typhus/Typhus-2008-2018.pdf, accessed 6 August 2021).
Generally, infections with R. typhi are highly endemic in coastal tropical and subtropical regions in low-income countries in Asia [20,21,22,23], Africa [24], and South America, e.g., Mexico [25]. In Laos, it was found that the infection with O. tsutsugamushi and R. typhi was responsible for a high proportion of central nervous system infections (12% and 11%, respectively) with a high mortality rate [26]. In Europe, rickettsial infections are predominantly detected in travelers who acquired the infection abroad. Nonetheless, e.g., R. typhi also appears in Europe (Greece, Cypres, Spain, Portugal [27,28,29,30,31,32,33,34,35]). Especially homeless people are at enhanced risk. In France, the seropositivity of the homeless in Marseille dramatically increased in the past 20 years from 0.054% in the years 2000–2003 to 22% in the years 2010–2013 [36]. In addition to that, sporadic outbreaks of rickettsial infections occur that are usually associated with poor hygienic conditions. After the earthquake in Nepal in 2015, increasing cases of febrile illnesses were observed in refugee camps. These were revealed to be caused by the infection with O. tsutsugamushi and had relatively high mortality of 5.7% [37]. Since then, the transmission of O. tsutsugamushi is ongoing in Nepal. Other examples are the large outbreak of epidemic typhus during civil war in Burundi in 1995 in a jail and in refugee camps [38,39,40] and a smaller outbreak of epidemic typhus in Russia in 1997 [41].
A major problem in the recognition and treatment of rickettsial diseases is that diagnostic methods are still limited, not standardized, expensive, and often not accessible. Misdiagnosis, delayed antibiotic treatment, and treatment with inappropriate antibiotics often result in more severe or fatal disease. This was also the reason for the high mortality of O. tsutsugamushi infections after the earthquake in Nepal, where clinicians treated the patients with cefexime or ceftraiaxone and imipenem [37]. Rickettsiae and orientiae respond to only a few antibiotics (doxycycline, rifampin, chloramphenicol), with doxycycline being the treatment of choice. The development of antibiotic resistance is a great threat, and also, in cases of doxycycline intolerance, effective alternative antibiotics are missing. Finally, some rickettsial species can persist and reoccur despite antibiotic treatment, which is well known for R. prowazekii. These bacteria can reappear several years after primary infection and cause Brill-Zinsser disease [42]. The persistence of other rickettsial species in humans (R. rickettsii [43,44], R. typhi [12], as well as of O. tsutsugamushi [45]) is anticipated but has not yet been proven. At least for O. tsutsugamushi, relapse of patients that had been initially treated with antibiotics and recovered from the disease was observed months to years after the infection [45].
For the reasons mentioned above, a prophylactic vaccine against rickettsial infections is urgently needed. Vaccine design requires the understanding of protective immunity and also immunopathological reactions. This review provides a brief overview of current knowledge on immunity and immunopathology in rickettsial infections and focuses on experimental vaccination approaches and perspectives on vaccine design against these bacteria.
2. Adaptive Immunity Is Essential for Defense against Rickettsial Infections
Immunodeficient mice that lack T and B cells are highly susceptible to the infection with various rickettsiae [12,46,47], while common laboratory wild-type mouse strains such as C57BL/6 and BALB/c mice are resistant. This clearly indicates that adaptive immunity is essential for defense against rickettsiae.
Cytotoxic CD8+ T cells generally play a major role in defense against most intracellular pathogens and are also activated in the infection of mice with various rickettsiae as well as O. tsutsugamushi. The cells express IFNγ and show enhanced cytotoxic activity [48,49,50,51,52]. In case of the infection with SFG (R. rickettsii, R. conorii), transitional rickettsiae (R. australis), and O. tsutsugamushi, CD8+ T cells seem to be indispensable for defense as reflected by the observations that CD8+ T cell-deficient or depleted mice show reduced survival, enhanced bacterial burden and pathology in the infection with R. conorii, R. australis and O. tsutsugamushi [48,51,52,53]. Furthermore, mice are protected against the infection with R. conorii as well as with O. tsutsugamushi after the adoptive transfer of immune CD8+ T cells [51,53]. In the case of O. tsutsugamushi, a long-lasting CD8+ T cell response seems to be important for the control of persisting bacteria as the depletion of CD8+ T cells months after the infection leads to reactivation of the bacteria in mice [51]. Similar seems to be true for the infection with R. typhi that has also been demonstrated to persist in C57BL/6 mice as well as in BALB/c mice [12]. In concordance with the persistence of R. typhi, both mouse strains show a long-lasting CD8+ as well as a CD4+ T cell response that is even sporadically reactivated in BALB/c mice over time [49,50], indicating that both cell populations are needed for protection against recurrence.
The protective activity of CD8+ T cells in defense against the infection with SFG and transitional rickettsiae as well as O. tsutsugamushi mainly relies on the cytotoxic activity of CD8+ T cells rather than the production of IFNγ. Perforin-knockout mice that lack the cytotoxic activity of CD8+ T cells show higher susceptibility to R. australis than mice that lack the expression of IFNγ [48]. These mice also succumb to the infection with O. tsutsugamushi with enhanced bacterial burden in several organs [51].
The importance of CD8+ T cells and cytotoxic activity of these cells, however, may vary in defense against different rickettsial species. CD8+ T cells from R. typhi-infected mice also express IFNγ and granzyme B [49,50], indicating enhanced cytotoxic activity. Although it has been described that the depletion of CD8+ T cells leads to enhanced bacterial burden and pathology in R. typhi-infected C3H/HeN mice [54], other studies show that CD4+ T cells are sufficient for protection against this pathogen. In contrast to the infection with R. australis, CD8+ T cell-deficient C57BL/6 mice are not susceptible to the infection with R. typhi [50]. Moreover, the adoptive transfer of either CD8+ or CD4+ T cells into immunodeficient mice of different genetic backgrounds (C57BL/6, BALB/c) protects the animals from R. typhi-mediated disease and leads to bacterial elimination [49,50]. In contrast to the infection with SFG rickettsiae or R. australis, however, the cytotoxic activity seems to be dispensable for the protective effect of CD8+ T cells against R. typhi. This is demonstrated by the observation that BALB/c perforin-knockout mice are not susceptible to infection with this pathogen. Moreover, the transfer of Perforin-knockout CD8+ T cells still protects immunodeficient mice BALB/c CB17 SCID mice against R. typhi. Interestingly, CD8+ IFNγ-/- T cells were revealed to be less efficient than CD8+ Perforin-/- T cells to keep persisting R. typhi below the qPCR detection limit in this infection model [49,50], suggesting that the production of IFNγ by CD8+ T cells is more important than the cytotoxic activity for long-term control of the bacteria.
The protective capacity of CD4+ T cells has been demonstrated in murine infection models of SFG as well as of TG rickettsiae. Although the depletion of CD4+ T cells does not alter the course of the disease in the infection of C3H/HeN mice with a sublethal dose of R. conorii, adoptive transfer of immune CD4+ T cells protects these animals against challenge with a normally lethal dose of this pathogen [53]. Similarly, adoptive transfer of CD4+ T cells protects R. typhi-infected immunodeficient BALB/c CB17 SCID mice as well C57BL/6 RAG1-/- mice [49,50]. In these experiments, adoptive transfer of either CD8+ or CD4+ T cells was comparably protective against R. typhi, although CD8+ T cells were clearly quicker in bacterial elimination. These findings indicate that CD4+ T cells are sufficient for protection against TG rickettsiae, at least R. typhi.
CD4+ T cells differentiate into TH1 cells that produce IFNγ and TNFα in the infection with rickettsiae and orientiae. Both cytokines activate the expression of inducible nitric oxide synthase (iNOS) in infected target cells such as ECs and MΦ, which leads to the production of bactericidal nitric oxide (NO) and bacterial killing [49,55,56]. Both cytokines are important in defense against SFG as well as TG rickettsiae and orientiae [49,57], whereby IFNγ may play a more critical role in the infection with SFG and transitional rickettsiae. For example, IFNγ-deficient C57BL/6 mice show enhanced susceptibility to R. conorii as well as R. australis [48,57]. In contrast, IFNγ-knockout BALB/c mice were shown to be resistant to R. typhi [49]. In this case, it can be assumed that CD8+ T cells and the cytotoxic activity of these cells may compensate for the absence of IFNγ. However, adoptive transfer of CD4+ T cells from BALB/c IFNγ-knockout mice still protects congenic immunodeficient mice against R. typhi [49]. These cells show a TH17 phenotype and produce IL-17, TNFα, and IL-22, indicating that also TH17 cells can confer protection against R. typhi. However, TH17 cells were also shown to have pathological effects via the coproduction of IL-17 and TNFα. Neutralization of one or the other cytokine led to enhanced survival of CD4+ TH17 recipients [49].
The role of the humoral response is considered to be less important than the cellular arm of adaptive immunity in primary defense because B cells start to produce high-affinity antibodies relatively late in the infection with rickettsiae (>day 15 in the infection with R. typhi, >day 16 and >day 25 in the infection with R. conorii and R. africae) [58,59]. Nonetheless, antibodies can contribute to the protection, as demonstrated by passive immunization experiments. Administration of polyclonal immune serum from R. conorii-infected C3H/HeN mice into C3H SCID mice protects the animals against a lethal challenge with R. conorii [47]. Even in already infected C3H SCID mice, the application of immune serum leads to prolonged survival and reduced bacterial load [47]. Targets of the humoral response are likely surface proteins of the bacteria that are easily accessible for antibodies. Bound to surface proteins, antibodies can opsonize the bacteria for the uptake by phagocytes, inhibit the receptor-mediated uptake of the bacteria into target cells, or induce complement activation and bacterial destruction.
3. Immunopathology in Rickettsial Infections
Only a few descriptions of immunopathological mechanisms in rickettsial infections are found in the literature. These relate to the infection with O. tsutsugamushi and R. typhi.
O. tsutsugamushi enters MΦ and replicates in these cells. Unlike many other intracellular bacteria, it induces an M1 phenotype. O. tsutsugamushi-infected human as well as murine MΦ produce NO as well as enhanced levels of inflammatory cytokines including IL-1β and TNFα [60,61,62]. It has recently further been shown that O. tsutsugamushi not only survives and replicates in murine MΦ despite the presence of NO, but that NO even enhances bacterial replication in MΦ [63]. This M1 polarization likely depends on TLR2 as O. tsutsugamushi has been demonstrated to use this receptor to induce the secretion of TNFα and IL-6 in DCs [64]. Although IL-1β, TNFα, and other pro-inflammatory cytokines that are produced by O. tsutsugamushi-infected MΦ and DCs contribute to a protective TH1-polarized immune response, they also induce inflammatory reactions in the tissue environment. Therefore, M1 MΦ are considered to be largely responsible for tissue pathology that is observed in scrub typhus patients [62]. In support of this, it was shown that O. tsutsugamushi-infected Toll-like receptor 2 (TLR2)-/- C57BL/6 mice were even better protected from lethal infection compared to wild-type mice and showed lower bacterial burden and milder symptoms of disease [64]. These observations indicate that the inflammatory effects of MΦ and maybe also DCs are responsible for more severe disease and that TLR2 is dispensable for the induction of protective immunity.
In addition to that, also CD8+ T cells have been shown to be involved in MΦ-mediated tissue pathology in experimentally infected mice. O. tsutsugamushi-infected C57BL/6 mice show lung inflammation and hepatic injury. The latter was shown to be dependent on the infiltration of CD8+ T cells, followed by MΦ infiltration. Furthermore, inflammation of the lung could be attributed to CD8+ T cells [51]. These observations indicate a positive feedback mechanism between activated CD8+ T cells and MΦ, most likely via CD8+ T cell-derived IFNγ as an activator of MΦ, that accelerates the inflammatory response and leads to enhanced pathology in scrub typhus disease. On the other hand, O. tsutsugamushi-infected CD8+ T cell-deficient mice show enhanced lethality and uncontrolled bacterial growth, although these mice produce enhanced IFNγ levels and show stronger MΦ responses in the organs, which is correlated to enhanced tissue damage [51]. In this case, it is speculated that the absence of CD8+ T cells results in enhanced activation of CD4+ T cells as a compensatory mechanism and that IFNγ that is produced by these cells drives MΦ activation and pathology. Together these observations indicate that especially IFNγ, produced by either CD8+ or CD4+ T cells, can enhance tissue pathology by activating MΦ.
Other, yet unclear mechanisms, that may contribute to pathology are the development of anti-nuclear antibodies (ANAs) that are observed in around 40% of scrub typhus patients [65] and the release of IL-17. Levels of IL-17 are generally enhanced in scrub typhus patients and higher in patients who suffer from headaches [66], suggesting a causal relationship.
Inflammatory MΦ can clearly enhance tissue damage. Other cells of the innate immune system that can be involved in pathology also include neutrophils. R. typhi-infected immunodeficient BALB/c CB17 SCID mice develop severe liver necrosis. In the absence of neutrophils upon depletion of this cell population, the mice succumb to the infection with the same kinetics and develop comparable bacterial loads in all organs as control groups, but the depletion of neutrophils completely prevents liver damage [46]. In addition to neutrophils, also MΦ play clearly a role in pathology in the infection with R. typhi. In contrast to the infection with O. tsutsugamushi, however, MΦ hardly respond to R. typhi in vitro and do not show an M1 phenotype per se. They do not produce inflammatory cytokines or NO upon infection with R. typhi but exclusively upregulate major histocompatibility class I (MHCI) [46]. This indicates that the bacteria are either not recognized in a classical manner, e.g., via TLR, or that the bacteria actively suppress MΦ activation. In BALB/c CB17 SCID mice, R. typhi predominantly resides in MΦ [46]. MΦ also expressed iNOS and produced, together with NK cells, high amounts of IFNγ. The expression of iNOS, however, was restricted to those MΦ that did not harbor R. typhi [46], indicating that activation of these cells appears through indirect mechanisms, probably endogenous danger signals that are released from damaged tissue or IFNγ produced by NK cells. In another model of R. typhi infection (immunodeficient C57BL/6 RAG1-/- mice), the bacteria are also found predominantly in MΦ. These mice develop severe central nervous system (CNS) inflammation, which is due to massive accumulation and activation of microglia as well as to the presence of infiltrating MΦ. In contrast to BALB/c CB17 SCID mice, these infiltrating MΦ carry R. typhi and express iNOS [12]. Here, the expression of iNOS and CNS inflammation is largely enhanced by the adoptive transfer of immune CD4+ T cells but not CD8+ T cells relatively late in the infection, although the bacteria were efficiently eliminated by both cell populations [50]. This observation suggests that brain inflammation in this model is mainly due to immunopathology rather than cellular destruction by the bacteria themselves. This MΦ activation can be put down to the release of IFNγ by CD4+ T cells [50] and again, as in the infection with O. tsutsugamushi, demonstrates that the T cell-derived IFNγ-MΦ axis, although essential for protection, has pathological side effects.
Whether immunopathology plays a role in the infection with SFG rickettsiae is unclear. R. conorii has been demonstrated to induce an MΦ M2 phenotype with reduced production of reactive oxygen species (ROS), among other effects that inhibit pro-inflammatory signaling and M1 polarization [10]. This argues against a major contribution of MΦ to pathology in the infection with SFG rickettsiae agents.
4. Vaccination against Rickettsiae with Whole-Cell Antigen (WCA)
The first attempts of immunization against rickettsiae were made with inactivated intact bacteria that were either produced in arthropods, embryonated chicken eggs, embryonal chicken fibroblasts, or infected animals. The first whole-cell antigen (WCA) vaccines against R. prowazekii and R. rickettsii were produced already in the 1920s. R. L. Weigl produced R. prowazekii by intrarectal injection of the bacteria into lice and fed the arthropods on humans. The bacteria were prepared from the gut of the lice and inactivated in phenol. This vaccine not only protected guinea pigs from disease [67] but was also used for the vaccination of German soldiers during World War II [68]. A similar vaccine was produced by the U.S. military at the same time where R. prowazekii was grown in chicken egg yolk sacs and inactivated in formalin. Vaccination of U.S. soldiers during World War II ameliorated disease [69]. Another vaccine against epidemic typhus was produced by isolation of R. prowazekii from the lungs of infected rabbits (Castaneda vaccine) [69] or the tunica vaginalis and the peritoneum from infected rats (Zinsser-Castaneda vaccine) and inactivation of the bacteria in formalin [70]. The application of three doses of the Zinsser-Castaneda vaccine was sufficient to protect guinea pigs from the disease [71].
Similarly, in 1924 the first WCA vaccine against RMSF was developed by growing R. rickettsii in ticks that were fed on guinea pigs. The bacteria were isolated by triturating the arthropods and inactivated in phenol and formalin [72]. In another attempt, R. rickettsii was grown and isolated from embryonated chicken eggs. In this way, the Cox vaccine, also inactivated R. rickettsii, was produced [73]. Administration of these inactivated bacteria leads to milder disease in humans [73] and the production of antibodies but does not completely prevent infection and disease [74]. Similarly, formalin-inactivated R. rickettsii that were produced by the U.S. military in the 1970s in embryonal chicken fibroblasts [75,76] protected cynomolgus and rhesus monkeys after two times immunization [77,78]. This vaccine ameliorated disease in humans but did not prevent the infection [79].
Formalin-inactivated bacteria were also used for first attempts for vaccination against scrub typhus. Here, O. tsutsugamushi was isolated from homogenized formalin-fixed lungs from infected cotton rats [80,81] or purified, followed by formalin-inactivation [82]. The vaccination with such inactivated O. tsutsugamushi only partially protected mice against challenge with the homologous bacterial strain in early studies from the 1940s [83,84], while a more recent study described protection of C3H/HeN mice against challenge with the homologous strain and long-term immunity (>8 months) [85]. Vaccination of humans, however, did not prevent infection and disease [82].
Phenol or formalin treatment of the bacteria can result in the modification of antigenic determinants, which could explain the ineffectiveness of such vaccines. Other possibilities of inactivation that can preserve antigenic structures include heat-inactivation at 56 °C or irradiation. Irradiated O. tsutsugamushi was found to protect mice against challenge with homologous bacteria [86,87,88], and heat-inactivated R. rickettsii protected dogs from severe RMSF [89], indicating a higher protective capacity compared to formalin-fixed bacteria.
Another possibility is the use of avirulent or attenuated bacteria. Examples are the vaccination with a low-virulence strain of O. tsutsugamushi that efficiently induces immunity in humans [90] and vaccination with R. prowazekii strain Madrid E. This strain was isolated during World War II and lost virulence during several passages through embryonated chicken eggs. R. prowazekii Madrid E has been successfully used for the vaccination of humans and induces long-term immunity up to approximately five years [91,92]. The use of such strains, however, bears the risk of reversion to a pathogenic form. Avirulence of R. prowazekii Madrid E is due to a mutation in the methyltransferase that is responsible for methylation of surface proteins, including OmpB. In R. prowazekii Madrid E, this protein, as well as other surface proteins, is hypomethylated [93]. After passage through mice, R. prowazekii Madrid E shows a reversion of this mutation, and reisolates of these bacteria (R. prowazekii Evir) are pathogenic again [94].
Stably attenuated rickettsial strains that can be produced by the introduction of mutations or the deletion of virulence genes may provide a safer way of vaccination. Genetic manipulation of rickettsiae is possible, and an attenuated strain of R. prowazekii was produced by site-directed knockout of the gene encoding for phospholipase D [95] that is involved in phagosomal escape [96]. Guinea pigs that were immunized with these bacteria were protected against lethal challenge with virulent R. prowazekii [95].
Although attenuated mutant or knockout strains are promising vaccine candidates, virulence factors that are essential for infectivity and pathogenicity are largely unknown and still need to be identified. These may include other proteins that are involved in bacterial adherence and invasion, e.g., OmpA and OmpB. Knockout of OmpA, however, did not influence the infectivity of R. rickettsii in guinea pigs [97]. Another problem with this kind of vaccine is that large-scale production is time-consuming and expensive, and hardly applicable for the immunization of a larger portion of people in affected areas.
Therefore, other strategies and vaccines that can be produced much more easily at larger amounts are needed.
5. Immunogenic Determinants and Vaccine Candidates
The development of such vaccines requires the knowledge of immunodominant rickettsial antigens that can induce protective adaptive immune responses as well as the elucidation of the optimal way of antigen delivery.
So far, only a few rickettsial antigens have been described. Most of these have been identified because they are recognized by antibodies. The most prominent ones belong to the surface cell antigen (Sca) autotransporter family (Sca 0–5) that are involved in bacterial adherence and uptake into target cells. Among this family, especially the outer membrane protein A (OmpA/Sca0), which is not expressed by TG rickettsiae, and OmpB/Sca5 represent immunodominant surface antigens that are recognized by antibodies and also by T cells in infected mice and patients [98]. Passive immunization with antibodies against OmpA and OmpB protects C3H/HeN mice and guinea pigs from normally lethal challenges with R. rickettsii and R. conorii [99,100,101,102] and even C3H SCID mice against infection with R. conorii [47]. Antibodies against OmpA and OmpB have been shown to enhance the uptake of R. conorii by phagocytic cells [103], to inhibit adherence of R. rickettsii to L929 cells [104] as well as to mediate complement-mediated killing of the bacteria [102] so that all three mechanisms may contribute to protection.
The majority of other antigens described in the literature are also surface-expressed proteins that are predominantly recognized by antibodies. Exceptions are Sca4 and the molecular chaperone GroEL, both of which are cytosolic proteins. GroEL, however, has also been demonstrated to be surface-exposed in SFG as well as TG rickettsiae and to be recognized by antibodies [105,106] that can enhance bacterial uptake into phagocytes [106]. Table 2 provides an overview of all so-far-identified rickettsial antigens.

Table 2.
Overview on experimentally identified rickettsial immunogens, their localization, and recognition by B and/or T cells. The table provides an overview of so-far-known antigens from rickettsiae and orientia, their localization and function, and whether they are recognized by B and/or T cells. OM: outer membrane, IM: inner membrane, C: cytoplasm, P: periplasm, EC: extracellular, √: experimentally proven recognition. Empty fields: not described.
Only a few of these proteins (OmpA, OmpB, Adr2, YbgF, and ScaA from orientia) have been shown to be also detected by T cells. Experimental evidence for the recognition by B and T cells of these antigens is reviewed elsewhere in more detail [113]. Generally, data on antigen-specific T cell responses, however, are rare, and immunodominant antigens that are recognized by CD4+ and/or CD8+ T cells still need to be identified. Experimentally, this can be achieved by immunoprecipitation of MHCII from professional APCs such as DCs and MΦ treated with live or inactivated bacteria, or of MHCI from cells infected with rickettsiae. Bound peptides can then be identified by mass spectrometry. Such studies, however, are still missing.
Other possibilities include the use of bioinformatic tools. Meanwhile, several bioinformatic tools are available that can assist in the determination of antigenic proteins and vaccine design by predicting the general immunogenicity of a protein (Vaxign and Vaxitope [114], VaxiJen [115]), potential B cell epitopes (ANTIGENpro, APBpred, Epitome [116,117,118]), potential CD4+ and CD8+ T cell epitopes and the probability of MHCI or MHCII presentation (PREDBALB/c, PRED(TAP), MHCPred, NetMHCpan, NetMHCIIpan, IEBD Analysis Resource, RANKPEP and SYFPEITHI [119,120,121,122,123,124,125,126]). In addition, knowledge of the predicted localization of a protein (SOSUIGramN, pSORTb, SignalP, SecretomeP [127,128,129,130]) and its function can be helpful to estimate whether it might be accessible for protective antibodies or the MHCI and MHCII presentation pathways to be recognized by CD4+ or CD8+ T cells. Bioinformatic approaches have been successfully used for the identification of five antigens from R. prowazekii that are recognized by CD8+ T cells (RP403, RP598, RP739, RP778, RP884) [131,132]. These antigens were expressed in SVEC 4–10 cells, and immunization of mice with these cells induced antigen-specific CD8+ T cells that produced IFNγ and granzyme B and protected the mice from lethal challenge with R. typhi [131,132].
Except for RP884, which is a cytosolic protein, the other four proteins are surface-exposed. Generally, it can be assumed that surface-exposed proteins or proteins that are released by the bacteria are accessible for the proteasome in the cytosol for degradation and transport into the MHCI presentation pathway for recognition by CD8+ T cells.
6. Experimental Approaches of Vaccination against Rickettsiae
Because of the important role of CD8+ T and CD4+ T cells in protection against rickettsiae, it stands to reason that a vaccine should address cellular immune responses, ideally in addition to the production of antibodies. While the induction of CD4+ T cell responses can be easily achieved by the application of recombinant protein, the difficulty in addressing CD8+ T cells with a vaccine lies in the delivery of the antigen into the cytosol of host cells to gain access to the MHCI presentation pathway. Antigen delivery into the cytoplasm of host cells can be achieved by different methods such as immunization with nucleotides, vector-based vaccines, or the use of APCs that express rickettsial antigens. Experimental approaches to vaccination against rickettsiae are described in the following. Figure 1 provides an overview of all experimental vaccination approaches described so far in the literature.
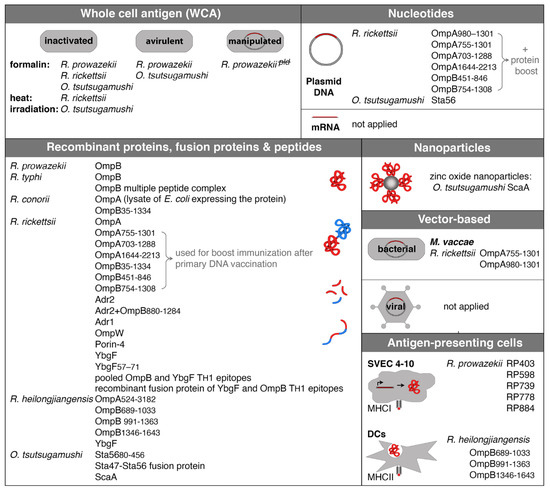
Figure 1.
Experimental approaches of vaccination against rickettsiae. The figure shows all so far described approaches of vaccination against rickettsiae and names strains and antigens. WCA immunization was performed with either inactivated bacteria or avirulent strains. In addition, an attenuated knockout strain of R. prowazekii that lacks phospholipase D was generated and used for immunization in experimental infection of mice. The vast majority of vaccinations were performed with recombinant proteins, fusion proteins, or peptides. Other methods include recombinant protein coupled to nanoparticles, bacteria (M. vaccae), or transfected cells that express rickettsial antigens and DCs that were pulsed with recombinant protein. mRNA vaccination and vaccination with adenoviral vectors as they are used now for the immunization against SARS-Cov2 with great success have not been applied yet, but represent great new tools that should be taken under consideration.
6.1. Immunization with Recombinant Proteins and Peptides
A conventional way of immunization is the application of recombinant proteins, and most approaches of vaccination against rickettsial infections in experimental animal models used either proteins, protein fragments, fusion proteins, or peptides.
OmpA and OmpB are clearly immunodominant antigens that have been extensively used for the experimental vaccination of mice. Both proteins are recognized by B as well as by T cells in the infection of animals and humans. T cells from R. rickettsii, R. typhi and R. felis react to MΦ that express fragments of R. rickettsii OmpB with the release of IL-2 and IFNγ, indicating the recognition of peptides presented by MHCI by CD8+ T cells and cross-reaction of T cells to conserved OmpB epitopes between SFG and TG rickettsiae [133]. This is an important point with regard to vaccination because conserved proteins such as OmpB have the potential to mediate immunity against various rickettsial species. In the case of OmpA and OmpB, recombinant proteins or protein fragments have been used for experimental immunization of animals, mainly mice or guinea pigs. Vaccination of guinea pigs with recombinant OmpA from R. rickettsii or truncated OmpA from R. heilongjiangensis protects the animals against challenges with the homologous bacteria [134,135]. In the case of the immunization with R. heilongjiangensis OmpA, also cross-protection against R. rickettsii was achieved [135]. Here, antibody production may well play a role in protection. The transfer of monoclonal antibodies against OmpA has been shown to protect immunodeficient mice from fatal infection with R. conorii [47]. The same was true for the application of monoclonal antibodies against OmpB [47]. In another study, it was shown that guinea pigs were protected against challenge with R. conorii and partially protected against the infection R. rickettsii upon immunization with a lysate from E. coli that expressed OmpA [136].
Similarly, vaccination of guinea pigs with OmpB from R. typhi protected the animals against challenge with this pathogen [137]. The immunization of rabbits with OmpB from R. prowazekii induces antibody production, and B cell epitopes were identified by the analysis of antibody binding to overlapping synthetic peptides (Table 2) [138]. An additional B cell epitope was determined from R. typhi OmpB and from R. typhi Sca1, Sca2, Sca3, and Sca4 [139]. All of these peptides are recognized by antibodies upon immunization of rabbits with a multiple peptide antigen conjugate [139].
Further, also epitopes that are recognized by CD4+ T cells and CD8+ T cells have been identified from R. rickettsii and R. conorii OmpB and from another protein, YbgF, from R. rickettsii (Table 2). Immunization of C3H/HeN mice with pooled CD4+ T cell epitopes from OmpB and YbgF or a fusion protein of these epitopes resulted in the induction of CD4+ TH1 cells that produced TNFα and IFNγ as well as in enhanced IgG1 and IgG2a production and reduced bacterial load upon infection with R. rickettsii [140].
The immunization of C3H/HeN mice with recombinant YbgF protein leads to enhanced proliferation and IFNγ release by both CD4+ and CD8+ T cells, prolonged IgG2a and IgG1 production, and reduced bacterial burden in the infection with R. rickettsii [141]. Vaccination with another recombinant immunogen from R. rickettsii, TolC, was less efficient than immunization with YbgF [141]. Similarly, the immunization of C3H/HeN mice with recombinant YbgF from R. heilongjiangensis results in reduced bacterial load upon infection with homologous bacteria [142]. The authors further demonstrate that YbgF is recognized by CD4+ T cells as well as by B cells in the infection with R. heilongjiangensis [142]. Other immunogenic proteins that have been used for experimental vaccination are Adr1, Adr2, OmpW, and Porin-4 from R. rickettsii. The immunization of C3H/HeN mice with recombinant Adr1, TolC, OmpW, or Porin-4 results in reduced bacterial load upon challenge with R. rickettsii [111]. Similarly, the immunization with recombinant Adr2 protected the animals from R. rickettsii infection and led to enhanced production of IFNγ by CD4+ T cells and TNFα by CD8+ T cells and increased IgG2a and IgG1 production [143]. Adr2 and OmpB have also been used in combination for experimental vaccination with the same effect [144].
In the case of orientia that phylogenetically differs from rickettsiae, three proteins have been used for experimental vaccination: Sta47, Sta56, and ScaA. O. tsutsugamushi-infected mice, as well as humans, develop Sta56-specific antibodies and CD4+ T cells [145,146,147] and antibodies against Sta47 [148]. vSta56-immunized mice produced Sta56-specific antibodies and showed enhanced proliferation of lymphocytes, which was associated with increased IFNγ and IL-2 production. Moreover, the mice were protected against challenge with the homologous O. tsutsugamushi strain, which produced enhanced antibody levels and lymphocytes showed increased proliferation and IFNγ and IL-2 release [149,150,151]. In a more recent study, conserved blocks of the Sta56 protein were used for the immunization of mice. This vaccination not only conferred protection against the infection with homologous bacteria but also heterologous orientia genotypes [152]. The authors further synthesized overlapping peptides from the Sta56 protein and could identify 39 peptides that are recognized by CD8+ T cells. Immunization with a mixture of these peptides also provided protection against lethal challenge with O. tsutsugamushi [152], underlining the protective activity of cytotoxic CD8+ T cells. In addition, a fusion protein of Sta56 and Sta47 has been used for experimental vaccination, which was, however, only partially protective against the infection with homologous orientia [153], and the vaccination of primates (Macaca fascicularis) with a recombinant Sta56 fragment (Sta5680–456) was only weakly protective and did not prevent disease and rickettsemia [154].
Other immunogenic proteins from orientia are the surface proteins Sta22, ScaA, ScaC, ScaD, and ScaE. All of these antigens have been shown to be recognized by antibodies in the infection with O. tsutsugamushi with a stronger response to ScaA and ScaC compared to ScaE [155,156]. In the case of Sta22, it has been shown that O. tsutsugamushi-infected mice develop Sta22-specific CD4+ T cells [155]. Of these proteins, only recombinant ScaA and ScaC have been used for experimental vaccination. Of these, only the immunization with ScaA protected mice from challenge with homologous as well as heterologous orientia strains [157]. The authors further showed that ScaA-specific antibodies inhibit the uptake of orientia by non-phagocytic HeLa cells.
6.2. Immunization with Antigen-Coupled Nanoparticles
There is one description in the literature where antigen-coupled nanoparticles were used for the immunization of mice against O. tsutsugamushi. Recombinant ScaA protein from O. tsutsugamushi was coupled to zinc oxide nanoparticles. These particles were taken up by DCs in vitro. Immunization of C57BL/6 mice with these particles induced CD4+ as well as CD8+ T cells that produced IFNγ as well as the generation of antibodies. Moreover, ScaA/nanoparticle immunization protected the animals against lethal challenge with O. tsutsugamushi [158]. The use of nanoparticles for vaccine development has gained interest in the past years because nanoparticles can stabilize antigens and enhance the uptake of antigen by APCs, and in this way, overcome otherwise probably low immunogenicity. Furthermore, the use of nanoparticles allows targeted antigen delivery and slow release [159]. However, there are no further descriptions of the use of nanoparticles for experimental immunization against rickettsia.
6.3. Immunization with Nucleotides
One possibility of cytotoxic CD8+ and CD4+ TH cell-oriented vaccination is the use of DNA. DNA immunization has been proven in various animal models of infections to efficiently induce cellular immunity. Upon intramuscular or intradermal application, the DNA is taken up by muscle cells and monocytes that then start to express the encoded protein. Intracellular cytosolic processing of the protein results in the generation of antigenic peptides that are presented by MHCI molecules for recognition by CD8+ T cells. In addition, CD4+ T cells can be induced by APCs that engulf the protein when released from the cells and present antigenic peptides via MHCII molecules [160,161].
DNA vaccination has been successfully applied for the induction of protective immunity against SFG rickettsiae and O. tsutsugamushi in experimental murine infection models. Heterologous prime-boost immunization was used for vaccination against SFG rickettsiae. DNA encoding for fragments of the OmpA protein (OmpA703–1288, OmpA755–1301 or OmpA980–1301 or OmpA1644–2213) from R. conorii in addition to a plasmid encoding for IL-12 was used for primary immunization followed by boost immunization with the corresponding recombinant protein fragments. In this way, lymphocytes were induced that produced IFNγ upon in vitro restimulation with R. conorii whole-cell antigen, and the mice were protected against normally lethal challenge with R. conorii [162,163]. Similarly, primary immunization with DNA encoding for fragments of the OmpB protein from R. conorii (OmpB451–846 or OmpB754–1308) followed by boost immunization with the corresponding recombinant protein fragments led to the same result, and combined DNA immunization with plasmids encoding for four protein fragments of OmpA and OmpB (OmpA703–1288 and OmpA1644–2213 or OmpB451–846 and OmpB754–1308) was protective against lethal challenge with the pathogen [163]. In the case of O. tsutsugamushi, DNA encoding for the Sta56 protein was used for the immunization of mice. Here, single immunization with the plasmid vector was not sufficient for protection, while partial protection (60% of the mice) was achieved after four immunizations with plasmid DNA [164].
The application of DNA, however, bears the risk of integration and persistence of the introduced DNA in the cellular genome. In addition, DNA vaccination can induce the generation of anti-DNA antibodies that can have serious side effects. A more elegant, safer, and modern way is the immunization with mRNA, which is shortly described in the following and reviewed in more detail elsewhere [165]. The mRNA encodes for an antigen or a part of an antigen and instructs the cells to transiently produce the encoded protein without the need to pass the nucleus membrane and without integration into the cellular genome. Conventional mRNAs carry the coding sequence of an antigen flanked by regulatory regions. Another form of mRNA vaccine is based on the modification of the genome of positive-stranded RNA viruses to obtain self-amplifying mRNA encoding for the antigen of choice, which ensures prolonged and robust expression of the antigen and subsequently better induction of adaptive immune responses. Conventional as well as self-amplifying mRNA vaccines are usually delivered packed with lipid nanoparticles (LNPs) as a vehicle that enhances the uptake of the material into the cell. Another method of delivery is the complexation of the mRNA with nucleotide-binding peptides such as protamine that stabilizes the mRNA and enhances its uptake into the cell. Apart from that, protamine acts as adjuvants by activating innate cells via the pattern recognition receptors (PRR) TLR7 and 8 [166,167,168]. In addition, bacterial and viral RNAs have been shown to be recognized by TLRs 3 and 7 [169], and in this way, possess an intrinsic adjuvant effect themselves [170,171]. This adjuvant effect, especially on professional APCs, is needed for efficient induction of adaptive immune response, and the complexation of mRNA with protamine has been demonstrated to enhance cytotoxic CD8+ T cell and CD4+ TH1 responses [167].
As these responses are also desired in protection against rickettsiae, mRNA vaccination is a promising strategy but has not been applied yet. Generally, the design of DNA as well as mRNA vaccines is quite flexible and offers the opportunity to combine several antigenic determinants from different proteins to obtain a broader spectrum of antigen-specific adaptive immune responses.
Regarding DNA and also mRNA immunization, one could also think of constructs that encode for a combination of antigenic determinants from different proteins, probably as a fusion protein. For example, CD4+ T cell epitopes have been identified from the R. rickettsii OmpB protein (OmpB152–166 (QNVVVQFNNGAAIDN), OmpB399–413 (NTDFGNLAAQIKVPN), OmpB563–577 (TIDLQANGGTIKLTS), OmpB698–712 (TNPLAEINFGSKGVN) and OmpB1411–1425 (NLMIGAAIGITKTDI)) and from the R. rickettsii YbgF protein (YbgF57–71 (LQHKIDLLTQNSNIS) [140]. These peptides, used alone or pooled or expressed as a recombinant fusion protein, induced protective immunity in C3H/HeN mice with the induction of CD4+ TH1 cells and antibody response [140]. A comparable vaccine that primarily addresses CD4+ T cells may also be sufficient for protection against R. typhi, and DNA or mRNA constructs could be designed for the expression of such fusion proteins.
Furthermore, five CD8+ T cell epitopes from the R. conorii OmpB protein have been described (OmpB708–716 (SKGVNVDTV), OmpB789–797 (ANSTLQIGG), OmpB812–820 (IVEFVNTGP), OmpB735–743 (ANVGSFVFN), and OmpB749–757 (IVSGTVGGQ) [172] that could also be integsrated into a fusion construct to obtain a CD8+ T cell response in addition to CD4+ T cells and antibody production.
The development of efficient DNA or mRNA vaccines may require codon-optimization to enable robust expression of rickettsial antigens in eukaryotic cells because the rickettsial genome possesses a very high A/T content. In addition, the efficacy of DNA and mRNA immunization can be generally significantly improved by several methods [173], e.g., the use of liposomes that facilitate the uptake into the cells after injection, the use of adjuvants or bicistronic constructs encoding for the antigen of choice in addition to costimulatory molecules or cytokines such as IL-12 that contribute to more efficient immune induction.
6.4. Vector-Based Immunization: Adenoviral Vectors
Genetically engineered replication-incompetent adenoviral vectors allow the efficient introduction of the transported genetic material into eukaryotic cells and have the potential to induce potent humoral as well as cellular responses. Different adenoviral vectors are in use for vaccination against SARS-Cov2, and adenoviral vectors have been studied as carriers for vaccinating antigens from several pathogens such as human immunodeficiency virus type I (HIV-1), Plasmodium falciparum, and Mycobacterium (M.) tuberculosis [174,175,176]. Different types of human adenoviral vectors have been studied. Human adenoviral vectors may be recognized by preexisting antibodies that are found in a very large proportion of the population. These antibodies can reduce the vector uptake and expression of the transgene, leading to reduced specific immune responses [177,178,179]. A chimpanzee adenoviral vector is an alternative. The immunizing effect of adenoviral vectors is generally very promising. Use for vaccination against rickettsiae has not been described yet, but should be taken into consideration in the future. This method offers similar opportunities as the design of DNA or mRNA vaccines with regard to flexibility in the combination of different antigens.
6.5. Vaccination with Genetically Modified Bacterial Vectors
Mycobacterium (M.) vaccae is an environmental member of the mycobacterial family and non-pathogenic for humans. It belongs to the same genus as M. tuberculosis, contains many homologous antigens, and is a promising vaccine in humans to prevent tuberculosis (e.g., Vaccae™ vaccine) used in an irradiation-killed or heat-killed form [180]. Immunization of mice with heat-killed M. vaccae itself induces cytotoxic CD8+ T cells that react to M. tuberculosis-infected MΦ and produce IFNγ and [181] and triggers a CD4+ TH1 response [182]. M. vaccae was also used for the expression of M. tuberculosis antigens. Applied to mice, such vaccine induces a TH1-biased M. tuberculosis antigen-specific response [183].
Similarly, genetically modified M. vaccae can potentially be used to induce immunity against other pathogens, including rickettsiae, as described in one study. Here, a plasmid encoding for fragments of the OmpA protein from R. rickettsii (OmpA755–1301 or OmpA980–1301) was introduced into M. vaccae. The engineered bacteria were then used for the immunization of C3H/HeN mice, followed by a boost immunization with recombinant OmpA755–1301 or OmpA980–1301 protein. In this way, IFNγ-producing rickettsia-specific T cells were induced, and the immunization mediated partial protection against challenge with R. conorii at a normally lethal dose [162].
6.6. Immunization with Antigen-Expressing Cells or Antigen-Pulsed APCs
Recently, CD8+ T cell antigens from R. prowazekii have been identified by bioinformatic approaches in reverse vaccinology (RP403, RP598, RP739, RP778, RP884) [131,132]. These antigens were recombinantly expressed in SVEC 4–10 cells to be presented by MHCI. Transfected SVEC 4–10 cells were then used for the immunization of C3H/HeN mice. The antigens were recognized by CD8+ T cells, and the immunization induced protective immunity to lethal challenge with R. typhi [131,132]. These are the only descriptions of the use of antigen-expressing cells for immunization, however.
Another possibility to induce CD4+ T cell responses is the use of professional APCs that are pulsed with recombinant antigenic proteins. This approach has been applied for immunization against the infection with R. heilongjiangensis. Bone marrow-derived DCs (bmDCs) from C3H/HeN mice were pulsed with recombinant fragments of the OmpB protein from the bacteria (OmpB689–1033, OmpB991–1363, OmpB1346–1643) and transferred into naïve C3H/HeN mice followed by challenge with R. heilongjiangensis 14 days afterward. The immunization resulted in reduced bacterial load and led to the activation of CD4+ as well as CD8+ T cells that produced IFNγ and TNFα, indicating a CD4+ TH1-biased and cytotoxic CD8+ T cell response [184].
These methods are highly interesting for the determination of immunogenic parts of a protein but would only be applicable for the immunization of individuals because the MHC haplotype must match for the recognition by T cells.
7. Conclusions
Several animal models for the infection with various rickettsiae have been used for experimental vaccination against these pathogens with different methods and some success. A limiting factor still is missing knowledge about immunogenic rickettsial determinants in general and especially about those that are recognized by T cells. As the cellular arm of the adaptive immune response clearly plays a dominant role in defense against rickettsial infections, such antigens need to be identified. Another focus should be the way of antigen delivery. So far, recombinant proteins and plasmid DNA immunization have been predominantly used for experimental vaccination of animals. Other promising ways of antigen delivery include the use of mRNA and adenoviral vectors, both of which are now successfully in use against the SARS-Cov2 pandemic. Other aspects that should be addressed and taken into consideration include the use of appropriate adjuvants and heterologous or homologous prime/boost regimens.
Funding
The author is funded by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG; No OS583/1-1).
Institutional Review Board Statement
Not applicable. This review article does not contain studies involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The origin of all data mentioned in the text is cited in the reference list.
Acknowledgments
I thank Bernhard Fleischer for carefully reading the manuscript and discussions.
Conflicts of Interest
The author declares no conflict of interest.
References
- Izzard, L.; Fuller, A.; Blacksell, S.D.; Paris, D.H.; Richards, A.L.; Aukkanit, N.; Nguyen, C.; Jiang, J.; Fenwick, S.; Day, N.P.; et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010, 48, 4404–4409. [Google Scholar] [CrossRef]
- Abarca, K.; Martinez-Valdebenito, C.; Angulo, J.; Jiang, J.; Farris, C.M.; Richards, A.L.; Acosta-Jamett, G.; Weitzel, T. Molecular Description of a Novel Orientia Species Causing Scrub Typhus in Chile. Emerg. Infect. Dis. 2020, 26, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Abdad, M.Y.; Abou Abdallah, R.; Fournier, P.E.; Stenos, J.; Vasoo, S. A Concise Review of the Epidemiology and Diagnostics of Rickettsioses: Rickettsia and Orientia spp. J. Clin. Microbiol. 2018, 56, e01728-17. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, P.; Vitale, G.; Cascio, A.; Seidita, A.; Pepe, I.; Carroccio, A.; di Rosa, S.; Rini, G.B.; Cillari, E.; Walker, D.H. New insight into immunity and immunopathology of Rickettsial diseases. Clin. Dev. Immunol. 2012, 2012, 967852. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.K.; Rydkina, E. Host-cell interactions with pathogenic Rickettsia species. Future Microbiol. 2009, 4, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.J.; Bond, S.B. Infection of human vascular endothelial cells by Rickettsia rickettsii. J. Infect. Dis. 1984, 149, 201–206. [Google Scholar] [CrossRef]
- Hackstadt, T. The biology of rickettsiae. Infect. Agents Dis. 1996, 5, 127–143. [Google Scholar]
- Kim, M.J.; Kim, M.K.; Kang, J.S. Involvement of lipid rafts in the budding-like exit of Orientia tsutsugamushi. Microb. Pathog. 2013, 63, 37–43. [Google Scholar] [CrossRef]
- Radulovic, S.; Price, P.W.; Beier, M.S.; Gaywee, J.; Macaluso, J.A.; Azad, A. Rickettsia-macrophage interactions: Host cell responses to Rickettsia akari and Rickettsia typhi. Infect. Immun. 2002, 70, 2576–2582. [Google Scholar] [CrossRef][Green Version]
- Curto, P.; Riley, S.P.; Simoes, I.; Martinez, J.J. Macrophages Infected by a Pathogen and a Non-pathogen Spotted Fever Group Rickettsia Reveal Differential Reprogramming Signatures Early in Infection. Front. Cell. Infect. Microbiol. 2019, 9, 97. [Google Scholar] [CrossRef]
- Drevets, D.A.; Leenen, P.J.; Greenfield, R.A. Invasion of the central nervous system by intracellular bacteria. Clin. Microbiol. Rev. 2004, 17, 323–347. [Google Scholar] [CrossRef]
- Osterloh, A.; Papp, S.; Moderzynski, K.; Kuehl, S.; Richardt, U.; Fleischer, B. Persisting Rickettsia typhi Causes Fatal Central Nervous System Inflammation. Infect. Immun. 2016, 84, 1615–1632. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.J.; Traeger, M.S.; Humpherys, D.; Mahoney, D.L.; Martinez, M.; Emerson, G.L.; Tack, D.M.; Geissler, A.; Yasmin, S.; Lawson, R.; et al. Risk factors for fatal outcome from rocky mountain spotted Fever in a highly endemic area-Arizona, 2002–2011. Clin. Infect. Dis. 2015, 60, 1659–1666. [Google Scholar] [CrossRef]
- Kuloglu, F. Rickettsial infections. Dis. Mol. Med. 2013, 1, 39–45. [Google Scholar] [CrossRef]
- Raoult, D.; Woodward, T.; Dumler, J.S. The history of epidemic typhus. Infect. Dis. Clin. N. Am. 2004, 18, 127–140. [Google Scholar] [CrossRef]
- Li, Z.; Xin, H.; Sun, J.; Lai, S.; Zeng, L.; Zheng, C.; Ray, S.E.; Weaver, N.D.; Wang, L.; Yu, J.; et al. Epidemiologic Changes of Scrub Typhus in China, 1952–2016. Emerg. Infect. Dis. 2020, 26, 1091–1101. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Murine typhus—Hawaii, 2002. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 1224–1226. [Google Scholar]
- Centers for Disease Control and Prevention. Outbreak of Rickettsia typhi infection—Austin, Texas, 2008. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 1267–1270. [Google Scholar]
- Adjemian, J.; Parks, S.; McElroy, K.; Campbell, J.; Eremeeva, M.E.; Nicholson, W.L.; McQuiston, J.; Taylor, J. Murine typhus in Austin, Texas, USA, 2008. Emerg. Infect. Dis. 2010, 16, 412–417. [Google Scholar] [CrossRef]
- Parola, P.; Miller, R.S.; McDaniel, P.; Telford, S.R., 3rd; Rolain, J.M.; Wongsrichanalai, C.; Raoult, D. Emerging rickettsioses of the Thai-Myanmar border. Emerg. Infect. Dis. 2003, 9, 592–595. [Google Scholar] [CrossRef]
- Suputtamongkol, Y.; Suttinont, C.; Niwatayakul, K.; Hoontrakul, S.; Limpaiboon, R.; Chierakul, W.; Losuwanaluk, K.; Saisongkork, W. Epidemiology and clinical aspects of rickettsioses in Thailand. Ann. N. Y. Acad. Sci. 2009, 1166, 172–179. [Google Scholar] [CrossRef]
- Phongmany, S.; Rolain, J.M.; Phetsouvanh, R.; Blacksell, S.D.; Soukkhaseum, V.; Rasachack, B.; Phiasakha, K.; Soukkhaseum, S.; Frichithavong, K.; Chu, V.; et al. Rickettsial infections and fever, Vientiane, Laos. Emerg. Infect. Dis. 2006, 12, 256–262. [Google Scholar] [CrossRef]
- Maude, R.R.; Maude, R.J.; Ghose, A.; Amin, M.R.; Islam, M.B.; Ali, M.; Bari, M.S.; Majumder, M.I.; Tanganuchitcharnchai, A.; Dondorp, A.M.; et al. Serosurveillance of Orientia tsutsugamushi and Rickettsia typhi in Bangladesh. Am. J. Trop. Med. Hyg. 2014, 91, 580–583. [Google Scholar] [CrossRef]
- Dupont, H.T.; Brouqui, P.; Faugere, B.; Raoult, D. Prevalence of antibodies to Coxiella burnetti, Rickettsia conorii, and Rickettsia typhi in seven African countries. Clin. Infect. Dis. 1995, 21, 1126–1133. [Google Scholar] [CrossRef]
- Acuna-Soto, R.; Calderon-Romero, L.; Romero-Lopez, D.; Bravo-Lindoro, A. Murine typhus in Mexico City. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 45. [Google Scholar] [CrossRef]
- Dittrich, S.; Rattanavong, S.; Lee, S.J.; Panyanivong, P.; Craig, S.B.; Tulsiani, S.M.; Blacksell, S.D.; Dance, D.A.; Dubot-Peres, A.; Sengduangphachanh, A.; et al. Orientia, rickettsia, and leptospira pathogens as causes of CNS infections in Laos: A prospective study. Lancet. Glob. Health 2015, 3, e104–e112. [Google Scholar] [CrossRef]
- Koliou, M.; Psaroulaki, A.; Georgiou, C.; Ioannou, I.; Tselentis, Y.; Gikas, A. Murine typhus in Cyprus: 21 paediatric cases. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Christou, C.; Chochlakis, D.; Tsiligianni, I.; Sandalakis, V.; Georgalis, L.; Ioannou, I.; Giorgalas, G.; Tselentis, Y. Murine typhus in Cyprus: A 9-year survey. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Chaliotis, G.; Kritsotakis, E.I.; Psaroulaki, A.; Tselentis, Y.; Gikas, A. Murine typhus in central Greece: Epidemiological, clinical, laboratory, and therapeutic-response features of 90 cases. Int. J. Infect. Dis. 2012, 16, e591–e596. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lledo, L.; Gegundez, M.I.; Medina, J.; Gonzalez, J.V.; Alamo, R.; Saz, J.V. Epidemiological study of Rickettsia typhi infection in two provinces of the north of Spain: Analysis of sera from the general population and sheep. Vector Borne Zoonotic Dis. 2005, 5, 157–161. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; del Toro, M.D.; Nogueras, M.M.; Muniain, M.A.; Cardenosa, N.; Marquez, F.J.; Segura, F.; Pachon, J. Seroepidemiological study of Rickettsia felis, Rickettsia typhi, and Rickettsia conorii infection among the population of southern Spain. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 375–381. [Google Scholar] [CrossRef]
- Nogueras, M.M.; Cardenosa, N.; Sanfeliu, I.; Munoz, T.; Font, B.; Segura, F. Serological evidence of infection with Rickettsia typhi and Rickettsia felis among the human population of Catalonia, in the northeast of Spain. Am. J. Trop. Med. Hyg. 2006, 74, 123–126. [Google Scholar] [CrossRef]
- Espinosa, N.; Canas, E.; Bernabeu-Wittel, M.; Martin, A.; Viciana, P.; Pachon, J. The changing etiology of fever of intermediate duration. Enferm. Infecc. Y Microbiol. Clin. 2010, 28, 416–420. [Google Scholar] [CrossRef]
- Andre, E.; Correia, R.; Castro, P.; Neto, M.; Rola, J.; Bacelar, F.; Oliveira, I.; Velosa, I.; Feio, A.; Filipe, A. Murine typhus in Portugal. Acta Med. Port. 1998, 11, 81–85. [Google Scholar] [PubMed]
- Bacellar, F.; Lencastre, I.; Filipe, A.R. Is murine typhus re-emerging in Portugal? Eurosurveillance 1998, 3, 18–20. [Google Scholar] [CrossRef]
- Badiaga, S.; Benkouiten, S.; Hajji, H.; Raoult, D.; Brouqui, P. Murine typhus in the homeless. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Dhimal, M.; Dumre, S.P.; Sharma, G.N.; Khanal, P.; Ranabhat, K.; Shah, L.P.; Lal, B.K.; Jha, R.; Upadhyaya, B.P.; Acharya, B.; et al. An outbreak investigation of scrub typhus in Nepal: Confirmation of local transmission. BMC Infect. Dis. 2021, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Bise, G.; Coninx, R. Epidemic typhus in a prison in Burundi. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 133–134. [Google Scholar] [CrossRef]
- Raoult, D.; Roux, V.; Ndihokubwayo, J.B.; Bise, G.; Baudon, D.; Marte, G.; Birtles, R. Jail fever (epidemic typhus) outbreak in Burundi. Emerg. Infect. Dis. 1997, 3, 357–360. [Google Scholar] [CrossRef]
- Raoult, D.; Ndihokubwayo, J.B.; Tissot-Dupont, H.; Roux, V.; Faugere, B.; Abegbinni, R.; Birtles, R.J. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet 1998, 352, 353–358. [Google Scholar] [CrossRef]
- Tarasevich, I.; Rydkina, E.; Raoult, D. Outbreak of epidemic typhus in Russia. Lancet 1998, 352, 1151. [Google Scholar] [CrossRef]
- Lutwick, L.I. Brill-Zinsser disease. Lancet 2001, 357, 1198–1200. [Google Scholar] [CrossRef]
- Parker, R.T.; Menon, P.G.; Merideth, A.M.; Snyder, M.J.; Woodward, T.E. Persistence of Rickettsia rickettsii in a patient recovered from Rocky Mountain spotted fever. J. Immunol. 1954, 73, 383–386. [Google Scholar]
- Hove, M.G.; Walker, D.H. Persistence of rickettsiae in the partially viable gangrenous margins of amputated extremities 5 to 7 weeks after onset of Rocky Mountain spotted fever. Arch. Pathol. Lab. Med. 1995, 119, 429–431. [Google Scholar]
- Chung, M.H.; Lee, J.S.; Baek, J.H.; Kim, M.; Kang, J.S. Persistence of Orientia tsutsugamushi in humans. J. Korean Med. Sci. 2012, 27, 231–235. [Google Scholar] [CrossRef]
- Papp, S.; Moderzynski, K.; Rauch, J.; Heine, L.; Kuehl, S.; Richardt, U.; Mueller, H.; Fleischer, B.; Osterloh, A. Liver Necrosis and Lethal Systemic Inflammation in a Murine Model of Rickettsia typhi Infection: Role of Neutrophils, Macrophages and NK Cells. PLoS Negl. Trop. Dis. 2016, 10, e0004935. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.M.; Whitworth, T.; Olano, J.P.; Popov, V.L.; Walker, D.H. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 2004, 72, 2222–2228. [Google Scholar] [CrossRef]
- Walker, D.H.; Olano, J.P.; Feng, H.M. Critical role of cytotoxic T lymphocytes in immune clearance of rickettsial infection. Infect. Immun. 2001, 69, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Moderzynski, K.; Heine, L.; Rauch, J.; Papp, S.; Kuehl, S.; Richardt, U.; Fleischer, B.; Osterloh, A. Cytotoxic effector functions of T cells are not required for protective immunity against fatal Rickettsia typhi infection in a murine model of infection: Role of TH1 and TH17 cytokines in protection and pathology. PLoS Negl. Trop. Dis. 2017, 11, e0005404. [Google Scholar] [CrossRef]
- Moderzynski, K.; Papp, S.; Rauch, J.; Heine, L.; Kuehl, S.; Richardt, U.; Fleischer, B.; Osterloh, A. CD4+ T Cells Are as Protective as CD8+ T Cells against Rickettsia typhi Infection by Activating Macrophage Bactericidal Activity. PLoS Negl. Trop. Dis. 2016, 10, e0005089. [Google Scholar] [CrossRef]
- Hauptmann, M.; Kolbaum, J.; Lilla, S.; Wozniak, D.; Gharaibeh, M.; Fleischer, B.; Keller, C.A. Protective and Pathogenic Roles of CD8+ T Lymphocytes in Murine Orientia tsutsugamushi Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004991. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Mendell, N.L.; Liang, Y.; Shelite, T.R.; Goez-Rivillas, Y.; Soong, L.; Bouyer, D.H.; Walker, D.H. CD8+ T cells provide immune protection against murine disseminated endotheliotropic Orientia tsutsugamushi infection. PLoS Negl. Trop. Dis. 2017, 11, e0005763. [Google Scholar] [CrossRef]
- Feng, H.; Popov, V.L.; Yuoh, G.; Walker, D.H. Role of T lymphocyte subsets in immunity to spotted fever group Rickettsiae. J. Immunol. 1997, 158, 5314–5320. [Google Scholar]
- Walker, D.H.; Popov, V.L.; Feng, H.M. Establishment of a novel endothelial target mouse model of a typhus group rickettsiosis: Evidence for critical roles for gamma interferon and CD8 T lymphocytes. Lab. Investig. A J. Tech. Methods Pathol. 2000, 80, 1361–1372. [Google Scholar] [CrossRef][Green Version]
- Feng, H.M.; Walker, D.H. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect. Immun. 2000, 68, 6729–6736. [Google Scholar] [CrossRef]
- Turco, J.; Winkler, H.H. Gamma-interferon-induced inhibition of the growth of Rickettsia prowazekii in fibroblasts cannot be explained by the degradation of tryptophan or other amino acids. Infect. Immun. 1986, 53, 38–46. [Google Scholar] [CrossRef]
- Feng, H.M.; Popov, V.L.; Walker, D.H. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: Impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect. Immun. 1994, 62, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Taylor, J.P.; Walker, D.H. Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA 1991, 266, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Jensenius, M.; Laferl, H.; Vene, S.; Raoult, D. Kinetics of antibody responses in Rickettsia africae and Rickettsia conorii infections. Clin. Diagn. Lab. Immunol. 2002, 9, 324–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tantibhedhyangkul, W.; Prachason, T.; Waywa, D.; El Filali, A.; Ghigo, E.; Thongnoppakhun, W.; Raoult, D.; Suputtamongkol, Y.; Capo, C.; Limwongse, C.; et al. Orientia tsutsugamushi stimulates an original gene expression program in monocytes: Relationship with gene expression in patients with scrub typhus. PLoS Negl. Trop. Dis. 2011, 5, e1028. [Google Scholar] [CrossRef]
- Koo, J.E.; Hong, H.J.; Dearth, A.; Kobayashi, K.S.; Koh, Y.S. Intracellular invasion of Orientia tsutsugamushi activates inflammasome in asc-dependent manner. PLoS ONE 2012, 7, e39042. [Google Scholar] [CrossRef]
- Tantibhedhyangkul, W.; Ben Amara, A.; Textoris, J.; Gorvel, L.; Ghigo, E.; Capo, C.; Mege, J.L. Orientia tsutsugamushi, the causative agent of scrub typhus, induces an inflammatory program in human macrophages. Microb. Pathog. 2013, 55, 55–63. [Google Scholar] [CrossRef]
- Ogawa, M.; Satoh, M.; Kataoka, M.; Ando, S.; Saijo, M. Nitric oxide enhanced the growth of an obligated intracellular bacterium Orientia tsutsugamushi in murine macrophages. Microb. Pathog. 2017, 107, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, M.; Hagedorn, M.; Lilla, S.; Hauptmann, M.; Heine, H.; Fleischer, B.; Keller, C. Toll-Like Receptor 2 Recognizes Orientia tsutsugamushi and Increases Susceptibility to Murine Experimental Scrub Typhus. Infect. Immun. 2016, 84, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.V.; Griffith, M.F.; Prakash, J.A.; Chrispal, A.; Pichamuthu, K.; Varghese, G.M. Anti-nuclear antibody expression in severe scrub typhus infection: Preliminary observations. J. Glob. Infect. Dis. 2014, 6, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Lee, M.S.; Ki, M.; Ihm, C.; Kim, D.; Kim, Y.; Yoo, S.M. Does IL-17 play a role in hepatic dysfunction of scrub typhus patients? Vector Borne Zoonotic Dis. 2010, 10, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Weigl, R.L. Die Methoden der aktiven Fleckfieberimmunisierung. Bull. Int. Acad. Pol. Sci. Et Lett. 1930, 7, 25–62. [Google Scholar]
- Weigl, R. Immunization against typhus fever in Poland during World War II. Tex. Rep. Biol. Med. 1947, 5, 177–179. [Google Scholar]
- Walker, D.H. The realities of biodefense vaccines against Rickettsia. Vaccine 2009, 27 (Suppl. 4), D52–D55. [Google Scholar] [CrossRef]
- Zinsser, H.; Castaneda, M.R. Studies on Typhus Fever: Vii. Active Immunization against Mexican Typhus Fever with Dead Virus. J. Exp. Med. 1931, 53, 493–497. [Google Scholar] [CrossRef][Green Version]
- Veintemillas, F. Vaccination against typhus fever with the Zinsser-Castaneda Vaccine. J. Immunol. 1939, 36, 339–348. [Google Scholar]
- Spencer, R.R.; Parker, R.R. Rocky Mountain spotted fever: Vaccination of monkeys and man. Public Health Rep. 1925, 40, 2159–2167. [Google Scholar] [CrossRef]
- Ecke, R.S.; Gilliam, A.G.; Snyder, J.C.; Yeomans, A.; Zarafonetis, C.J.; Murray, E.S. The effect of Cox-type vaccine on louse-borne typhus fever; an account of 61 cases of naturally occurring typhus fever in patients who had previously received one or more injections of Cox-type vaccine. Am. J. Trop. Med. Hyg. 1945, 25, 447–462. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Hornick, R.B.; Dawkins, A.T.; Heiner, G.G.; Fabrikant, I.B.; Wisseman, C.L., Jr.; Woodward, T.E. Rocky Mountain spotted fever: A comparative study of the active immunity induced by inactivated and viable pathogenic Rickettsia rickettsii. J. Infect. Dis. 1973, 128, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, R.H.; Pedersen, C.E., Jr. Preparation of Rocky Mountain spotted fever vaccine suitable for human immunization. J. Clin. Microbiol. 1975, 1, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, R.H.; Sammons, L.S.; Pedersen, C.E., Jr. Comparison of three rocky mountain spotted fever vaccines. J. Clin. Microbiol. 1975, 2, 300–304. [Google Scholar] [CrossRef]
- Gonder, J.C.; Kenyon, R.H.; Pedersen, C.E., Jr. Evaluation of a killed Rocky Mountain spotted fever vaccine in cynomolgus monkeys. J. Clin. Microbiol. 1979, 10, 719–723. [Google Scholar] [CrossRef]
- Maugh, T.H., 2nd. Rickettsiae: A new vaccine for Rocky Mountain spotted fever. Science 1978, 201, 604. [Google Scholar] [CrossRef]
- Clements, M.L.; Wisseman, C.L., Jr.; Woodward, T.E.; Fiset, P.; Dumler, J.S.; McNamee, W.; Black, R.E.; Rooney, J.; Hughes, T.P.; Levine, M.M. Reactogenicity, immunogenicity, and efficacy of a chick embryo cell-derived vaccine for Rocky Mountain spotted fever. J. Infect. Dis. 1983, 148, 922–930. [Google Scholar] [CrossRef]
- Buckland, F.E.; Dudgeon, A. Scrubtyphus vaccine; large-scale production. Lancet 1945, 2, 734–737. [Google Scholar] [CrossRef]
- Card, W.I.; Walker, J.M. Scrub-typhus vaccine; field trial in South-east Asia. Lancet 1947, 1, 481–483. [Google Scholar] [CrossRef]
- Berge, T.O.; Gauld, R.L.; Kitaoka, M. A field trial of a vaccine prepared from the Volner strain of Rickettsia tsutsugamushi. Am. J. Hyg. 1949, 50, 337–342. [Google Scholar] [CrossRef]
- Bailey, C.A.; Diercks, F.H.; Proffitt, J.E. Preparation of a serological antigen and a vaccine for experimental tsutsugamushi disease. J. Immunol. 1948, 60, 431–441. [Google Scholar]
- Rights, F.L.; Smadel, J.E. Studies on scrib typhus; tsutsugamushi disease; heterogenicity of strains of R. tsutsugamushi as demonstrated by cross-vaccination studies. J. Exp. Med. 1948, 87, 339–351. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, K.S.; Kim, T.Y.; Cheong, H.S.; Ahn, B.Y. Long-term egg-yolk adaptation of the Orientia tsutsugamushi for preparation of a formalinized immunogen. Vaccine 2006, 24, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, G.H., Jr.; Osterman, J.V. Gamma-irradiated scrub typhus immunogens: Broad-spectrum immunity with combinations of rickettsial strains. Infect. Immun. 1979, 26, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, G.H., Jr.; Osterman, J.V. Gamma-irradiated scrub typhus immunogens: Development and duration of immunity. Infect. Immun. 1978, 22, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, G.H., Jr.; Osterman, J.V. Experimental scrub typhus immunogens: Gamma-irradiated and formalinized rickettsiae. Infect. Immun. 1977, 15, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.; Liu, H.; McGill, J.; Cerezo, A.; Jakkula, L.; Nair, A.D.S.; Winkley, E.; Olson, S.; Marlow, D.; Sahni, A.; et al. Rickettsia rickettsii Whole-Cell Antigens Offer Protection against Rocky Mountain Spotted Fever in the Canine Host. Infect. Immun. 2019, 87, e00628-18. [Google Scholar] [CrossRef]
- Kawamura, R.; Kasahar, S.; Toyama, T.; Nishinarita, F.; Tsubaki, S. On the prevention of tsutsugamushi. Results of preventive inoculations for people in the endemic region, and laboratory tests with the Pescadores strain. Trop. Dis. Bull. 1940, 37, 269–270. [Google Scholar]
- Fox, J.P.; Jordan, M.E.; Gelfand, H.M. Immunization of man against epidemic typhus by infection with avirulent Rickettsia prowazeki strain E. IV. Persistence of immunity and a note as to differing complement-fixation antigen requirements in post-infection and post-vaccination sera. J. Immunol. 1957, 79, 348–354. [Google Scholar] [PubMed]
- Wisseman, C.L., Jr. Concepts of louse-borne typhus control in developing countries: The use of the living attenuated E strain typhus vaccine in epidemic and endemic situations. Adv. Exp. Med. Biol. 1972, 31, 97–130. [Google Scholar]
- Ching, W.M.; Wang, H.; Davis, J.; Dasch, G.A. Amino acid analysis and multiple methylation of lysine residues in the surface protein antigen of Rickettsia prowazekii. In Techniques in Protein Chemistry IV; Angeletti, R.H., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1993; pp. 307–314. [Google Scholar]
- Zhang, J.Z.; Hao, J.F.; Walker, D.H.; Yu, X.J. A mutation inactivating the methyltransferase gene in avirulent Madrid E strain of Rickettsia prowazekii reverted to wild type in the virulent revertant strain Evir. Vaccine 2006, 24, 2317–2323. [Google Scholar] [CrossRef]
- Driskell, L.O.; Yu, X.J.; Zhang, L.; Liu, Y.; Popov, V.L.; Walker, D.H.; Tucker, A.M.; Wood, D.O. Directed mutagenesis of the Rickettsia prowazekii pld gene encoding phospholipase D. Infect. Immun. 2009, 77, 3244–3248. [Google Scholar] [CrossRef]
- Whitworth, T.; Popov, V.L.; Yu, X.J.; Walker, D.H.; Bouyer, D.H. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica serovar Typhimurium mediates phagosomal escape. Infect. Immun. 2005, 73, 6668–6673. [Google Scholar] [CrossRef] [PubMed]
- Noriea, N.F.; Clark, T.R.; Hackstadt, T. Targeted knockout of the Rickettsia rickettsii OmpA surface antigen does not diminish virulence in a mammalian model system. MBio 2015, 6, e00323-15. [Google Scholar] [CrossRef] [PubMed]
- Teysseire, N.; Raoult, D. Comparison of Western immunoblotting and microimmunofluorescence for diagnosis of Mediterranean spotted fever. J. Clin. Microbiol. 1992, 30, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Anacker, R.L.; List, R.H.; Mann, R.E.; Hayes, S.F.; Thomas, L.A. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J. Infect. Dis. 1985, 151, 1052–1060. [Google Scholar] [CrossRef]
- Anacker, R.L.; McDonald, G.A.; List, R.H.; Mann, R.E. Neutralizing activity of monoclonal antibodies to heat-sensitive and heat-resistant epitopes of Rickettsia rickettsii surface proteins. Infect. Immun. 1987, 55, 825–827. [Google Scholar] [CrossRef]
- Lange, J.V.; Walker, D.H. Production and characterization of monoclonal antibodies to Rickettsia rickettsii. Infect. Immun. 1984, 46, 289–294. [Google Scholar] [CrossRef]
- Chan, Y.G.; Riley, S.P.; Chen, E.; Martinez, J.J. Molecular basis of immunity to rickettsial infection conferred through outer membrane protein B. Infect. Immun. 2011, 79, 2303–2313. [Google Scholar] [CrossRef]
- Feng, H.M.; Whitworth, T.; Popov, V.; Walker, D.H. Effect of antibody on the rickettsia-host cell interaction. Infect. Immun. 2004, 72, 3524–3530. [Google Scholar] [CrossRef]
- Li, H.; Walker, D.H. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb. Pathog. 1998, 24, 289–298. [Google Scholar] [CrossRef]
- Qi, Y.; Xiong, X.; Wang, X.; Duan, C.; Jia, Y.; Jiao, J.; Gong, W.; Wen, B. Proteome analysis and serological characterization of surface-exposed proteins of Rickettsia heilongjiangensis. PLoS ONE 2013, 8, e70440. [Google Scholar] [CrossRef]
- Rauch, J.; Barton, J.; Kwiatkowski, M.; Wunderlich, M.; Steffen, P.; Moderzynski, K.; Papp, S.; Höhn, K.; Schwanke, H.; Witt, S.; et al. GroEL is an immunodominant surface-exposed antigen of Rickettsia typhi. PLoS ONE 2021, 16, e0253084. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.H.; Gouin, E.; Cossart, P.; Izard, T. The rickettsia surface cell antigen 4 applies mimicry to bind to and activate vinculin. J. Biol. Chem. 2011, 286, 35096–35103. [Google Scholar] [CrossRef]
- Fish, A.I.; Riley, S.P.; Singh, B.; Riesbeck, K.; Martinez, J.J. The Rickettsia conorii Adr1 Interacts with the C-Terminus of Human Vitronectin in a Salt-Sensitive Manner. Front. Cell. Infect. Microbiol. 2017, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Riley, S.P.; Patterson, J.L.; Nava, S.; Martinez, J.J. Pathogenic Rickettsia species acquire vitronectin from human serum to promote resistance to complement-mediated killing. Cell. Microbiol. 2014, 16, 849–861. [Google Scholar] [CrossRef][Green Version]
- Garza, D.A.; Riley, S.P.; Martinez, J.J. Expression of Rickettsia Adr2 protein in E. coli is sufficient to promote resistance to complement-mediated killing, but not adherence to mammalian cells. PLoS ONE 2017, 12, e0179544. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Xiong, X.; Qi, Y.; Jiao, J.; Duan, C.; Wen, B. Identification of novel surface-exposed proteins of Rickettsia rickettsii by affinity purification and proteomics. PLoS ONE 2014, 9, e100253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Renesto, P.; Azza, S.; Dolla, A.; Fourquet, P.; Vestris, G.; Gorvel, J.P.; Raoult, D. Proteome analysis of Rickettsia conorii by two-dimensional gel electrophoresis coupled with mass spectrometry. FEMS Microbiol. Lett. 2005, 245, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, A. The neglected challenge: Vaccination against rickettsiae. PLoS Negl. Trop. Dis. 2020, 14, e0008704. [Google Scholar] [CrossRef]
- He, Y.; Racz, R.; Sayers, S.; Lin, Y.; Todd, T.; Hur, J.; Li, X.; Patel, M.; Zhao, B.; Chung, M.; et al. Updates on the web-based VIOLIN vaccine database and analysis system. Nucleic Acids Res. 2014, 42, D1124–D1132. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef]
- Magnan, C.N.; Zeller, M.; Kayala, M.A.; Vigil, A.; Randall, A.; Felgner, P.L.; Baldi, P. High-throughput prediction of protein antigenicity using protein microarray data. Bioinformatics 2010, 26, 2936–2943. [Google Scholar] [CrossRef]
- Saha, S.; Raghava, G.P. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 2006, 65, 40–48. [Google Scholar] [CrossRef]
- Schlessinger, A.; Ofran, Y.; Yachdav, G.; Rost, B. Epitome: Database of structure-inferred antigenic epitopes. Nucleic Acids Res. 2006, 34, D777–D780. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Worning, P.; Lauemoller, S.L.; Lamberth, K.; Buus, S.; Brunak, S.; Lund, O. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. 2003, 12, 1007–1017. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Worning, P.; Hvid, C.S.; Lamberth, K.; Buus, S.; Brunak, S.; Lund, O. Improved prediction of MHC class I and class II epitopes using a novel Gibbs sampling approach. Bioinformatics 2004, 20, 1388–1397. [Google Scholar] [CrossRef]
- Lin, H.H.; Ray, S.; Tongchusak, S.; Reinherz, E.L.; Brusic, V. Evaluation of MHC class I peptide binding prediction servers: Applications for vaccine research. BMC Immunol. 2008, 9, 8. [Google Scholar] [CrossRef]
- Rammensee, H.; Bachmann, J.; Emmerich, N.P.; Bachor, O.A.; Stevanovic, S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics 1999, 50, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A.; Reinherz, E.L. Prediction of peptide-MHC binding using profiles. Methods Mol. Biol. 2007, 409, 185–200. [Google Scholar] [CrossRef]
- Zhang, G.L.; Srinivasan, K.N.; Veeramani, A.; August, J.T.; Brusic, V. PREDBALB/c: A system for the prediction of peptide binding to H2d molecules, a haplotype of the BALB/c mouse. Nucleic Acids Res. 2005, 33, W180–W183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, G.L.; Petrovsky, N.; Kwoh, C.K.; August, J.T.; Brusic, V. PRED(TAP): A system for prediction of peptide binding to the human transporter associated with antigen processing. Immunome Res. 2006, 2, 3. [Google Scholar] [CrossRef][Green Version]
- Guan, P.; Doytchinova, I.A.; Zygouri, C.; Flower, D.R. MHCPred: Bringing a quantitative dimension to the online prediction of MHC binding. Appl. Bioinform. 2003, 2, 63–66. [Google Scholar]
- Imai, K.; Asakawa, N.; Tsuji, T.; Akazawa, F.; Ino, A.; Sonoyama, M.; Mitaku, S. SOSUI-GramN: High performance prediction for sub-cellular localization of proteins in gram-negative bacteria. Bioinformation 2008, 2, 417–421. [Google Scholar] [CrossRef]
- Gardy, J.L.; Spencer, C.; Wang, K.; Ester, M.; Tusnady, G.E.; Simon, I.; Hua, S.; deFays, K.; Lambert, C.; Nakai, K.; et al. PSORT-B: Improving protein subcellular localization prediction for Gram-negative bacteria. Nucleic Acids Res. 2003, 31, 3613–3617. [Google Scholar] [CrossRef] [PubMed]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, J.D.; Jensen, L.J.; Blom, N.; Von Heijne, G.; Brunak, S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng. Des. Sel. PEDS 2004, 17, 349–356. [Google Scholar] [CrossRef]
- Gazi, M.; Caro-Gomez, E.; Goez, Y.; Cespedes, M.A.; Hidalgo, M.; Correa, P.; Valbuena, G. Discovery of a protective Rickettsia prowazekii antigen recognized by CD8+ T cells, RP884, using an in vivo screening platform. PLoS ONE 2013, 8, e76253. [Google Scholar] [CrossRef]
- Caro-Gomez, E.; Gazi, M.; Goez, Y.; Valbuena, G. Discovery of novel cross-protective Rickettsia prowazekii T-cell antigens using a combined reverse vaccinology and in vivo screening approach. Vaccine 2014, 32, 4968–4976. [Google Scholar] [CrossRef]
- Dzul-Rosado, K.; Balam-Romero, J.; Valencia-Pacheco, G.; Lugo-Caballero, C.; Arias-Leon, J.; Peniche-Lara, G.; Zavala-Castro, J. Immunogenicity of OmpA and OmpB antigens from Rickettsia rickettsii on mononuclear cells from Rickettsia positive Mexican patients. J. Vector Borne Dis. 2017, 54, 317–327. [Google Scholar] [CrossRef]
- Sumner, J.W.; Sims, K.G.; Jones, D.C.; Anderson, B.E. Protection of guinea-pigs from experimental Rocky Mountain spotted fever by immunization with baculovirus-expressed Rickettsia rickettsii rOmpA protein. Vaccine 1995, 13, 29–35. [Google Scholar] [CrossRef]
- Jiao, Y.; Wen, B.; Chen, M.; Niu, D.; Zhang, J.; Qiu, L. Analysis of immunoprotectivity of the recombinant OmpA of Rickettsia heilongjiangensis. Ann. N. Y. Acad. Sci. 2005, 1063, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, S.; McDonald, G.A.; Watkins, N.G. A recombinant Rickettsia conorii vaccine protects guinea pigs from experimental boutonneuse fever and Rocky Mountain spotted fever. Infect. Immun. 1990, 58, 646–653. [Google Scholar] [CrossRef]
- Bourgeois, A.L.; Dasch, G.A. The species-specific surface protein antigen of Rickettsia typhi: Immunogenicity and protective efficacy in guinea pigs. In Rickettsiae and Rickettsial Diseases; Burgdorfer, W., Anacker, R.L., Eds.; Academic Press: New York, NY, USA, 1981; pp. 71–80. [Google Scholar]
- Ching, W.M.; Wang, H.; Jan, B.; Dasch, G.A. Identification and characterization of epitopes on the 120-kilodalton surface protein antigen of Rickettsia prowazekii with synthetic peptides. Infect. Immun. 1996, 64, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Sears, K.T.; Ceraul, S.M.; Gillespie, J.J.; Allen, E.D., Jr.; Popov, V.L.; Ammerman, N.C.; Rahman, M.S.; Azad, A.F. Surface proteome analysis and characterization of surface cell antigen (Sca) or autotransporter family of Rickettsia typhi. PLoS Pathog. 2012, 8, e1002856. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, X.; Jiao, J.; Yang, X.; Jiang, Y.; Wen, B.; Gong, W. Th1 epitope peptides induce protective immunity against Rickettsia rickettsii infection in C3H/HeN mice. Vaccine 2017, 35, 7204–7212. [Google Scholar] [CrossRef]
- Gong, W.; Qi, Y.; Xiong, X.; Jiao, J.; Duan, C.; Wen, B. Rickettsia rickettsii outer membrane protein YbgF induces protective immunity in C3H/HeN mice. Hum. Vaccines Immunother. 2015, 11, 642–649. [Google Scholar] [CrossRef]
- Qi, Y.; Xiong, X.; Duan, C.; Jiao, J.; Gong, W.; Wen, B. Recombinant protein YbgF induces protective immunity against Rickettsia heilongjiangensis infection in C3H/HeN mice. Vaccine 2013, 31, 5643–5650. [Google Scholar] [CrossRef]
- Gong, W.; Xiong, X.; Qi, Y.; Jiao, J.; Duan, C.; Wen, B. Surface protein Adr2 of Rickettsia rickettsii induced protective immunity against Rocky Mountain spotted fever in C3H/HeN mice. Vaccine 2014, 32, 2027–2033. [Google Scholar] [CrossRef]
- Gong, W.; Wang, P.; Xiong, X.; Jiao, J.; Yang, X.; Wen, B. Enhanced protection against Rickettsia rickettsii infection in C3H/HeN mice by immunization with a combination of a recombinant adhesin rAdr2 and a protein fragment rOmpB-4 derived from outer membrane protein B. Vaccine 2015, 33, 985–992. [Google Scholar] [CrossRef]
- Chen, W.J.; Niu, D.S.; Zhang, X.Y.; Chen, M.L.; Cui, H.; Wei, W.J.; Wen, B.H.; Chen, X.R. Recombinant 56-kilodalton major outer membrane protein antigen of Orientia tsutsugamushi Shanxi and its antigenicity. Infect. Immun. 2003, 71, 4772–4779. [Google Scholar] [CrossRef]
- Seong, S.Y.; Park, S.G.; Huh, M.S.; Jang, W.J.; Kim, H.R.; Han, T.H.; Choi, M.S.; Chang, W.H.; Kim, I.S. Mapping of antigenic determinant regions of the Bor56 protein of Orientia tsutsugamushi. Infect. Immun. 1997, 65, 5250–5256. [Google Scholar] [CrossRef]
- Ramaiah, A.; Koralur, M.C.; Dasch, G.A. Complexity of type-specific 56 kDa antigen CD4 T-cell epitopes of Orientia tsutsugamushi strains causing scrub typhus in India. PLoS ONE 2018, 13, e0196240. [Google Scholar] [CrossRef]
- Chen, H.W.; Zhang, Z.; Huber, E.; Mutumanje, E.; Chao, C.C.; Ching, W.M. Kinetics and magnitude of antibody responses against the conserved 47-kilodalton antigen and the variable 56-kilodalton antigen in scrub typhus patients. Clin. Vaccine Immunol. 2011, 18, 1021–1027. [Google Scholar] [CrossRef]
- Seong, S.Y.; Huh, M.S.; Jang, W.J.; Park, S.G.; Kim, J.G.; Woo, S.G.; Choi, M.S.; Kim, I.S.; Chang, W.H. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect. Immun. 1997, 65, 1541–1545. [Google Scholar] [CrossRef]
- Seong, S.Y.; Kim, H.R.; Huh, M.S.; Park, S.G.; Kang, J.S.; Han, T.H.; Choi, M.S.; Chang, W.H.; Kim, I.S. Induction of neutralizing antibody in mice by immunization with recombinant 56 kDa protein of Orientia tsutsugamushi. Vaccine 1997, 15, 1741–1747. [Google Scholar] [CrossRef]
- Choi, S.; Jeong, H.J.; Ju, Y.R.; Gill, B.; Hwang, K.J.; Lee, J. Protective immunity of 56-kDa type-specific antigen of Orientia tsutsugamushi causing scrub typhus. J. Microbiol. Biotechnol. 2014, 24, 1728–1735. [Google Scholar] [CrossRef]
- Kim, H.I.; Ha, N.Y.; Kim, G.; Min, C.K.; Kim, Y.; Yen, N.T.H.; Choi, M.S.; Cho, N.H. Immunization with a recombinant antigen composed of conserved blocks from TSA56 provides broad genotype protection against scrub typhus. Emerg. Microbes Infect. 2019, 8, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wen, B.; Wen, B.; Niu, D.; Chen, M.; Qiu, L. Induction of protective immunity against scrub typhus with a 56-kilodalton recombinant antigen fused with a 47-kilodalton antigen of Orientia tsutsugamushi Karp. Am. J. Trop. Med. Hyg. 2005, 72, 458–464. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Jiang, J.; Chan, T.C.; Manetz, T.S.; Chao, C.C.; Ching, W.M.; Richards, A.L. Scrub typhus vaccine candidate Kp r56 induces humoral and cellular immune responses in cynomolgus monkeys. Infect. Immun. 2005, 73, 5039–5047. [Google Scholar] [CrossRef]
- Hickman, C.J.; Stover, C.K.; Joseph, S.W.; Oaks, E.V. Molecular cloning and sequence analysis of a Rickettsia tsutsugamushi 22 kDa antigen containing B- and T-cell epitopes. Microb. Pathog. 1991, 11, 19–31. [Google Scholar] [CrossRef]
- Ha, N.Y.; Kim, Y.; Choi, J.H.; Choi, M.S.; Kim, I.S.; Kim, Y.S.; Cho, N.H. Detection of antibodies against Orientia tsutsugamushi Sca proteins in scrub typhus patients and genetic variation of sca genes of different strains. Clin. Vaccine Immunol. 2012, 19, 1442–1451. [Google Scholar] [CrossRef]
- Ha, N.Y.; Sharma, P.; Kim, G.; Kim, Y.; Min, C.K.; Choi, M.S.; Kim, I.S.; Cho, N.H. Immunization with an autotransporter protein of Orientia tsutsugamushi provides protective immunity against scrub typhus. PLoS Negl. Trop. Dis. 2015, 9, e0003585. [Google Scholar] [CrossRef]
- Ha, N.Y.; Shin, H.M.; Sharma, P.; Cho, H.A.; Min, C.K.; Kim, H.I.; Yen, N.T.; Kang, J.S.; Kim, I.S.; Choi, M.S.; et al. Generation of protective immunity against Orientia tsutsugamushi infection by immunization with a zinc oxide nanoparticle combined with ScaA antigen. J. Nanobiotechnol. 2016, 14, 76. [Google Scholar] [CrossRef]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Youn, J.W.; Sung, Y.C. Cross-priming as a predominant mechanism for inducing CD8(+) T cell responses in gene gun DNA immunization. J. Immunol. 2001, 167, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Weiner, D.B. Developing DNA vaccines that call to dendritic cells. J. Clin. Investig. 2004, 114, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Crocquet-Valdes, P.A.; Diaz-Montero, C.M.; Feng, H.M.; Li, H.; Barrett, A.D.; Walker, D.H. Immunization with a portion of rickettsial outer membrane protein A stimulates protective immunity against spotted fever rickettsiosis. Vaccine 2001, 20, 979–988. [Google Scholar] [CrossRef]
- Diaz-Montero, C.M.; Feng, H.M.; Crocquet-Valdes, P.A.; Walker, D.H. Identification of protective components of two major outer membrane proteins of spotted fever group Rickettsiae. Am. J. Trop. Med. Hyg. 2001, 65, 371–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ni, Y.S.; Chan, T.C.; Chao, C.C.; Richards, A.L.; Dasch, G.A.; Ching, W.M. Protection against scrub typhus by a plasmid vaccine encoding the 56-KD outer membrane protein antigen gene. Am. J. Trop. Med. Hyg. 2005, 73, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef]
- Scheel, B.; Teufel, R.; Probst, J.; Carralot, J.P.; Geginat, J.; Radsak, M.; Jarrossay, D.; Wagner, H.; Jung, G.; Rammensee, H.G.; et al. Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur. J. Immunol. 2005, 35, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Kallen, K.J.; Heidenreich, R.; Schnee, M.; Petsch, B.; Schlake, T.; Thess, A.; Baumhof, P.; Scheel, B.; Koch, S.D.; Fotin-Mleczek, M. A novel, disruptive vaccination technology: Self-adjuvanted RNActive((R)) vaccines. Hum. Vaccines Immunother. 2013, 9, 2263–2276. [Google Scholar] [CrossRef]
- Rauch, S.; Lutz, J.; Kowalczyk, A.; Schlake, T.; Heidenreich, R. RNActive(R) Technology: Generation and Testing of Stable and Immunogenic mRNA Vaccines. Methods Mol. Biol. 2017, 1499, 89–107. [Google Scholar] [CrossRef]
- Chen, N.; Xia, P.; Li, S.; Zhang, T.; Wang, T.T.; Zhu, J. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life 2017, 69, 297–304. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Doener, F.; Zanzinger, K.; Noth, J.; Baumhof, P.; Fotin-Mleczek, M.; Heidenreich, R. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine 2016, 34, 3882–3893. [Google Scholar] [CrossRef]
- Edwards, D.K.; Jasny, E.; Yoon, H.; Horscroft, N.; Schanen, B.; Geter, T.; Fotin-Mleczek, M.; Petsch, B.; Wittman, V. Adjuvant effects of a sequence-engineered mRNA vaccine: Translational profiling demonstrates similar human and murine innate response. J. Transl. Med. 2017, 15, 1. [Google Scholar] [CrossRef]
- Li, Z.; Diaz-Montero, C.M.; Valbuena, G.; Yu, X.J.; Olano, J.P.; Feng, H.M.; Walker, D.H. Identification of CD8 T-lymphocyte epitopes in OmpB of Rickettsia conorii. Infect. Immun. 2003, 71, 3920–3926. [Google Scholar] [CrossRef]
- Saade, F.; Petrovsky, N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev. Vaccines 2012, 11, 189–209. [Google Scholar] [CrossRef]
- Harro, C.; Sun, X.; Stek, J.E.; Leavitt, R.Y.; Mehrotra, D.V.; Wang, F.; Bett, A.J.; Casimiro, D.R.; Shiver, J.W.; DiNubile, M.J.; et al. Safety and immunogenicity of the Merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clin. Vaccine Immunol. 2009, 16, 1285–1292. [Google Scholar] [CrossRef]
- Hill, A.V.; Reyes-Sandoval, A.; O’Hara, G.; Ewer, K.; Lawrie, A.; Goodman, A.; Nicosia, A.; Folgori, A.; Colloca, S.; Cortese, R.; et al. Prime-boost vectored malaria vaccines: Progress and prospects. Hum. Vaccin 2010, 6, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Radosevic, K.; Wieland, C.W.; Rodriguez, A.; Weverling, G.J.; Mintardjo, R.; Gillissen, G.; Vogels, R.; Skeiky, Y.A.; Hone, D.M.; Sadoff, J.C.; et al. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect. Immun. 2007, 75, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.; Tatsis, N.; Korioth-Schmitz, B.; Lasaro, M.O.; Hensley, S.E.; Lin, S.W.; Li, Y.; Giles-Davis, W.; Cun, A.; Zhou, D.; et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 2007, 81, 6594–6604. [Google Scholar] [CrossRef]
- Pichla-Gollon, S.L.; Lin, S.W.; Hensley, S.E.; Lasaro, M.O.; Herkenhoff-Haut, L.; Drinker, M.; Tatsis, N.; Gao, G.P.; Wilson, J.M.; Ertl, H.C.; et al. Effect of preexisting immunity on an adenovirus vaccine vector: In vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J. Virol. 2009, 83, 5567–5573. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, Y.; Cun, A.; Yang, W.; Ellenberg, S.; Switzer, W.M.; Kalish, M.L.; Ertl, H.C. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 2006, 12, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Liang, Y.; Wu, X. The current status, challenges, and future developments of new tuberculosis vaccines. Hum. Vaccines Immunother. 2018, 14, 1697–1716. [Google Scholar] [CrossRef]
- Skinner, M.A.; Yuan, S.; Prestidge, R.; Chuk, D.; Watson, J.D.; Tan, P.L. Immunization with heat-killed Mycobacterium vaccae stimulates CD8+ cytotoxic T cells specific for macrophages infected with Mycobacterium tuberculosis. Infect. Immun. 1997, 65, 4525–4530. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Cui, Z.; Yang, W.; Yue, L.; Ma, Y.; Shi, S.; Wang, C.; Wang, C.; Qian, A. Mycobacterium vaccae induces a strong Th1 response that subsequently declines in C57BL/6 mice. J. Vet. Sci. 2016, 17, 505–513. [Google Scholar] [CrossRef]
- Safar, H.A.; Mustafa, A.S.; Amoudy, H.A.; El-Hashim, A. The effect of adjuvants and delivery systems on Th1, Th2, Th17 and Treg cytokine responses in mice immunized with Mycobacterium tuberculosis-specific proteins. PLoS ONE 2020, 15, e0228381. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xiong, X.; Qi, Y.; Duan, C.; Gong, W.; Jiao, J.; Wen, B. Protective immunity against Rickettsia heilongjiangensis in a C3H/HeN mouse model mediated by outer membrane protein B-pulsed dendritic cells. Sci. China Life Sci. 2015, 58, 287–296. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).