Chlamydia trachomatis Cross-Serovar Protection during Experimental Lung Reinfection in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chlamydial Culture
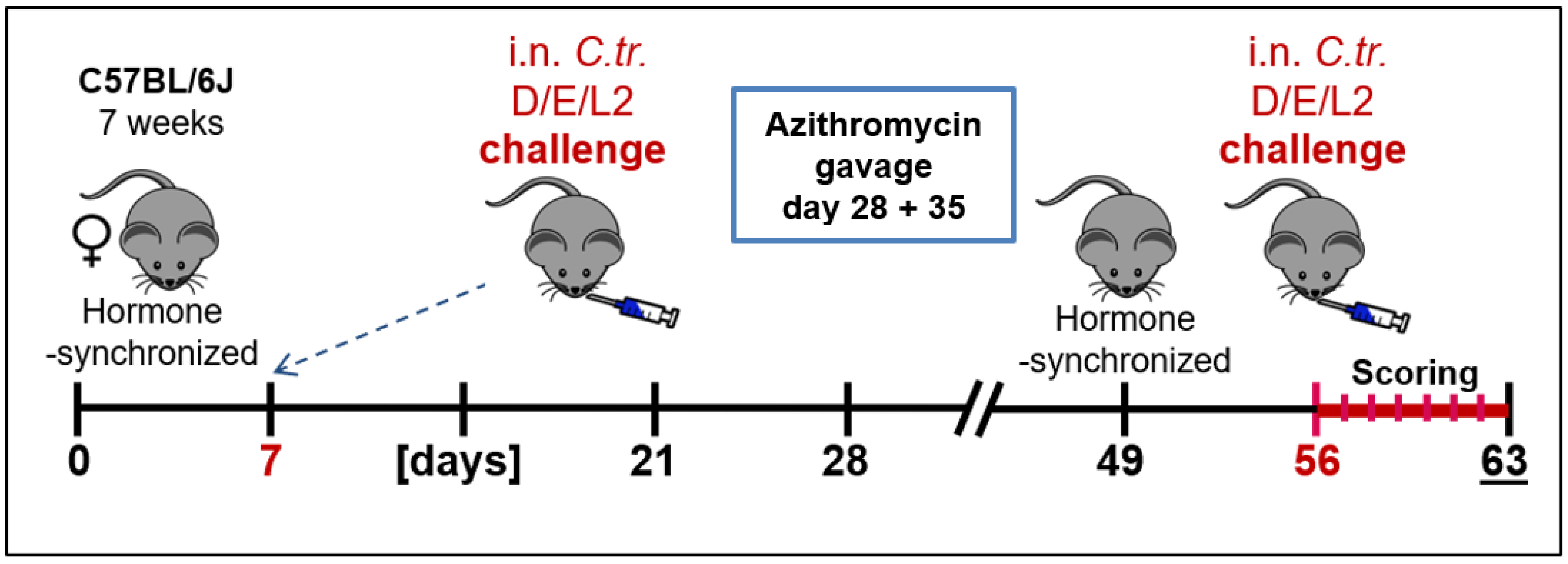
2.2. Animal Experiments and Clinical Scoring
2.3. Determination of Bacterial Load in Mouse Lung with Flow Cytometry
2.4. Quantification of Cytokine and Myeloperoxidase Levels
2.5. Determination of Chlamydia-Specific IgG by ELISA
2.6. Statistics and Group Sizes
3. Results
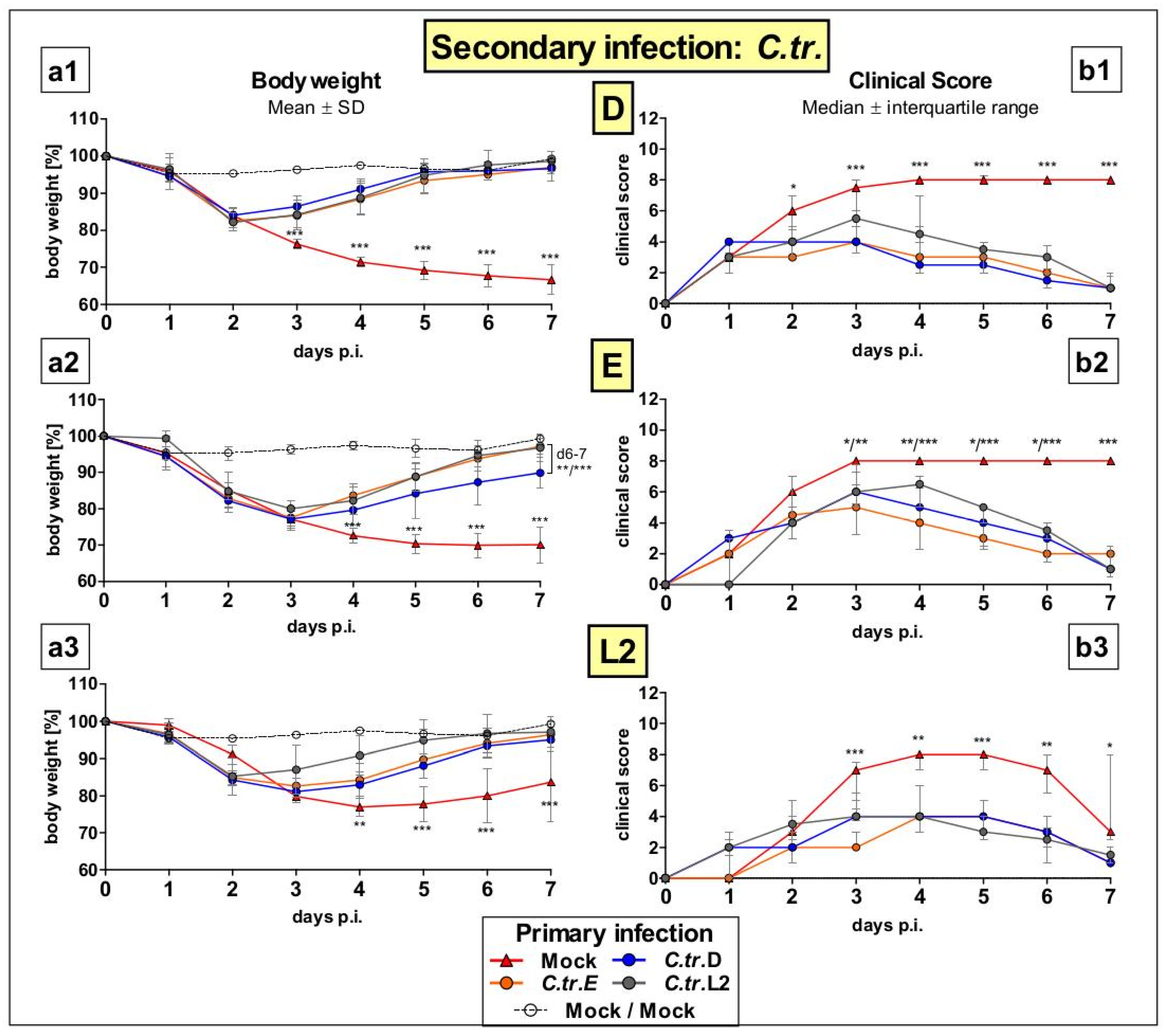
3.1. Previous i.n. Infections with C. trachomatis Serovars D, E, and L2 Improve Body Weight and Clinical Score during a Later Cross-Serovar Infection
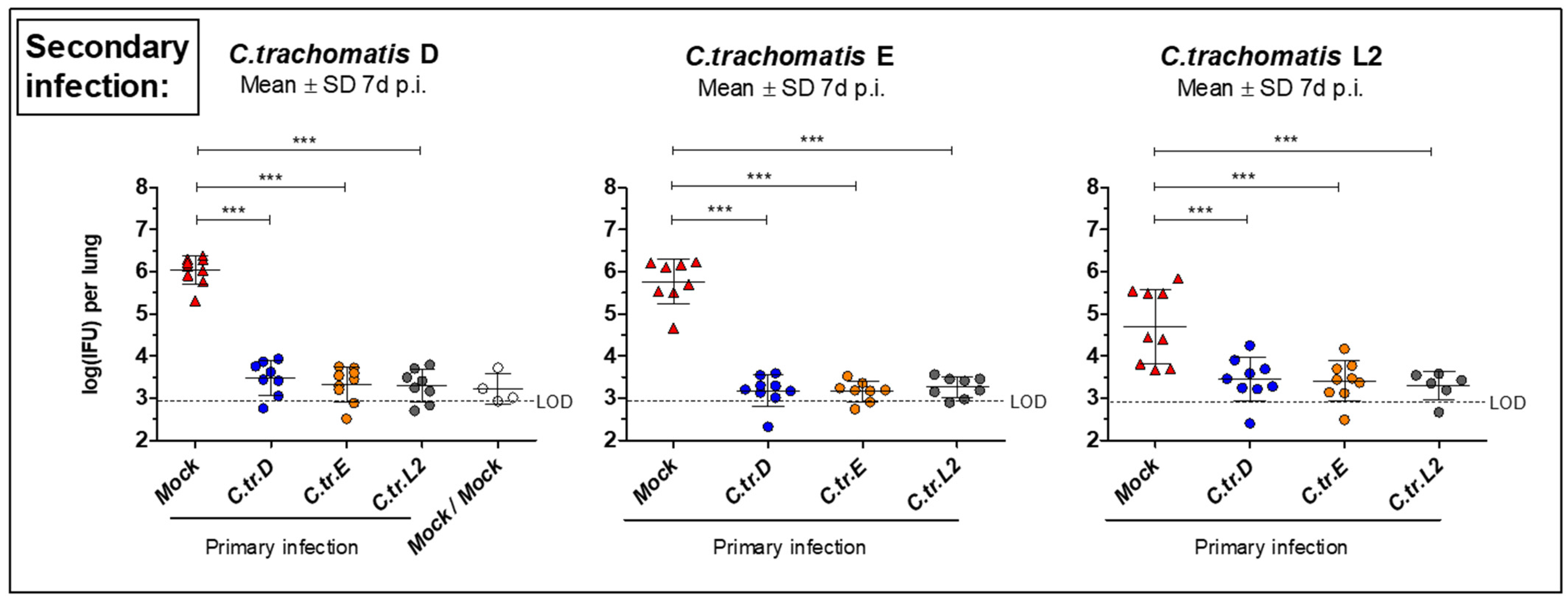
3.2. Initial i.n. Infections with C. trachomatis Serovars D, E, and L2 Lead to Similar Improved Bacterial Clearance after Secondary Infection with the Same or Other Serovars
3.3. Myeloperoxidase Levels in Lung Homogenate Are Similarly Reduced during C. trachomatis D, E, and L2 Secondary Infection in Mice Previously Infected with Serovar E
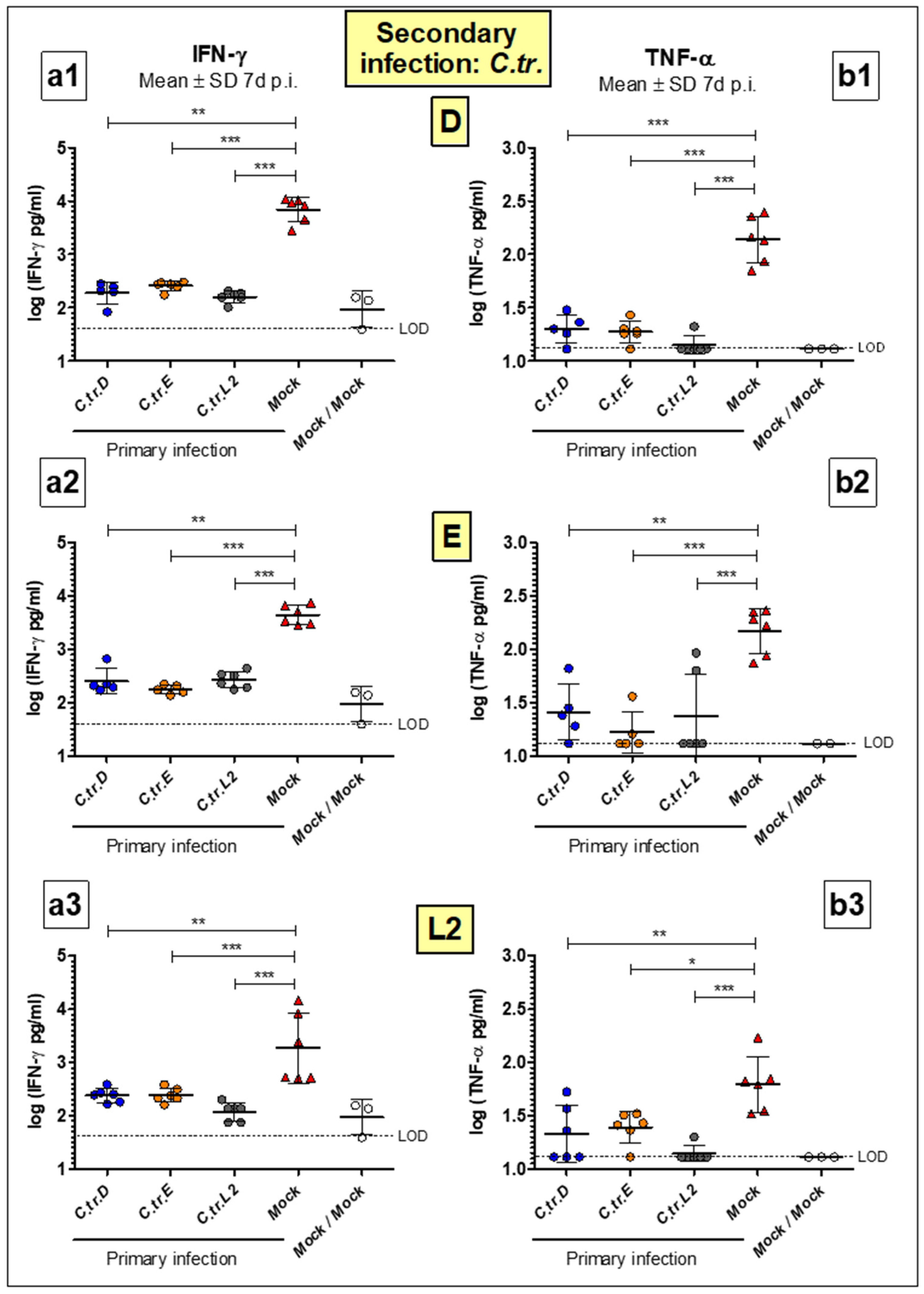
3.4. Levels of Key Cytokines IFN-γ and TNF-α Are Decreased to a Similar Extent during Homologous or Heterologous Secondary Infection with C. trachomatis D, E or L2
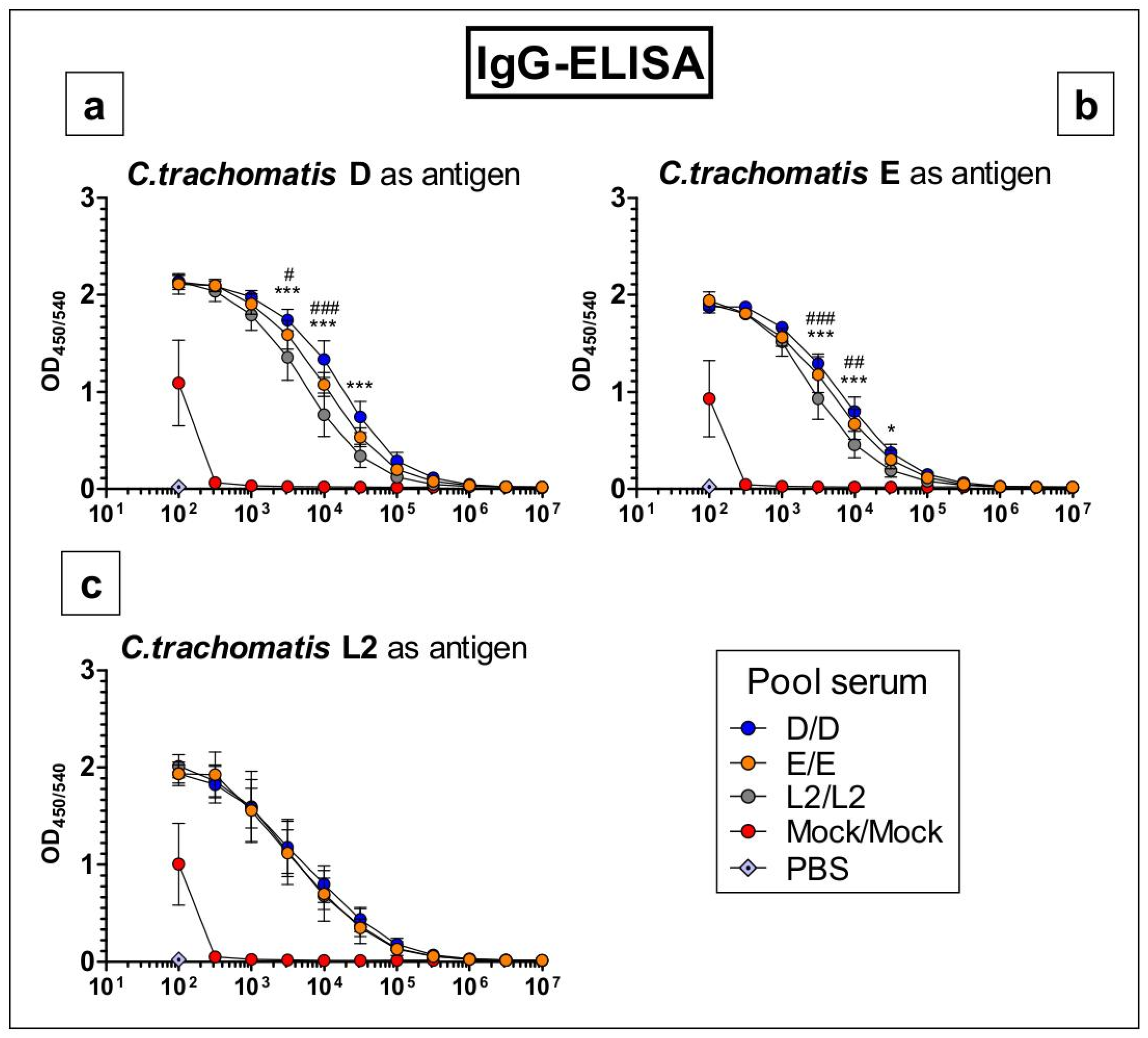
3.5. Antibodies Raised by Repetitive C. trachomatis Serovars D, E or L2 Lung Infection Almost Do Not Distinguish between Crude Antigen Preparations of the Three Serovars
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chlamydia Biology: From Genome to Disease; Tan, M.; Hegemann, J.H.; Sütterlin, C. (Eds.) Caister Academic Press: Poole, UK, 2020. [Google Scholar]
- Rank, R.G.; Yeruva, L. Hidden in plain sight: Chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect. Immun. 2014, 82, 1362–1371. [Google Scholar] [CrossRef] [Green Version]
- Morré, S.A.; Rozendaal, L.; van Valkengoed, I.G.M.; Boeke, A.J.P.; van Voorst Vader, P.C.; Schirm, J.; de Blok, S.; van den Hoek, J.A.R.; van Doornum, G.J.J.; Meijer, C.J.L.M.; et al. Urogenital Chlamydia trachomatis Serovars in Men and Women with a Symptomatic or Asymptomatic Infection: An Association with Clinical Manifestations? J. Clin. Microbiol. 2000, 38, 2292–2296. [Google Scholar] [CrossRef]
- Hoenderboom, B.M.; Van Benthem, B.H.B.; Van Bergen, J.E.A.M.; Dukers-Muijrers, N.H.T.M.; Götz, H.M.; Hoebe, C.J.P.A.; Hogewoning, A.A.; Land, J.A.; Van Der Sande, M.A.B.; Morré, S.A.; et al. Relation between Chlamydia trachomatis infection and pelvic inflammatory disease, ectopic pregnancy and tubal factor infertility in a Dutch cohort of women previously tested for chlamydia in a chlamydia screening trial. Sex. Transm. Infect. 2019, 95, 300–306. [Google Scholar] [CrossRef] [Green Version]
- WHO Guidelines for the Treatment of Chlamydia trachomatis; World Health Organization: Geneva, Switzerland, 2016; ISBN 9789241549714.
- Sethi, S.; Roy, A.; Garg, S.; Sree Venkatesan, L.; Bagga, R. Detection of Chlamydia trachomatis infections by polymerase chain reaction in asymptomatic pregnant women with special reference to the utility of the pooling of urine specimens. Indian J. Med. Res. Suppl. 2017, 146, 59–63. [Google Scholar] [CrossRef]
- Gaston, J.S.H. Immunological basis of chlamydia induced reactive arthritis. Sex. Transm. Infect. 2000, 76, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlott, T.; Eiffert, H.; Bohne, W.; Landgrebe, J.; Brunner, E.; Spielbauer, B.; Knight, B. Chlamydia trachomatis modulates expression of tumor suppressor gene caveolin-1 and oncogene C-myc in the transformation zone of non-neoplastic cervical tissue. Gynecol. Oncol. 2005, 98, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Status of Elimination of Trachoma as a Public Health Problem. Available online: https://apps.who.int/neglected_diseases/ntddata/trachoma/trachoma.html (accessed on 15 January 2021).
- Phillips, S.; Quigley, B.L.; Timms, P. Seventy years of Chlamydia vaccine research—Limitations of the past and directions for the future. Front. Microbiol. 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachter, J. Infection and Disease Epidemiology. In Chlamydia; ASM Press: Washington, DC, USA, 2014; pp. 139–169. [Google Scholar]
- Stephens, R.S.; Sanchez-Pescador, R.; Wagar, E.A.; Inouye, C.; Urdea, M.S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 1987, 169, 3879–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, M.P.; Twin, J.; Fairley, C.K.; Donovan, B.; Tan, S.E.; Yu, J.; Garland, S.M.; Tabrizi, S.N. Development and evaluation of an ompA quantitative real-time PCR assay for Chlamydia trachomatis serovar determination. J. Clin. Microbiol. 2010, 48, 2060–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Theodor, I.; Peterson, E.M.; De la Maza, L.M. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 2001, 69, 6240–6247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: A first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2019, 19, 1091–1100. [Google Scholar] [CrossRef]
- Joseph, S.J.; Didelot, X.; Rothschild, J.; De Vries, H.J.C.; Morré, S.A.; Read, T.D.; Dean, D. Population genomics of chlamydia trachomatis: Insights on drift, selection, recombination, and population structure. Mol. Biol. Evol. 2012, 29, 3933–3946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, V.; Cordeiro, D.; Salas, A.I.; Lodhia, Z.; Correia, C.; Isidro, J.; Fernandes, C.; Rodrigues, A.M.; Azevedo, J.; Alves, J.; et al. Chlamydia trachomatis: When the virulence-associated genome backbone imports a prevalence-associated major antigen signature. Microb. Genomics 2019, 5, e000313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, E.; Hegemann, J.H. All subtypes of the Pmp adhesin family are implicated in chlamydial virulence and show species-specific function. Microbiologyopen 2014, 3, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Takahashi, N.; Swanson, A.F.; Ozeki, Y.; Hakomori, S.I. An N-linked high-mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J. Clin. Investig. 1996, 98, 2813–2818. [Google Scholar] [CrossRef]
- Zhang, J.P.; Stephens, R.S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 1992, 69, 861–869. [Google Scholar] [CrossRef]
- Vasilevsky, S.; Stojanov, M.; Greub, G.; Baud, D. Chlamydial polymorphic membrane proteins: Regulation, function and potential vaccine candidates. Virulence 2016, 7, 11–22. [Google Scholar] [CrossRef]
- Crane, D.D.; Carlson, J.H.; Fischer, E.R.; Bavoil, P.; Hsia, R.C.; Tan, C.; Kuo, C.C.; Caldwell, H.D. Chlamydia tracomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc. Natl. Acad. Sci. USA 2006, 103, 1894–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Karunakaran, K.P.; Jiang, X.; Shen, C.; Andersen, P.; Brunham, R.C. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect. Immun. 2012, 80, 1510–1518. [Google Scholar] [CrossRef] [Green Version]
- Karunakaran, K.P.; Yu, H.; Jiang, X.; Chan, Q.; Moon, K.M.; Foster, L.J.; Brunham, R.C. Outer membrane proteins preferentially load MHC class II peptides: Implications for a Chlamydia trachomatis T cell vaccine. Vaccine 2015, 33, 2159–2166. [Google Scholar] [CrossRef] [Green Version]
- Lanfermann, C.; Wintgens, S.; Ebensen, T.; Kohn, M.; Laudeley, R.; Schulze, K.; Rheinheimer, C.; Hegemann, J.H.; Guzmán, C.A.; Klos, A. Prophylactic multi-subunit vaccine against chlamydia trachomatis: In vivo evaluation in mice. Vaccines 2021, 9, 609. [Google Scholar] [CrossRef]
- Dutow, P.; Wask, L.; Bothe, M.; Fehlhaber, B.; Laudeley, R.; Rheinheimer, C.; Yang, Z.; Zhong, G.; Glage, S.; Klos, A. An optimized, fast-to-perform mouse lung infection model with the human pathogen Chlamydia trachomatis for in vivo screening of antibiotics, vaccine candidates and modified host-pathogen interactions. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E.; Kalmar, I.; Vanrompay, D. Animal models for studying female genital tract infection with chlamydia trachomatis. Infect. Immun. 2013, 81, 3060–3067. [Google Scholar] [CrossRef] [Green Version]
- O’Meara, C.P.; Andrew, D.W.; Beagley, K.W. The Mouse Model of Chlamydia Genital Tract Infection: A Review of Infection, Disease, Immunity and Vaccine Development. Curr. Mol. Med. 2014, 14, 396–421. [Google Scholar] [CrossRef] [PubMed]
- Verweij, S.P.; Lanjouw, E.; Bax, C.J.; Quint, K.D.; Oostvogel, P.M.; Dörr, P.J.; Pleijster, J.; de Vries, H.J.C.; Peters, R.P.H.; Ouburg, S.; et al. Serovar D and E of serogroup B induce highest serological responses in urogenital Chlamydia trachomatis infections. BMC Infect. Dis. 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- Lesiak-Markowicz, I.; Schötta, A.M.; Stockinger, H.; Stanek, G.; Markowicz, M. Chlamydia trachomatis serovars in urogenital and ocular samples collected 2014–2017 from Austrian patients. Sci. Rep. 2019, 9, 1–4. [Google Scholar] [CrossRef]
- Kuo, C.C.; Wang, S.P.; Holmes, K.K.; Grayston, J.T. Immunotypes of Chlamydia trachomatis isolates in Seattle, Washington. Infect. Immun. 1983, 41, 865–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, M.; Lanfermann, C.; Laudeley, R.; Glage, S.; Rheinheimer, C.; Klos, A. Complement and Chlamydia psittaci: Non-myeloid derived C3 predominantly induces protective adaptive immune responses in mouse lung infection. Front. Immunol. 2021, 12, 485. [Google Scholar] [CrossRef]
- Kohn, M.; Lanfermann, C.; Laudeley, R.; Glage, S.; Rheinheimer, C.; Andreas, K. Complement and Chlamydia psittaci: Early complement-dependent events are important for DC migration and protection during mouse lung infection. Front. Immunol. 2021, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- De La Maza, L.M.; Zhong, G.; Brunham, R.C. Update on Chlamydia trachomatis vaccinology. Clin. Vaccine Immunol. 2017, 24, e00543-16. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Peterson, E.M.; De La Maza, L.M. Vaccination with the chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 2005, 73, 8153–8160. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Tatarenkova, O.V.; de la Maza, L.M. A vaccine formulated with the major outer membrane protein can protect C3H/HeN, a highly susceptible strain of mice, from a Chlamydia muridarum genital challenge. Immunology 2015, 146, 432–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Frazer, L.C.; O’Connell, C.M.; Tarantal, A.F.; Andrews, C.W.; O’Connor, S.L.; Russell, A.N.; Sullivan, J.E.; Poston, T.B.; Vallejo, A.N.; et al. Comparable Genital Tract Infection, Pathology, and Immunity in Rhesus Macaques Inoculated with Wild-Type or Plasmid-Deficient Chlamydia trachomatis Serovar, D. Infect. Immun. 2015, 83, 4056–4067. [Google Scholar] [CrossRef] [Green Version]
- Rother, M.; Gonzalez, E.; Teixeira da Costa, A.R.; Wask, L.; Gravenstein, I.; Pardo, M.; Pietzke, M.; Gurumurthy, R.K.; Angermann, J.; Laudeley, R.; et al. Combined Human Genome-wide RNAi and Metabolite Analyses Identify IMPDH as a Host-Directed Target against Chlamydia Infection. Cell Host Microbe 2018, 23, 661–671.e8. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Feilzer, K.; Caldwell, H.D.; Morrison, R.P. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 1997, 65, 1993–1999. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.M.; Grubbs, B.G.; Pack, E.; Kelly, K.; Rank, R.G. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect. Immun. 1997, 65, 2876–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roan, N.R.; Starnbach, M.N. Immune-mediated control of Chlamydia infection. Cell. Microbiol. 2008, 10, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutow, P.; Fehlhaber, B.; Bode, J.; Laudeley, R.; Rheinheimer, C.; Glage, S.; Wetsel, R.A.; Pabst, O.; Klos, A. The Complement C3a Receptor Is Critical in Defense against Chlamydia psittaci in Mouse Lung Infection and Required for Antibody and Optimal T Cell Response. J. Infect. Dis. 2014, 209, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.X.; McSorley, S.J. B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Chlamydia muridarum Genital Tract Infection. PLoS Pathog. 2013, 9, e1003707. [Google Scholar] [CrossRef] [Green Version]
- Malaviarachchi, P.A.; Mercado, M.A.B.; McSorley, S.J.; Li, L. Antibody, but not B-cell-dependent antigen presentation, plays an essential role in preventing Chlamydia systemic dissemination in mice. Eur. J. Immunol. 2020, 50, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.G.; Morrison, R.P. A Predominant Role for Antibody in Acquired Immunity to Chlamydial Genital Tract Reinfection. J. Immunol. 2005, 175, 7536–7542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakshi, R.K.; Gupta, K.; Jordan, S.J.; Chi, X.; Lensing, S.Y.; Press, C.G.; Geisler, W.M. An Adaptive chlamydia trachomatis-specific IFN-γ-producing CD4+ T cell response is associated with protection against chlamydia reinfection in women. Front. Immunol. 2018, 9, 1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanfermann, C.; Kohn, M.; Laudeley, R.; Rheinheimer, C.; Klos, A. Chlamydia trachomatis Cross-Serovar Protection during Experimental Lung Reinfection in Mice. Vaccines 2021, 9, 871. https://doi.org/10.3390/vaccines9080871
Lanfermann C, Kohn M, Laudeley R, Rheinheimer C, Klos A. Chlamydia trachomatis Cross-Serovar Protection during Experimental Lung Reinfection in Mice. Vaccines. 2021; 9(8):871. https://doi.org/10.3390/vaccines9080871
Chicago/Turabian StyleLanfermann, Christian, Martin Kohn, Robert Laudeley, Claudia Rheinheimer, and Andreas Klos. 2021. "Chlamydia trachomatis Cross-Serovar Protection during Experimental Lung Reinfection in Mice" Vaccines 9, no. 8: 871. https://doi.org/10.3390/vaccines9080871
APA StyleLanfermann, C., Kohn, M., Laudeley, R., Rheinheimer, C., & Klos, A. (2021). Chlamydia trachomatis Cross-Serovar Protection during Experimental Lung Reinfection in Mice. Vaccines, 9(8), 871. https://doi.org/10.3390/vaccines9080871





