HIV in the Brain: Identifying Viral Reservoirs and Addressing the Challenges of an HIV Cure
Abstract
:1. Introduction
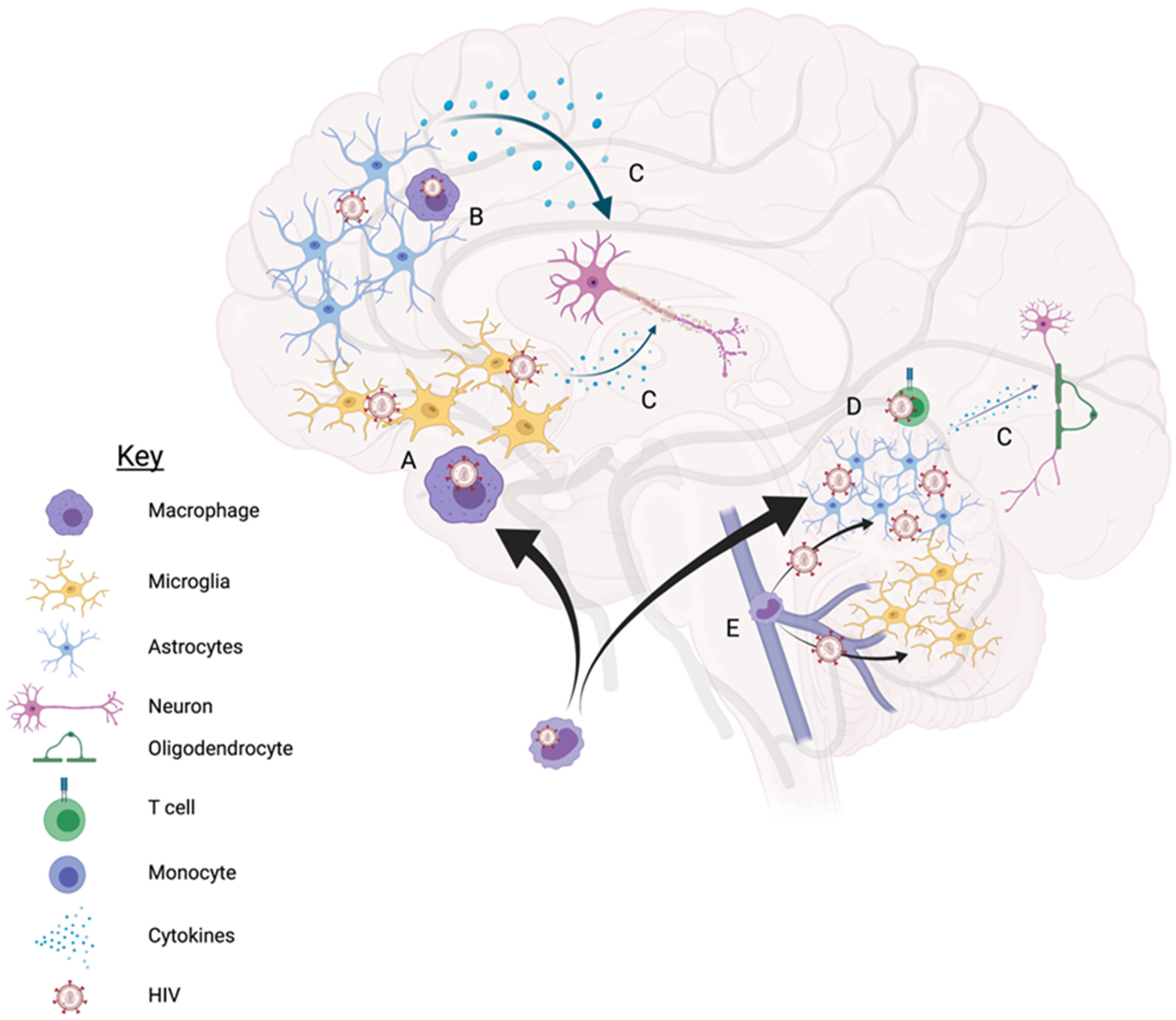
Cellular HIV Reservoirs
2. Microglia/Macrophages
3. Astrocytes
4. Outstanding Questions & Direction of the Field
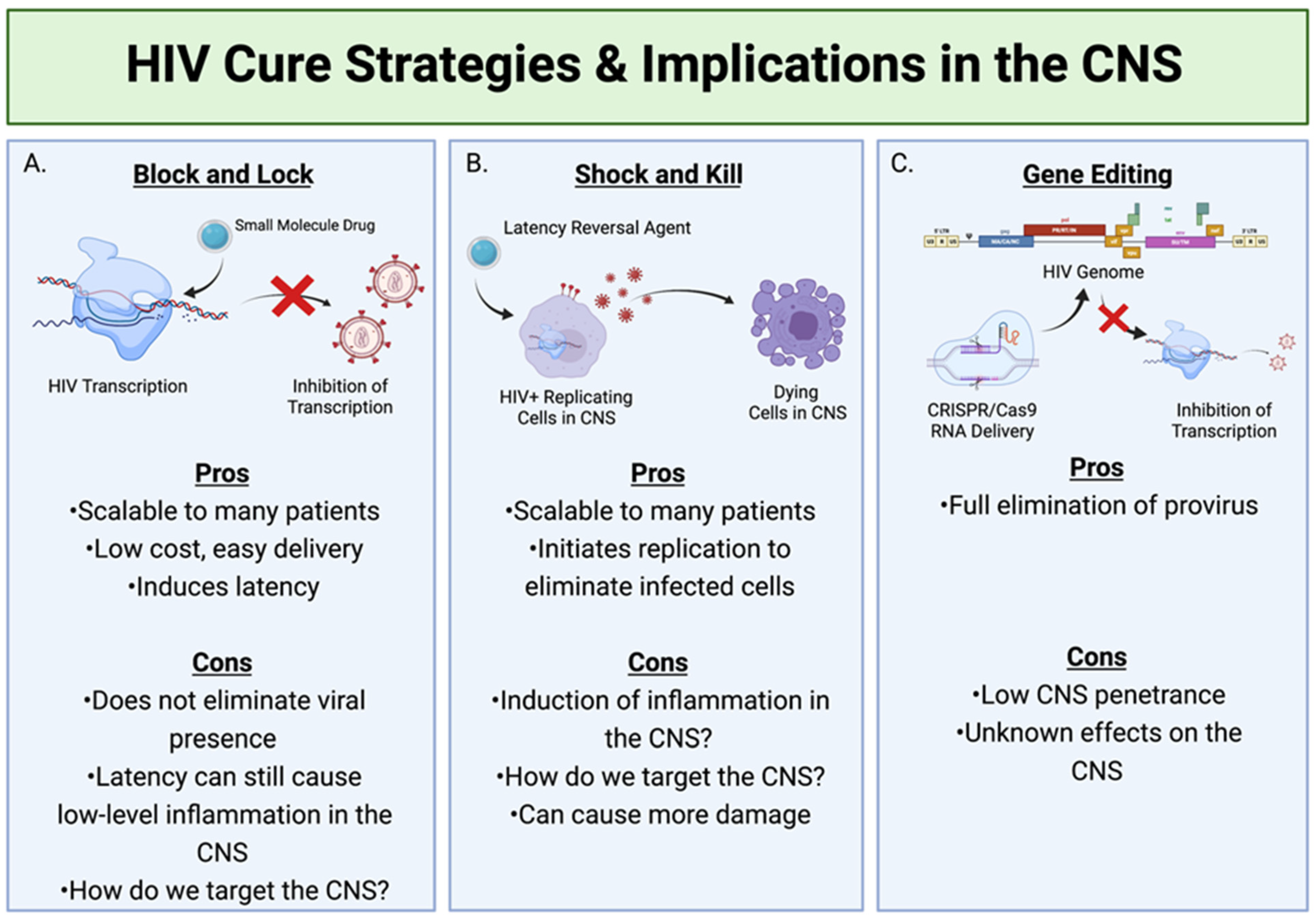
HIV Cure Strategies with Regard to CNS Reservoirs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blankson, J.N.; Persaud, D.; Siliciano, R.F. The Challenge of Viral Reservoirs in HIV-1 Infection. Annu. Rev. Med. 2002, 53, 557–593. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-C. Replication-competent non-induced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, T.; Nickle, D.C.; Justement, J.S.; Meyers, J.H.; Roby, G.; Hallahan, C.W.; Kottilil, S.; Moir, S.; Mican, J.M.; Mullins, J.I.; et al. Persistence of HIV in Gut-Associated Lymphoid Tissue despite Long-Term Antiretroviral Therapy. J. Infect. Dis. 2008, 197, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Van Marle, G. Compartmentalization of the gut viral reservoir in HIV-1 infected patients. Retrovirology 2007, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Bednar, M.M. Compartmentalization, Viral Evolution, and Viral Latency of HIV in the CNS. Curr. HIV/AIDS Rep. 2015, 12, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, A.M.; Sarmast, S.T.; Singh, R. Molecular Biology and Epidemiology of Neurotropic Viruses. Cureus 2020, 12, e9674. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, H.E.; Lipton, S.A.; Tardieu, M.; Bukrinsky, M.I.; Nottet, H.S. The neuropathogenesis of HIV-1 infection. J. Leukoc. Biol. 1994, 56, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.S. Acute SIV infection of the brain leads to upregulation of IL6 and interferon-regulated genes: Expression pat-terns throughout disease progression and impact on neuroAIDS. J. Neuroimmunol. 2004, 157, 81–92. [Google Scholar] [CrossRef]
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.A.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.L.; Michael, N.L.; Spudich, S.; et al. Central Nervous System Viral Invasion and Inflammation During Acute HIV Infection. J. Infect. Dis. 2012, 206, 275–282. [Google Scholar] [CrossRef]
- Ragin, A.B. Brain alterations within the first 100 days of HIV infection. Ann. Clin. Transl. Neurol. 2015, 2, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Hellmuth, J.; Slike, B.M.; Sacdalan, C.; Best, J.; Kroon, E.; Phanuphak, N.; Fletcher, J.L.K.; Prueksakaew, P.; Jagodzinski, L.L.; Valcour, V.; et al. Very Early Initiation of Antiretroviral Therapy During Acute HIV Infection is Associated with Normalized Levels of Immune Activation Markers in Cerebrospinal Fluid but Not in Plasma. J. Infect. Dis. 2019, 220, 1885–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Banks, W.A. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav. Immun. 2015, 45, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Eggers, C. HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, A.; Lu, H.; Miranda, A.S.; Ransohoff, R.M. Ontogeny and functions of CNS macrophages. J. Immunol. 2014, 193, 2615–2621. [Google Scholar] [CrossRef]
- Wang, X. Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood 2009, 113, 671–674. [Google Scholar] [CrossRef]
- Avalos, C.R. Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: A Func-tional Latent Reservoir. mBio 2017, 8, e01186-17. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.G. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology 2014, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Uzasci, L.; Nath, A.; Cotter, R. Oxidative Stress and the HIV-Infected Brain Proteome. Off. J. Neuroimmune Pharmacol. 2013, 8, 1167–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Harthi, L. Wnt/β-catenin and its Diverse Physiological Cell Signaling Pathways in Neurodegenerative and Neuropsychi-atric Disorders. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2012, 7, 725–730. [Google Scholar] [CrossRef]
- Aljawai, Y.; Richards, M.; Seaton, M.; Narasipura, S.; Al-Harthi, L. β-Catenin/TCF-4 Signaling Regulates Susceptibility of Macrophages and Resistance of Monocytes to HIV-1 Productive Infection. Curr. HIV Res. 2014, 12, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Prevedel, L.; Eugenin, E.A. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Joseph, S.B.; Arrildt, K.T.; Sturdevant, C.B.; Swanstrom, R. HIV-1 target cells in the CNS. J. Neuro. Virol. 2015, 21, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Bertin, J.; Jalaguier, P.; Barat, C.; Roy, M.-A.; Tremblay, M.J. Exposure of human astrocytes to leukotriene C4 promotes a CX3CL1/fractalkine-mediated transmigration of HIV-1-infected CD4+ T cells across an in vitro blood–brain barrier model. Virology 2014, 454–455, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Carbonell, D. Cross-talk between microglia and neurons regulates HIV latency. PLoS Pathog. 2019, 15, e1008249. [Google Scholar] [CrossRef] [Green Version]
- Guttenplan, K.A.; Liddelow, S.A. Astrocytes and microglia: Models and tools. J. Exp. Med. 2019, 216, 71–83. [Google Scholar] [CrossRef]
- Molofsky, A.V. Astrocytes and disease: A neurodevelopmental perspective. Genes Dev. 2012, 26, 891–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, J.G.; Macklis, J.D. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron. Glia. Biol. 2006, 2, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Fitting, S. Regional Heterogeneity and Diversity in Cytokine and Chemokine Production by Astroglia: Differential Responses to HIV-1 Tat, gp120, and Morphine Revealed by Multiplex Analysis. J. Proteome Res. 2010, 9, 1795–1804. [Google Scholar] [CrossRef] [Green Version]
- Al-Harthi, L.; Joseph, J.; Nath, A. Correction to: Astrocytes as an HIV CNS reservoir: Highlights and reflections of an NIMH-sponsored symposium. J. Neuro. Virol. 2019, 25, 616. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.F.; Kira, R.; Mori, H.; Okumura, A.; Torisu, H.; Yasumoto, S.; Shimizu, H.; Fujimoto, T.; Hanaoka, N.; Kusunoki, S.; et al. Clinical Features of Acute Flaccid Myelitis Temporally Associated With an Enterovirus D68 Outbreak: Results of a Nationwide Survey of Acute Flaccid Paralysis in Japan, August–December 2015. Clin. Infect. Dis. 2018, 66, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Churchill, M.J.; Gorry, P.R.; Cowley, D.; Lal, L.; Sonza, S.; Purcell, D.F.J.; Thompson, K.A.; Gabuzda, D.; McArthur, J.C.; Pardo, C.A.; et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J. Neuro. Virol. 2006, 12, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Gyorkey, F.; Melnick, J.L.; Gyorkey, P. Human immunodeficiency virus in brain biopsies of patients with AIDS and pro-gressive encephalopathy. J. Infect. Dis. 1987, 155, 870–876. [Google Scholar] [CrossRef]
- Ranki, A. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 1995, 9, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A. Astrocyte specific viral strains in HIV dementia. Ann. Neurol. 2004, 56, 873–877. [Google Scholar] [CrossRef]
- Tornatore, C.; Chandra, R.; Berger, J.R.; Major, E.O. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 1994, 44, 481–487. [Google Scholar] [CrossRef]
- Narasipura, S.D.; Kim, S.; Al-Harthi, L.; Silvestri, G. Epigenetic Regulation of HIV-1 Latency in Astrocytes. J. Virol. 2013, 88, 3031–3038. [Google Scholar] [CrossRef] [Green Version]
- Lutgen, V.; Narasipura, S.D.; Barbian, H.J.; Richards, M.; Wallace, J.; Razmpour, R.; Buzhdygan, T.; Ramirez, S.; Prevedel, L.; Eugenin, E.A.; et al. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog. 2020, 16, e1008381. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.A.; Clements, J.E.; Zink, M.C.; Berman, J.W. Human Immunodeficiency Virus Infection of Human Astrocytes Disrupts Blood-Brain Barrier Integrity by a Gap Junction-Dependent Mechanism. J. Neurosci. 2011, 31, 9456–9465. [Google Scholar] [CrossRef] [Green Version]
- Lutgen, V.; Narasipura, S.D.; Sharma, A.; Min, S.; Al-Harthi, L. β-Catenin signaling positively regulates glutamate uptake and metabolism in astrocytes. J. Neuroinflammation 2016, 13, 242. [Google Scholar] [CrossRef] [Green Version]
- Yu, C. HIV and drug abuse mediate astrocyte senescence in a β-catenin-dependent manner leading to neuronal toxici-ty. Aging Cell 2017, 16, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M. A new model for post-integration latency in macroglial cells to study HIV-1 reservoirs of the brain. AIDS 2015, 29, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Vincendeau, M.; Kramer, S.; Hadian, K.; Rothenaigner, I.; Bell, J.; Hauck, S.; Bickel, C.; Nagel, D.; Kremmer, E.; Werner, T.; et al. Control of HIV replication in astrocytes by a family of highly conserved host proteins with a common Rev-interacting domain (Risp). AIDS 2010, 24, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Rothenaigner, I.; Kramer, S.; Ziegler, M.; Wolff, H.; Kleinschmidt, A.; Brack-Werner, R. Long-term HIV-1 infection of neural progenitor populations. AIDS 2007, 21, 2271–2281. [Google Scholar] [CrossRef]
- Gray, L.R.; Turville, S.G.; Hitchen, T.L.; Cheng, W.-J.; Ellett, A.M.; Salimi, H.; Roche, M.J.; Wesselingh, S.L.; Gorry, P.R.; Churchill, M.J. HIV-1 Entry and Trans-Infection of Astrocytes Involves CD81 Vesicles. PLoS ONE 2014, 9, e90620. [Google Scholar] [CrossRef]
- Sturdevant, C.B.; Joseph, S.B.; Schnell, G.; Price, R.W.; Swanstrom, R.; Spudich, S. Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection. PLoS Pathog. 2015, 11, e1004720. [Google Scholar] [CrossRef] [Green Version]
- Brennan, T.P.; Woods, J.O.; Sedaghat, A.R.; Siliciano, J.D.; Siliciano, R.F.; Wilke, C.O. Analysis of Human Immunodeficiency Virus Type 1 Viremia and Provirus in Resting CD4 + T Cells Reveals a Novel Source of Residual Viremia in Patients on Antiretroviral Therapy. J. Virol. 2009, 83, 8470–8481. [Google Scholar] [CrossRef] [Green Version]
- Chun, T.W. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discon-tinuation of highly active anti-retroviral therapy. Nat. Med. 2000, 6, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-H.; Maric, D.; Major, E.O.; Nath, A. Productive HIV infection in astrocytes can be established via a nonclassical mechanism. AIDS 2020, 34, 963–978. [Google Scholar] [CrossRef]
- Chauhan, A.; Khandkar, M. Endocytosis of human immunodeficiency virus 1 (HIV-1) in astrocytes: A fiery path to its destination. Microb. Pathog. 2015, 78, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; He, J.J. Cell–cell contact viral transfer contributes to HIV infection and persistence in astrocytes. J. Neuro. Virol. 2015, 21, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Sandrone, S.; Moreno-Zambrano, D.; Kipnis, J.; Van Gijn, J. A (delayed) history of the brain lymphatic system. Nat. Med. 2019, 25, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Lamers, S.L.; Rose, R.; Ndhlovu, L.; Nolan, D.J.; Salemi, M.; Maidji, E.; Stoddart, C.A.; McGrath, M.S. The meningeal lymphatic system: A route for HIV brain migration? J. Neuro. Virol. 2016, 22, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bao, R.; Haigwood, N.L.; Persidsky, Y.; Ho, W.-Z. SIV infection of rhesus macaques of Chinese origin: A suitable model for HIV infection in humans. Retrovirology 2013, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Svensson Akusjärvi, S.; Sönnerborg, A.; Neogi, U. Characterization of Inducible Transcription and Transla-tion-Competent HIV-1 Using the RNAscope ISH Technology at a Single-Cell Resolution. Front. Microbiol. 2018, 9, 2538. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, Z.; Yu, J.; Choo, P.; Chen, L.; Choo, J. A SERS-based lateral flow assay biosensor for highly sensitive detection of HIV-1 DNA. Biosens. Bioelectron. 2016, 78, 530–537. [Google Scholar] [CrossRef]
- Bosman, K.J.; Nijhuis, M.; Van Ham, P.M.; Wensing, A.M.J.; Vervisch, K.; Vandekerckhove, L.; De Spiegelaere, W. Comparison of digital PCR platforms and semi-nested qPCR as a tool to determine the size of the HIV reservoir. Sci. Rep. 2015, 5, 13811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrich, T.J.; Hanhauser, E.; Marty, F.M.; Sirignano, M.N.; Keating, S.; Lee, T.H.; Robles, Y.P.; Davis, B.T.; Li, J.Z.; Heisey, A.; et al. Antiretroviral-Free HIV-1 Remission and Viral Rebound Following Allogeneic Stem Cell Transplantation: A Report of Two Cases. Ann. Intern. Med. 2014, 161, 319–327. [Google Scholar] [CrossRef]
- Estes, J.D.; Kityo, C.; Ssali, F.; Swainson, L.; Makamdop, K.N.; Del Prete, G.Q.; Deeks, S.; A Luciw, P.; Chipman, J.G.; Beilman, G.J.; et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 2017, 23, 1271–1276. [Google Scholar] [CrossRef] [Green Version]
- Gama, L.; Abreu, C.M.; Shirk, E.N.; Price, S.; Li, M.; Laird, G.M.; Pate, K.A.M.; Wietgrefe, S.W.; O’Connor, S.L.; Pianowski, L.; et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS 2017, 31, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Asahchop, E.L.; Meziane, O.; Mamik, M.K.; Chan, W.F.; Branton, W.; Resch, L.; Gill, M.J.; Haddad, E.; Guimond, J.V.; Wainberg, M.A.; et al. Reduced antiretroviral drug efficacy and concentration in HIV-infected microglia contributes to viral persistence in brain. Retrovirology 2017, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vansant, G.; Bruggemans, A.; Janssens, J.; Debyser, Z. Block-And-Lock Strategies to Cure HIV Infection. Viruses 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessing, C.F.; Nixon, C.C.; Li, C.; Tsai, P.; Takata, H.; Mousseau, G.; Ho, P.T.; Honeycutt, J.B.; Fallahi, M.; Trautmann, L.; et al. In Vivo Suppression of HIV Rebound by Didehydro-Cortistatin A, a “Block-and-Lock” Strategy for HIV-1 Treatment. Cell Rep. 2017, 21, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Méndez, C.; Ledger, S.; Petoumenos, K.; Ahlenstiel, C.; Kelleher, A. RNA-induced epigenetic silencing inhibits HIV-1 reactivation from latency. Retrovirology 2018, 15, 67. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.W. Monocytes Mediate HIV Neuropathogenesis: Mechanisms that Contribute to HIV Associated Neu-rocognitive Disorders. Curr. HIV Res. 2014, 12, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Spivak, A.M.; Planelles, V. Novel Latency Reversal Agents for HIV-1 Cure. Annu. Rev. Med. 2018, 69, 421–436. [Google Scholar] [CrossRef] [Green Version]
- Walker-Sperling, V.E.; Pohlmeyer, C.W.; Tarwater, P.M.; Blankson, J.N. The Effect of Latency Reversal Agents on Prima-ry CD8+ T Cells: Implications for Shock and Kill Strategies for Human Immunodeficiency Virus Eradication. eBio Med. 2016, 8, 217–229. [Google Scholar]
- Huang, Z.; Nair, M. A CRISPR/Cas9 guidance RNA screen platform for HIV provirus disruption and HIV/AIDS gene therapy in astrocytes. Sci. Rep. 2017, 7, 5955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunze, C.; Börner, K.; Kienle, E.; Orschmann, T.; Rusha, E.; Schneider, M.; Radivojkov-Blagojevic, M.; Drukker, M.; Desbordes, S.; Grimm, D.; et al. Synthetic AAV/CRISPR vectors for blocking HIV-1 expression in persistently infected astrocytes. Glia 2017, 66, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Kaminski, R.; Bella, R.; Su, H.; Mathews, S.; Ahooyi, T.M.; Chen, C.; Mancuso, P.; Sariyer, R.; Ferrante, P.; et al. Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice. Nat. Commun. 2019, 10, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letendre, S. Validation of the CNS Penetration-Effectiveness Rank for Quantifying Antiretroviral Penetration Into the Cen-tral Nervous System. Arch. Neurol. 2008, 65, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.K. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Hütter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Müssig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kücherer, C.; Blau, O.; et al. Long-Term Control of HIV byCCR5Delta32/Delta32 Stem-Cell Transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlin Patient: First Person Cured of HIV, Timothy Ray Brown, Dies. BBC News, 30 September 2020.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ash, M.K.; Al-Harthi, L.; Schneider, J.R. HIV in the Brain: Identifying Viral Reservoirs and Addressing the Challenges of an HIV Cure. Vaccines 2021, 9, 867. https://doi.org/10.3390/vaccines9080867
Ash MK, Al-Harthi L, Schneider JR. HIV in the Brain: Identifying Viral Reservoirs and Addressing the Challenges of an HIV Cure. Vaccines. 2021; 9(8):867. https://doi.org/10.3390/vaccines9080867
Chicago/Turabian StyleAsh, Michelle K., Lena Al-Harthi, and Jeffrey R. Schneider. 2021. "HIV in the Brain: Identifying Viral Reservoirs and Addressing the Challenges of an HIV Cure" Vaccines 9, no. 8: 867. https://doi.org/10.3390/vaccines9080867
APA StyleAsh, M. K., Al-Harthi, L., & Schneider, J. R. (2021). HIV in the Brain: Identifying Viral Reservoirs and Addressing the Challenges of an HIV Cure. Vaccines, 9(8), 867. https://doi.org/10.3390/vaccines9080867





