Effectiveness of the Adjuvanted Influenza Vaccine in Older Adults at High Risk of Influenza Complications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources and Linkage
2.3. Exposure Ascertainment
2.4. Study Population
2.5. Outcome Ascertainment
2.6. Covariates
2.7. Influenza Period
2.8. Statistical Methods
3. Results
3.1. Study Subjects
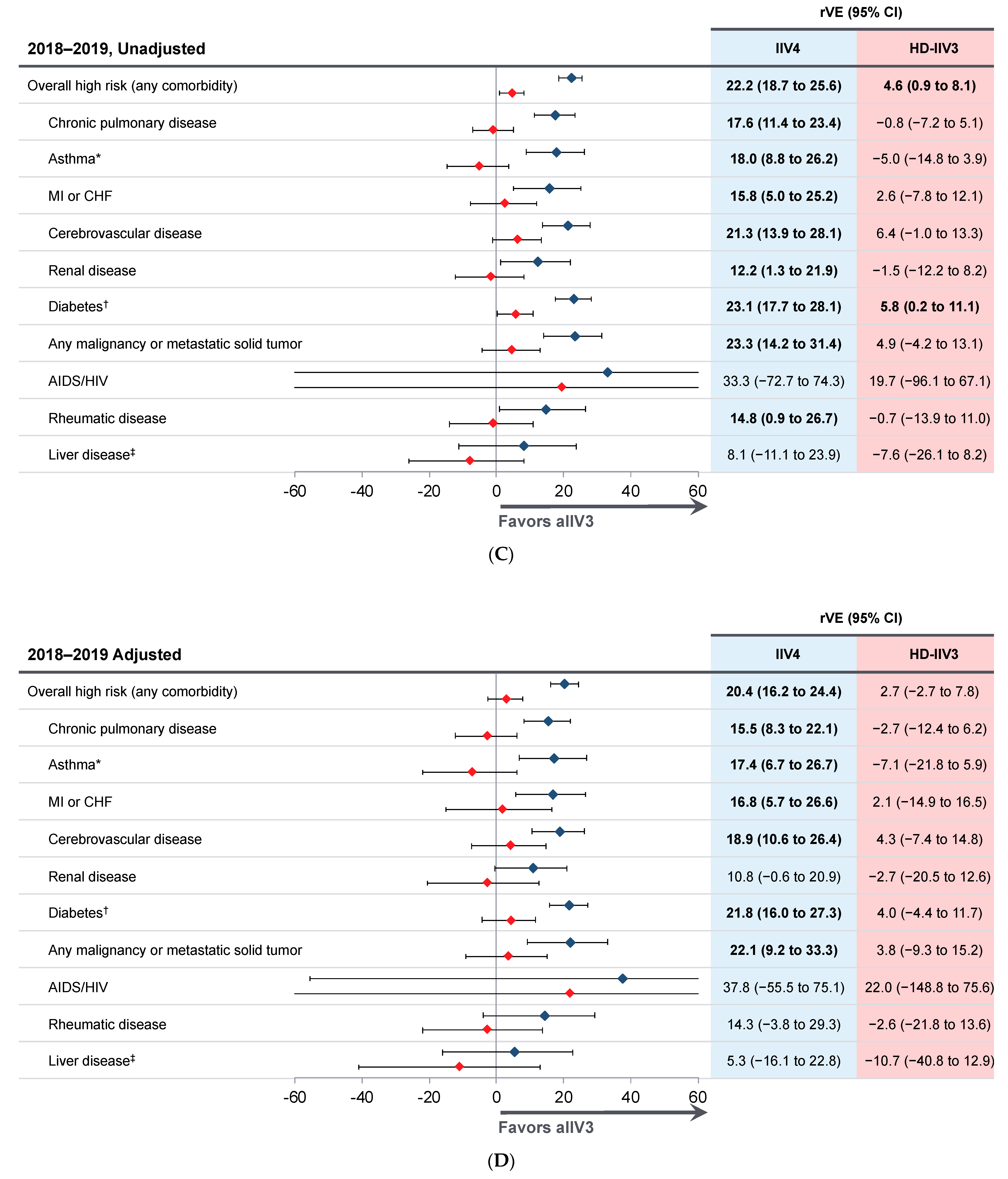
3.2. Relative Vaccine Effectiveness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Influenza Strategy, 2019–2030; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Pebody, R.; McLean, E.; Zhao, H.; Cleary, P.; Bracebridge, S.; Foster, K.; Charlett, A.; Hardelid, P.; Waight, P.; Ellis, J. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: Risk factors for death, April 2009 to March 2010. Eurosurveillance 2010, 15, 19571. [Google Scholar] [CrossRef]
- Nichol, K.L.; Wuorenma, J.; von Sternberg, T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch. Intern. Med. 1998, 158, 1769–1776. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices—United States, 2019–2020 influenza season. MMWR Recomm. Rep. 2019, 68, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2018–2019 Influenza Season. Available online: https://www.cdc.gov/flu/about/burden/2018-2019.html (accessed on 27 May 2021).
- Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 1057–1062. [Google Scholar]
- Rolfes, M.A.; Flannery, B.; Chung, J.; O’Halloran, A.; Garg, S.; Belongia, E.A.; Gaglani, M.; Zimmerman, R.; Jackson, M.L.; Monto, A.S.; et al. Effects of Influenza Vaccination in the United States during the 2017–2018 Influenza Season. Clin. Infect. Dis. 2019, 69, 1845–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellers, S.A.; Hagan, R.S.; Hayden, F.G.; Fischer, W.A., 2nd. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir. Viruses 2017, 11, 372–393. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.L.; Yang, W.; Ito, K.; Matte, T.D.; Shaman, J.; Kinney, P.L. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016, 1, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Restivo, V.; Costantino, C.; Bono, S.; Maniglia, M.; Marchese, V.; Ventura, G.; Casuccio, A.; Tramuto, F.; Vitale, F. Influenza vaccine effectiveness among high-risk groups: A systematic literature review and meta-analysis of case-control and cohort studies. Hum. Vaccines Immunother. 2018, 14, 724–735. [Google Scholar] [CrossRef] [Green Version]
- Lapi, F.; Marconi, E.; Simonetti, M.; Baldo, V.; Rossi, A.; Sessa, A.; Cricelli, C. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert Rev. Vaccines 2019, 18, 663–670. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Beyer, W.E.; McElhaney, J.; Smith, D.J.; Monto, A.S.; Nguyen-Van-Tam, J.S.; Osterhaus, A.D. Cochrane re-arranged: Support for policies to vaccinate elderly people against influenza. Vaccine 2013, 31, 6030–6033. [Google Scholar] [CrossRef] [PubMed]
- Castrucci, M.R. Factors affecting immune responses to the influenza vaccine. Hum. Vaccines Immunother. 2018, 14, 637–646. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubeck-Loebenstein, B.; Della Bella, S.; Iorio, A.M.; Michel, J.P.; Pawelec, G.; Solana, R. Immunosenescence and vaccine failure in the elderly. Aging Clin. Exp. Res. 2009, 21, 201–209. [Google Scholar] [CrossRef]
- Belongia, E.A.; McLean, H.Q. Influenza vaccine effectiveness: Defining the H3N2 problem. Clin. Infect. Dis. 2019, 69, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B. Vaccines for the elderly: Current use and future challenges. Immun. Ageing 2018, 15, 3. [Google Scholar] [CrossRef]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 2019, 124, 110632. [Google Scholar] [CrossRef]
- Vajo, Z.; Balaton, G.; Vajo, P.; Kalabay, L.; Erdman, A.; Torzsa, P. Dose sparing and the lack of a dose-response relationship with an influenza vaccine in adult and elderly patients—A randomized, double-blind clinical trial. Br. J. Clin. Pharmacol. 2017, 83, 1912–1920. [Google Scholar] [CrossRef] [Green Version]
- Vajo, Z.; Kalabay, L.; Vajo, P.; Balaton, G.; Rozsa, N.; Torzsa, P. Licensing the first reduced, 6 µg dose whole virion, aluminum adjuvanted seasonal influenza vaccine—A randomized-controlled multicenter trial. Vaccine 2019, 37, 258–264. [Google Scholar] [CrossRef]
- Ansaldi, F.; Zancolli, M.; Durando, P.; Montomoli, E.; Sticchi, L.; Del Giudice, G.; Icardi, G. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 2010, 28, 4123–4129. [Google Scholar] [CrossRef]
- Ansaldi, F.; Bacilieri, S.; Durando, P.; Sticchi, L.; Valle, L.; Montomoli, E.; Icardi, G.; Gasparini, R.; Crovari, P. Cross-protection by MF59-adjuvanted influenza vaccine: Neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine 2008, 26, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Van Buynder, P.G.; Konrad, S.; Van Buynder, J.L.; Brodkin, E.; Krajden, M.; Ramler, G.; Bigham, M. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013, 31, 6122–6128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domnich, A.; Arata, L.; Amicizia, D.; Puig-Barbera, J.; Gasparini, R.; Panatto, D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: A systematic review and meta-analysis. Vaccine 2017, 35, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Mannino, S.; Villa, M.; Apolone, G.; Weiss, N.S.; Groth, N.; Aquino, I.; Boldori, L.; Caramaschi, F.; Gattinoni, A.; Malchiodi, G.; et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am. J. Epidemiol. 2012, 176, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Chu, S.; Wernecke, M.; et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J. Infect. Dis. 2019, 220, 1255–1264. [Google Scholar] [CrossRef]
- Pebody, R.; Whitaker, H.; Zhao, H.; Andrews, N.; Ellis, J.; Donati, M.; Zambon, M. Protection provided by influenza vaccine against influenza-related hospitalisation in ≥65 year olds: Early experience of introduction of a newly licensed adjuvanted vaccine in England in 2018/19. Vaccine 2020, 38, 173–179. [Google Scholar] [CrossRef]
- Bella, A.; Gesualdo, F.; Orsi, A.; Arcuri, C.; Chironna, M.; Loconsole, D.; Napoli, C.; Orsi, G.B.; Manini, I.; Montomoli, E.; et al. Effectiveness of the trivalent MF59 adjuvated influenza vaccine in preventing hospitalization due to influenza B and A(H1N1)pdm09 viruses in the elderly in Italy, 2017–2018 season. Expert Rev. Vaccines 2019, 18, 671–679. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Wernecke, M.; MaCurdy, T.; et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018–2019. J. Infect. Dis. 2020, 222, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, J.; Han, T.; Liu, C.; Li, X.; Yan, L.; Yang, B.; Yang, X. Effectiveness, immunogenicity, and safety of influenza vaccines with MF59 adjuvant in healthy people of different age groups: A systematic review and meta-analysis. Medicine 2020, 99, e19095. [Google Scholar] [CrossRef] [PubMed]
- McConeghy, K.W.; Davidson, H.E.; Canaday, D.H.; Han, L.; Saade, E.; Mor, V.; Gravenstein, S. Cluster-randomized trial of adjuvanted vs. non-adjuvanted trivalent influenza vaccine in 823 U.S. nursing homes. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Van Aalst, R.; Gravenstein, S.; Mor, V.; Mahmud, S.M.; Wilschut, J.; Postma, M.; Chit, A. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: A retrospective cohort study. Vaccine 2020, 38, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative effectiveness of adjuvanted trivalent inactivated influenza vaccine versus egg-derived quadrivalent inactivated influenza vaccines and high-dose trivalent influenza vaccine in preventing influenza-related medical encounters in U.S. adults ≥65 years during the 2017–2018 and 2018–2019 influenza seasons. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; De Gregorio, E.; Seubert, A. The mechanism of action of MF59—An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Armed Forces Health Surveillance Center (AFHSC). AFHSC Standard Case Definitions: Influenza-Like Illness; Defense Health Agency: Falls Church, VA, USA, 2015. [Google Scholar]
- Eick-Cost, A.A.; Hunt, D.J. Assessment of ICD-9-based case definitions for influenza-like illness surveillance. MSMR 2015, 22, 2–7. [Google Scholar]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Hak, E.; Nordin, J.; Wei, F.; Mullooly, J.; Poblete, S.; Strikas, R.; Nichol, K.L. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin. Infect. Dis. 2002, 35, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Update: Influenza Activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. Morb. Mortal. Wkly. Rep. 2018, 67, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Seasonal Influenza Vaccine Effectiveness, 2017–2018. Available online: https://www.cdc.gov/flu/vaccines-work/2017-2018.html (accessed on 1 June 2021).
- Centers for Disease Control and Prevention. Update: Influenza Activity in the United States during the 2018–19 season and composition of the 2019–20 influenza vaccine. Morb. Mortal. Wkly. Rep. 2019, 68, 544–551. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Recommended Composition of Influenza Virus Vaccines for Use in the 2019–2020 Northern Hemisphere Influenza Season. Available online: https://www.who.int/influenza/vaccines/virus/recommendations/201902_recommendation.pdf (accessed on 22 July 2021).
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Vaccine Use. Available online: https://www.who.int/influenza/vaccines/use/en/ (accessed on 27 May 2021).
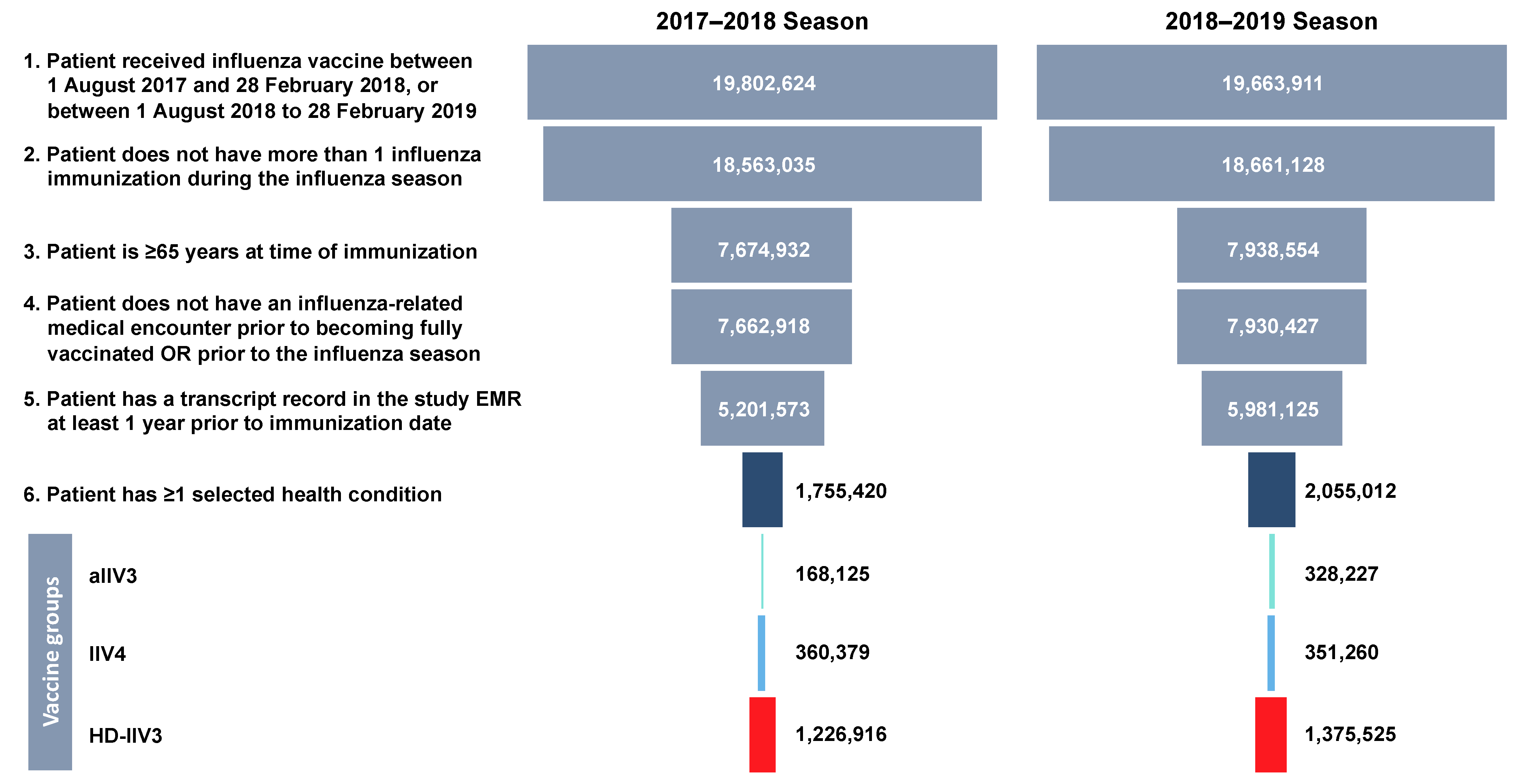

| 2017–2018 Season | 2018–2019 Season | |||||
|---|---|---|---|---|---|---|
| Characteristic | aIIV3 (n = 168,125) | IIV4 (n = 360,379) | HD-IIV3 (n = 1,226,916) | aIIV3 (n = 328,227) | IIV4 (n = 351,260) | HD-IIV3 (n = 1,375,525) |
| Mean age, years ± SD | 75.6 ± 6.7 | 74.9 ± 7.1 | 75.8 ± 6.8 | 75.7 ± 6.8 | 74.9 ± 7.2 | 75.8 ± 6.9 |
| Female, n (%) | 93,970 (56) | 202,670 (56) | 681,260 (56) | 182,214 (56) | 198,131 (56) | 767,661 (56) |
| Race, n (%) | ||||||
| White | 112,077 (67) | 230,571 (64) | 830,987 (68) | 215,363 (66) | 207,481 (59) | 896,117 (65) |
| Black | 9050 (5) | 24,707 (7) | 71,805 (6) | 16,043 (5) | 26,427 (8) | 81,066 (6) |
| Other | 11,669 (7) | 38,438 (11) | 90,175 (7) | 25,660 (8) | 37,301 (11) | 109,393 (8) |
| Not reported | 35,329 (21) | 66,663 (18) | 233,949 (19) | 71,161 (22) | 80,051 (23) | 288,949 (21) |
| Ethnicity, n (%) | ||||||
| Hispanic | 7982 (5) | 24,334 (7) | 45,310 (4) | 14,059 (4) | 27,732 (8) | 52,392 (4) |
| Non-Hispanic | 136,560 (81) | 289,830 (80) | 1,009,570 (82) | 267,577 (82) | 280,017 (80) | 1,123,324 (82) |
| Not reported | 23,583 (14) | 46,215 (13) | 172,036 (14) | 46,591 (14) | 43,511 (12) | 199,809 (15) |
| Geographic region, n (%) | ||||||
| Northeast | 22,459 (13) | 61,643 (17) | 251,559 (21) | 48,881 (15) | 63,524 (18) | 254,344 (18) |
| Midwest | 17,365 (10) | 69,256 (19) | 276,944 (23) | 45,554 (14) | 65,034 (19) | 306,667 (22) |
| South | 106,500 (63) | 138,853 (39) | 479,837 (39) | 192,007 (58) | 138,857 (40) | 541,477 (39) |
| West | 18,681 (11) | 85,298 (24) | 202,316 (16) | 35,671 (11) | 76,013 (22) | 254,799 (19) |
| Not reported/other | 3120 (2) | 5329 (1) | 16,260 (1) | 6114 (2) | 7832 (2) | 18,238 (1) |
| High-risk health condition | ||||||
| Chronic pulmonary disease | 46,020 (27) | 101,502 (28) | 341,912 (28) | 90,221 (27) | 102,422 (29) | 394,723 (29) |
| Myocardial infarction | 8101 (5) | 18,783 (5) | 62,436 (5) | 15,953 (5) | 17,182 (5) | 68,833 (5) |
| Congestive heart failure | 12,343 (7) | 34,350 (10) | 111,431 (9) | 24,036 (7) | 33,294 (9) | 122,219 (9) |
| Cerebrovascular disease | 19,562 (12) | 44,664 (12) | 157,325 (13) | 40,988 (12) | 46,549 (13) | 185,964 (14) |
| Peripheral vascular disease | 23,331 (14) | 59,620 (17) | 186,771 (15) | 45,314 (14) | 55,339 (16) | 206,224 (15) |
| Renal disease | 19,327 (11) | 50,437 (14) | 161,107 (13) | 39,964 (12) | 52,760 (15) | 197,466 (14) |
| Diabetes not chronic | 40,823 (24) | 107,093 (30) | 329,154 (27) | 78,948 (24) | 102,092 (29) | 362,658 (26) |
| Diabetes chronic | 62,692 (37) | 134,253 (37) | 422,923 (34) | 124,193 (38) | 143,480 (41) | 494,782 (36) |
| Any malignancy | 26,010 (15) | 50,567 (14) | 198,815 (16) | 55,147 (17) | 50,749 (14) | 230,268 (17) |
| Metastatic tumor | 8493 (5) | 13,198 (4) | 48,364 (4) | 14,583 (4) | 12,154 (3) | 52,711 (4) |
| AIDS/HIV | 266 (0) | 772 (0) | 1420 (0) | 577 (0) | 967 (0) | 2012 (0) |
| Rheumatic disease | 12,463 (7) | 25,370 (7) | 89,461 (7) | 23,753 (7) | 23,121 (7) | 96,184 (7) |
| Mild liver disease | 7875 (5) | 21,202 (6) | 65,472 (5) | 15,992 (5) | 19,440 (6) | 73,602 (5) |
| Liver disease | 403 (0) | 1233 (0) | 3645 (0) | 752 (0) | 1056 (0) | 3804 (0) |
| Hemiplegia or paraplegia | 884 (1) | 3146 (1) | 8163 (1) | 1836 (1) | 3448 (1) | 10,088 (1) |
| Dementia | 5474 (3) | 15,486 (4) | 45,716 (4) | 9766 (3) | 16,704 (5) | 53,106 (4) |
| Peptic ulcer disease | 4548 (3) | 9666 (3) | 35,361 (3) | 9220 (3) | 9080 (3) | 38,681 (3) |
| Charlson comorbidity index, mean ± SD | 2.2 ± 1.5 | 2.2 ± 1.4 | 2.1 ± 1.5 | 2.2 ± 1.5 | 2.2 ± 1.4 | 2.2 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boikos, C.; Imran, M.; Nguyen, V.H.; Ducruet, T.; Sylvester, G.C.; Mansi, J.A. Effectiveness of the Adjuvanted Influenza Vaccine in Older Adults at High Risk of Influenza Complications. Vaccines 2021, 9, 862. https://doi.org/10.3390/vaccines9080862
Boikos C, Imran M, Nguyen VH, Ducruet T, Sylvester GC, Mansi JA. Effectiveness of the Adjuvanted Influenza Vaccine in Older Adults at High Risk of Influenza Complications. Vaccines. 2021; 9(8):862. https://doi.org/10.3390/vaccines9080862
Chicago/Turabian StyleBoikos, Constantina, Mahrukh Imran, Van Hung Nguyen, Thierry Ducruet, Gregg C. Sylvester, and James A. Mansi. 2021. "Effectiveness of the Adjuvanted Influenza Vaccine in Older Adults at High Risk of Influenza Complications" Vaccines 9, no. 8: 862. https://doi.org/10.3390/vaccines9080862
APA StyleBoikos, C., Imran, M., Nguyen, V. H., Ducruet, T., Sylvester, G. C., & Mansi, J. A. (2021). Effectiveness of the Adjuvanted Influenza Vaccine in Older Adults at High Risk of Influenza Complications. Vaccines, 9(8), 862. https://doi.org/10.3390/vaccines9080862




