In Vivo Production of HN Protein Increases the Protection Rates of a Minicircle DNA Vaccine against Genotype VII Newcastle Disease Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Plasmids Construction
2.3. Determination of Synthesized HN Proteins from Both Prokaryotic and Eukaryotic Promoters
2.4. Determination of the Production of mcDNA in Transfected Cells
2.5. Chicken Immunization and Samples Collection
2.6. Cell Proliferation Assay
2.7. Challenge Study
2.8. Statistic Analysis
3. Results
3.1. Construction of Plasmid Expressing HN Protein with Both Prokaryotic and Eukaryotic Promoters
3.2. Confirmation of Desired HN Protein Synthesis from Both Prokaryotic and Eukaryotic Promoters
3.3. Increased HI Antibody Titers Induced by Double Promoter Design
3.4. Cell Proliferation
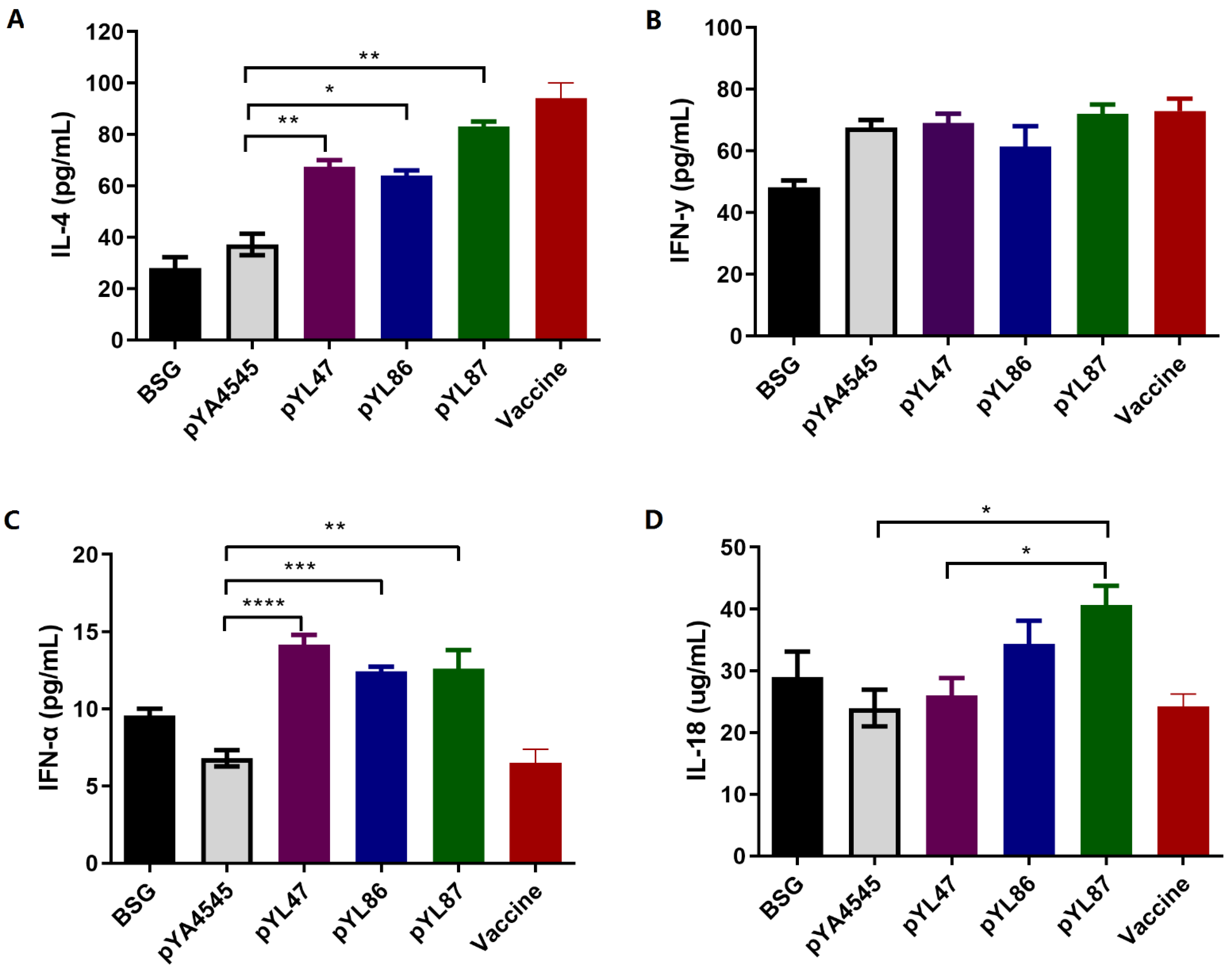
3.5. Cytokine Production
3.6. Dual Promoters Construction Increased the Survival Rate and Decreased the Viral Load
4. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rui, Z.; Juan, P.; Jingliang, S.; Jixun, Z.; Xiaoting, W.; Shouping, Z.; Xiaojiao, L.; Guozhong, Z. Phylogenetic characterization of Newcastle disease virus isolated in the mainland of China during 2001–2009. Vet. Microbiol. 2010, 141, 246–257. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, X.; Xu, K.; Wang, J.; Huang, H.; Shi, C.; Yang, W.; Kang, Y.; Curtiss, R., 3rd; Yang, G.; et al. A Novel Cre Recombinase-Mediated In Vivo Minicircle DNA (CRIM) Vaccine Provides Partial Protection against Newcastle Disease Virus. Appl. Environ. Microbiol. 2019, 85, e00407-19. [Google Scholar] [CrossRef]
- Li, J.; Valentin, A.; Kulkarni, V.; Rosati, M.; Beach, R.K.; Alicea, C.; Hannaman, D.; Reed, S.G.; Felber, B.K.; Pavlakis, G.N. HIV/SIV DNA vaccine combined with protein in a co-immunization protocol elicits highest humoral responses to envelope in mice and macaques. Vaccine 2013, 31, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bi, Y.; Xiao, H.; Yao, Y.; Liu, X.; Hu, Z.; Duan, J.; Yang, Y.; Li, Z.; Li, Y.; et al. A novel DNA and protein combination COVID-19 vaccine formulation provides full protection against SARS-CoV-2 in rhesus macaques. Emerg. Microbes Infect. 2021, 10, 342–355. [Google Scholar] [CrossRef]
- Sun, J.; Han, Z.; Zhao, R.; Ai, H.; Chen, L.; Li, L.; Liu, S. Protection of chicks from Newcastle disease by combined vaccination with a plasmid DNA and the pre-fusion protein of the virulent genotype VII of Newcastle disease virus. Vaccine 2020, 38, 7337–7349. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Zheng, M.; Xiong, F.; Liu, X.; Zhou, L.; Tan, W.; Chen, Z. Immunization with DNA prime-subunit protein boost strategy based on influenza H9N2 virus conserved matrix protein M1 and its epitope screening. Sci. Rep. 2020, 10, 4144. [Google Scholar] [CrossRef]
- Meunier, M.; Guyard-Nicodeme, M.; Vigouroux, E.; Poezevara, T.; Beven, V.; Quesne, S.; Amelot, M.; Parra, A.; Chemaly, M.; Dory, D. A DNA prime/protein boost vaccine protocol developed against Campylobacter jejuni for poultry. Vaccine 2018, 36, 2119–2125. [Google Scholar] [CrossRef]
- Stachyra, A.; Pietrzak, M.; Maciola, A.; Protasiuk, A.; Olszewska, M.; Smietanka, K.; Minta, Z.; Gora-Sochacka, A.; Kopera, E.; Sirko, A. A prime/boost vaccination with HA DNA and Pichia-produced HA protein elicits a strong humoral response in chickens against H5N1. Virus Res. 2017, 232, 41–47. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Shi, H.; Sun, W.; Roland, K.L.; Curtiss, R., 3rd. Comparison of a regulated delayed antigen synthesis system with in vivo-inducible promoters for antigen delivery by live attenuated Salmonella vaccines. Infect. Immun. 2011, 79, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Heithoff, D.M.; Conner, C.P.; Hanna, P.C.; Julio, S.M.; Hentschel, U.; Mahan, M.J. Bacterial infection as assessed by in vivo gene expression. Proc. Natl. Acad. Sci. USA 1997, 94, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.L.; Beer, K.B.; Loomis, W.P.; Olson, J.A.; Miller, S.I. An unusual pagC::TnphoA mutation leads to an invasion- and virulence-defective phenotype in Salmonellae. Infect. Immun. 1992, 60, 3763–3770. [Google Scholar] [CrossRef]
- Bumann, D. Regulated antigen expression in live recombinant Salmonella enterica serovar Typhimurium strongly affects colonization capabilities and specific CD4(+)-T-cell responses. Infect. Immun. 2001, 69, 7493–7500. [Google Scholar] [CrossRef][Green Version]
- Kong, W.; Brovold, M.; Koeneman, B.A.; Clark-Curtiss, J.; Curtiss, R., 3rd. Turning self-destructing Salmonella into a universal DNA vaccine delivery platform. Proc. Natl. Acad. Sci. USA 2012, 109, 19414–19419. [Google Scholar] [CrossRef]
- Kong, W.; Wanda, S.Y.; Zhang, X.; Bollen, W.; Tinge, S.A.; Roland, K.L.; Curtiss, R., 3rd. Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc. Natl. Acad. Sci. USA 2008, 105, 9361–9366. [Google Scholar] [CrossRef] [PubMed]
- Hmiel, S.P.; Snavely, M.D.; Florer, J.B.; Maguire, M.E.; Miller, C.G. Magnesium transport in Salmonella typhimurium: Genetic characterization and cloning of three magnesium transport loci. J. Bacteriol. 1989, 171, 4742–4751. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Kong, Q.; Roland, K.L.; Curtiss, R., 3rd. Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity. Int. J. Med. Microbiol. IJMM 2014, 304, 431–443. [Google Scholar] [PubMed]
- Cohen, R.N.; van der Aa, M.A.; Macaraeg, N.; Lee, A.P.; Szoka, F.C., Jr. Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. J. Control Release Off. J. Control. Release Soc. 2009, 135, 166–174. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, J.; Hu, S.; Liu, X.; Wang, X.; Zhu, J.; Liu, X. Strong innate immune response and cell death in chicken splenocytes infected with genotype VIId Newcastle disease virus. Virol. J. 2012, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Felber, B.K.; Lu, Z.; Hu, X.; Valentin, A.; Rosati, M.; Remmel, C.A.L.; Weiner, J.A.; Carpenter, M.C.; Faircloth, K.; Stanfield-Oakley, S.; et al. Co-immunization of DNA and Protein in the Same Anatomical Sites Induces Superior Protective Immune Responses against SHIV Challenge. Cell Rep. 2020, 31, 107624. [Google Scholar] [CrossRef]
- Gehring, S.; Gregory, S.H.; Kuzushita, N.; Wands, J.R. Type 1 interferon augments DNA-based vaccination against hepatitis C virus core protein. J. Med. Virol. 2005, 75, 249–257. [Google Scholar] [CrossRef]
- Gao, X.; Xu, K.; Yang, G.; Shi, C.; Huang, H.; Wang, J.; Yang, W.; Liu, J.; Liu, Q.; Kang, Y.; et al. Construction of a novel DNA vaccine candidate targeting F gene of genotype VII Newcastle disease virus and chicken IL-18 delivered by Salmonella. J. Appl. Microbiol. 2019, 126, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, H.B.; Jiang, Y.L.; Liu, J.; Gao, X.; Liu, Y.; Yang, W.T.; Shi, C.W.; Wang, D.; Wang, J.Z.; et al. Immunological evaluation of invasive Lactobacillus plantarum co-expressing EtMIC2 and chicken interleukin-18 against Eimeria tenella. Parasitol. Res. 2020, 119, 2885–2895. [Google Scholar] [CrossRef] [PubMed]


| Plasmids or Strains | Description | Source |
|---|---|---|
| Strains | ||
| χ11218 | Salmonella host strain for DNA vaccine study | Roy Curtiss III University of Florida |
| χ6212 | E.coli host strain for DNA cloning | Roy Curtiss III University of Florida |
| Plasmids | ||
| pYA4545 | Eukaryotic expression vector in Salmonella, arabinose dependent | Roy Curtiss III University of Florida |
| pYA3342-pagC | Asd+ plasmid, PpagC promoter | Lab collection |
| pYL66 | Asd+ plasmid, express HN under PpagC promoter | This study |
| pUC-HN | Kan+, complete HN gene of genotype VII NDV cloned into pUC57 vector | Lab collection |
| pYL46 | Minicircle DNA vector express EGFP under CMV promoter | Lab collection |
| pYL47 | Minicircle DNA vector express HN under CMV promoter | Lab collection |
| pYL86 | Insert PpagC-HN-TT cassette into pYL46 | This study |
| pYL87 | Insert PpagC-HN-TT cassette into pYL47 | This study |
| Primers | Sequences |
|---|---|
| HNopt1-NcoI-F | TGccatggGACCGCACGACCTCGCAGGTA |
| HNopt1-HindIII-R | GATaagcttCTATTACTTGTCGTCGTCGTCCTTGTAGTC |
| PagC-SacII-F | TCCccgcggGTTAACCACTCTTAATAAT |
| TT-SacII-R | TCCccgcggAAGAGTTTGTAGAAACGCAA |
| CMV-F1 | GTACGCCCCCTATTGACG |
| CMV-R1 | TTGGCATATGATACACTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhao, X.; Wang, Y.; Sun, C.; Sun, M.; Gao, X.; Jia, F.; Shan, C.; Yang, G.; Wang, J.; et al. In Vivo Production of HN Protein Increases the Protection Rates of a Minicircle DNA Vaccine against Genotype VII Newcastle Disease Virus. Vaccines 2021, 9, 723. https://doi.org/10.3390/vaccines9070723
Wang Z, Zhao X, Wang Y, Sun C, Sun M, Gao X, Jia F, Shan C, Yang G, Wang J, et al. In Vivo Production of HN Protein Increases the Protection Rates of a Minicircle DNA Vaccine against Genotype VII Newcastle Disease Virus. Vaccines. 2021; 9(7):723. https://doi.org/10.3390/vaccines9070723
Chicago/Turabian StyleWang, Zhannan, Xiaohan Zhao, Ying Wang, Chao Sun, Ming Sun, Xingyun Gao, Futing Jia, Chenxin Shan, Guilian Yang, Jianzhong Wang, and et al. 2021. "In Vivo Production of HN Protein Increases the Protection Rates of a Minicircle DNA Vaccine against Genotype VII Newcastle Disease Virus" Vaccines 9, no. 7: 723. https://doi.org/10.3390/vaccines9070723
APA StyleWang, Z., Zhao, X., Wang, Y., Sun, C., Sun, M., Gao, X., Jia, F., Shan, C., Yang, G., Wang, J., Huang, H., Shi, C., Yang, W., Qian, A., Wang, C., & Jiang, Y. (2021). In Vivo Production of HN Protein Increases the Protection Rates of a Minicircle DNA Vaccine against Genotype VII Newcastle Disease Virus. Vaccines, 9(7), 723. https://doi.org/10.3390/vaccines9070723





