Evaluation of Protective Efficacy of Influenza Virus Like Particles Prepared from H5N1 Virus of Clade 2.2.1.2 in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. VLPs Preparation and Characterization
2.2. Evaluation of the VLPs in SPF Chickens as a Candidate Vaccine
3. Results
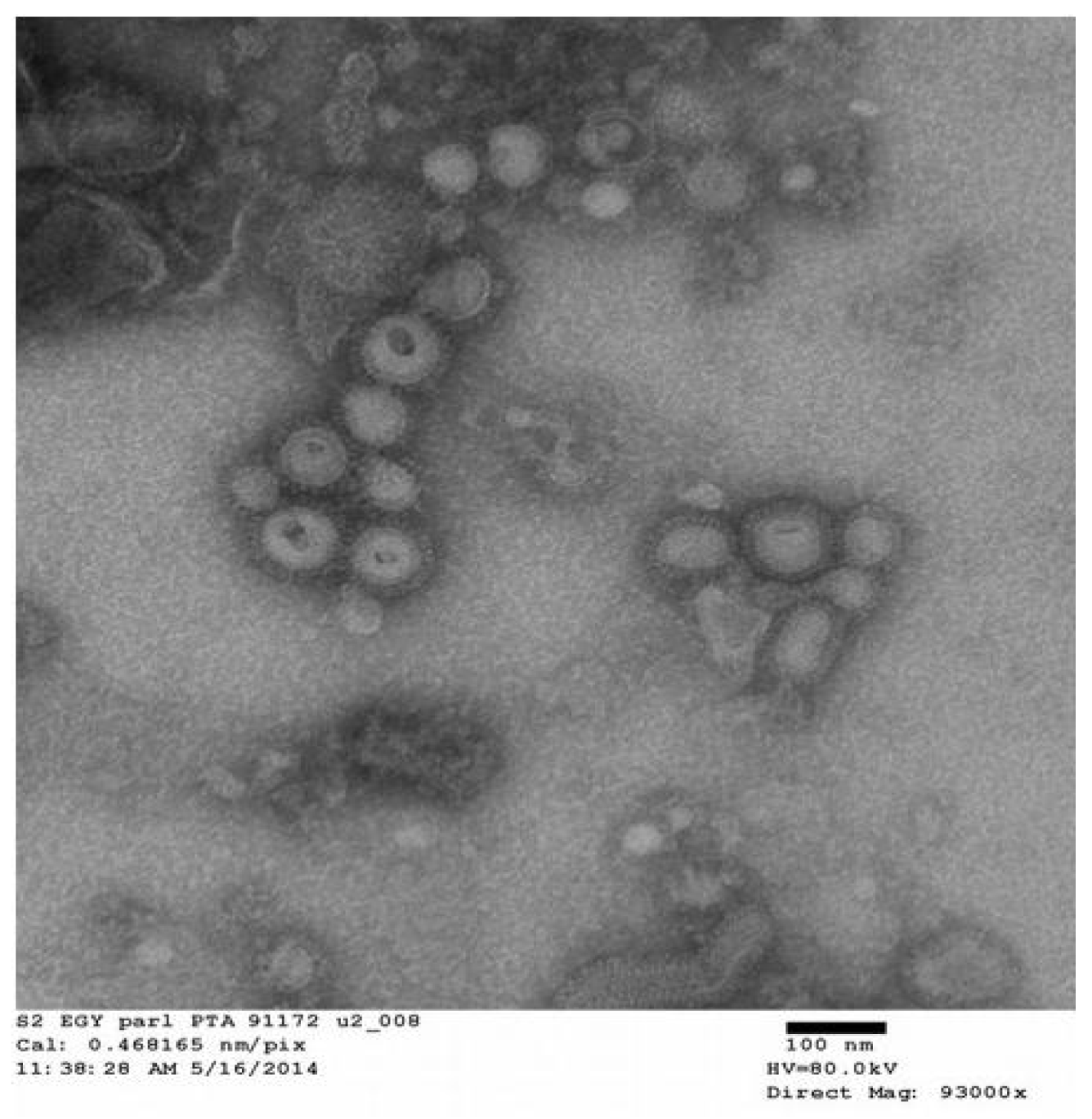
3.1. Morphology and Size of VLPs
3.2. Immunogenicity in Chickens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abdelwhab, E.M.; Hassan, M.K.; Abdel-Moneim, A.S.; Naguib, M.M.; Mostafa, A.; Hussein, I.T.M.; Arafa, A.; Erfan, A.M.; Kilany, W.H.; Agour, M.G.; et al. Introduction and enzootic of A/H5N1 in Egypt: Virus evolution, pathogenicity and vaccine efficacy ten years on. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 40, 80–90. [Google Scholar] [CrossRef]
- Arafa, A.S.; Naguib, M.M.; Luttermann, C.; Selim, A.A.; Kilany, W.H.; Hagag, N.; Samy, A.; Abdelhalim, A.; Hassan, M.K.; Abdelwhab, E.M.; et al. Emergence of a novel cluster of influenza A(H5N1) virus clade 2.2.1.2 with putative human health impact in Egypt, 2014/15. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2015, 20, 2–8. [Google Scholar] [CrossRef]
- Peyre, M.; Samaha, H.; Makonnen, Y.J.; Saad, A.; Abd-Elnabi, A.; Galal, S.; Ettel, T.; Dauphin, G.; Lubroth, J.; Roger, F.; et al. Avian influenza vaccination in Egypt: Limitations of the current strategy. J. Mol. Genet. Med. An Int. J. Biomed. Res. 2009, 3, 198–204. [Google Scholar] [CrossRef]
- Tosh, P.K.; Poland, G.A. Emerging vaccines for influenza. Expert Opin. Emerg. Drugs 2008, 13, 21–40. [Google Scholar] [CrossRef]
- Guyonnet, V.; Peters, A.R. Are current avian influenza vaccines a solution for smallholder poultry farmers? Gates Open Res. 2020, 4, 122. [Google Scholar] [CrossRef]
- Kang, S.M.; Song, J.M.; Quan, F.S.; Compans, R.W. Influenza vaccines based on virus-like particles. Virus Res. 2009, 143, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ninyio, N.N.; Ho, K.L.; Omar, A.R.; Tan, W.S.; Iqbal, M.; Mariatulqabtiah, A.R. Virus-like Particle Vaccines: A Prospective Panacea Against an Avian Influenza Panzootic. Vaccines 2020, 8, 694. [Google Scholar] [CrossRef]
- Wetzel, D.; Barbian, A.; Jenzelewski, V.; Schembecker, G.; Merz, J.; Piontek, M. Bioprocess optimization for purification of chimeric VLP displaying BVDV E2 antigens produced in yeast Hansenula polymorpha. J. Biotechnol. 2019, 306, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Roldão, A.; Mellado, M.C.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Pushko, P.; Tumpey, T.M.; Bu, F.; Knell, J.; Robinson, R.; Smith, G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 2005, 23, 5751–5759. [Google Scholar] [CrossRef]
- Buffin, S.; Peubez, I.; Barrière, F.; Nicolaï, M.C.; Tapia, T.; Dhir, V.; Forma, E.; Sève, N.; Legastelois, I. Influenza A and B virus-like particles produced in mammalian cells are highly immunogenic and induce functional antibodies. Vaccine 2019, 37, 6857–6867. [Google Scholar] [CrossRef]
- Pushko, P.; Tretyakova, I.; Hidajat, R.; Sun, X.; Belser, J.A.; Tumpey, T.M. Multi-clade H5N1 virus-like particles: Immunogenicity and protection against H5N1 virus and effects of beta-propiolactone. Vaccine 2018, 36, 4346–4353. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Hossain, J.; Yoo, D.G.; Lipatov, A.S.; Davis, C.T.; Quan, F.S.; Chen, L.M.; Hogan, R.J.; Donis, R.O.; Compans, R.W.; et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 2010, 405, 165–175. [Google Scholar] [CrossRef]
- Kapczynski, D.R.; Tumpey, T.M.; Hidajat, R.; Zsak, A.; Chrzastek, K.; Tretyakova, I.; Pushko, P. Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses. Vaccine 2016, 34, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Awad, E.T.; Gouda, E.; El-Husseiny, M.H.; Aly, M.M.; Pushko, P.; Tretyakova, I.; Arafa, A.S.M. Biochemical and immunogenicity studies on hemagglutinin protein rescued from H5N1 avian influenza virus like particles. J. Am. Sci. 2015, 11, 1–8. [Google Scholar]
- Lee, D.H.; Park, J.K.; Song, C.S. Progress and hurdles in the development of influenza virus-like particle vaccines for veterinary use. Clin. Exp. Vaccine Res. 2014, 3, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, L.M.; Cathcart, A.L.; Pujanauski, L.M.; Qi, L.; Kash, J.C.; Taubenberger, J.K. An Intranasal Virus-Like Particle Vaccine Broadly Protects Mice from Multiple Subtypes of Influenza A Virus. mBio 2015, 6, e01044. [Google Scholar] [CrossRef]
- Tretyakova, I.; Pearce, M.B.; Florese, R.; Tumpey, T.M.; Pushko, P. Intranasal vaccination with H5, H7 and H9 hemagglutinins co-localized in a virus-like particle protects ferrets from multiple avian influenza viruses. Virology 2013, 442, 67–73. [Google Scholar] [CrossRef]
- Shen, H.; Xue, C.; Lv, L.; Wang, W.; Liu, Q.; Liu, K.; Chen, X.; Zheng, J.; Li, X.; Cao, Y. Assembly and immunological properties of a bivalent virus-like particle (VLP) for avian influenza and Newcastle disease. Virus Res. 2013, 178, 430–436. [Google Scholar] [CrossRef]
- Jackwood, D.J. Multivalent virus-like-particle vaccine protects against classic and variant infectious bursal disease viruses. Avian Dis. 2013, 57, 41–50. [Google Scholar] [CrossRef]
- Liu, M.; Wood, J.M.; Ellis, T.; Krauss, S.; Seiler, P.; Johnson, C.; Hoffmann, E.; Humberd, J.; Hulse, D.; Zhang, Y.; et al. Preparation of a standardized, efficacious agricultural H5N3 vaccine by reverse genetics. Virology 2003, 314, 580–590. [Google Scholar] [CrossRef][Green Version]
- Spackman, E.; Swayne, D.E.; Pantin-Jackwood, M.J.; Wan, X.F.; Torchetti, M.K.; Hassan, M.; Suarez, D.L.; Sa e Silva, M. Variation in protection of four divergent avian influenza virus vaccine seed strains against eight clade 2.2.1 and 2.2.1.1. Egyptian H5N1 high pathogenicity variants in poultry. Influenza Other Respir. Viruses 2014, 8, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Naeem, K.; Siddique, N. Use of strategic vaccination for the control of avian influenza in Pakistan. Dev. Biol. 2006, 124, 145–150. [Google Scholar]
- Van Oers, M.M. Opportunities and challenges for the baculovirus expression system. J. Invertebr. Pathol. 2011, 107, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Sheppard, N.C.; Stewart-Jones, G.B.E.; Robson, C.L.; Chen, H.; Xu, X.; Krashias, G.; Bonomelli, C.; Scanlan, C.N.; Kwong, P.D.; et al. Expression-system-dependent modulation of HIV-1 envelope glycoprotein antigenicity and immunogenicity. J. Mol. Biol. 2010, 403, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Aguilar, J.C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488–496. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, D.H.; Yuk, S.S.; Tseren-Ochir, E.O.; Kwon, J.H.; Noh, J.Y.; Kim, B.Y.; Choi, S.W.; Kang, S.M.; Lee, J.B.; et al. Virus-like particle vaccine confers protection against a lethal newcastle disease virus challenge in chickens and allows a strategy of differentiating infected from vaccinated animals. Clin. Vaccine Immunol. CVI 2014, 21, 360–365. [Google Scholar] [CrossRef]
- Tretyakova, I.; Hidajat, R.; Hamilton, G.; Horn, N.; Nickols, B.; Prather, R.O.; Tumpey, T.M.; Pushko, P. Preparation of quadri-subtype influenza virus-like particles using bovine immunodeficiency virus gag protein. Virology 2016, 487, 163–171. [Google Scholar] [CrossRef]
- Meeusen, E.N.; Walker, J.; Peters, A.; Pastoret, P.P.; Jungersen, G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007, 20, 489–510. [Google Scholar] [CrossRef]
| Group. | Route | HI Geometric Mean Titer, log2, after 3wk of 1st Dose | HI geometric Mean Titer, log2, after 3wk of 2nd dose | Challenge Virus Titer | Mortality | Protection % |
|---|---|---|---|---|---|---|
| Group 1 (2 doses) | S/C | 22.5 | 26.5 | 106 EID50/0.1 mL | 0/9 | 100% * |
| Group 2 (1 dose) | S/C | 24.5 | ND | 106 EID50/0.1 mL | 3/9 | 70% * |
| Group 3 (Negative control) | ND | ND | 0 | ND | 0/9 | - |
| Group 4 (Positive Control) | ND | ND | 0 | 106 EID50/0.1 mL | 9/9 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Husseiny, M.H.; Hagag, N.M.; Pushko, P.; Tretyakova, I.; Naguib, M.M.; Arafa, A.S. Evaluation of Protective Efficacy of Influenza Virus Like Particles Prepared from H5N1 Virus of Clade 2.2.1.2 in Chickens. Vaccines 2021, 9, 715. https://doi.org/10.3390/vaccines9070715
El-Husseiny MH, Hagag NM, Pushko P, Tretyakova I, Naguib MM, Arafa AS. Evaluation of Protective Efficacy of Influenza Virus Like Particles Prepared from H5N1 Virus of Clade 2.2.1.2 in Chickens. Vaccines. 2021; 9(7):715. https://doi.org/10.3390/vaccines9070715
Chicago/Turabian StyleEl-Husseiny, Mohamed H., Naglaa M. Hagag, Peter Pushko, Irina Tretyakova, Mahmoud M. Naguib, and Abdel Satar Arafa. 2021. "Evaluation of Protective Efficacy of Influenza Virus Like Particles Prepared from H5N1 Virus of Clade 2.2.1.2 in Chickens" Vaccines 9, no. 7: 715. https://doi.org/10.3390/vaccines9070715
APA StyleEl-Husseiny, M. H., Hagag, N. M., Pushko, P., Tretyakova, I., Naguib, M. M., & Arafa, A. S. (2021). Evaluation of Protective Efficacy of Influenza Virus Like Particles Prepared from H5N1 Virus of Clade 2.2.1.2 in Chickens. Vaccines, 9(7), 715. https://doi.org/10.3390/vaccines9070715







