Development of SARS-CoV-2 Specific IgG and Virus-Neutralizing Antibodies after Infection with Variants of Concern or Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. SARS-CoV-2 S-Protein-Specific IgG Immunoassays
2.2. SARS-CoV-2 IgG Immunoblots and Analysis of IgG Avidity
2.3. Measurement of SARS-CoV-2-Neutralizing Antibodies
2.4. Data Evaluation and Statistical Calculations
3. Results
3.1. Composition of the Study Groups
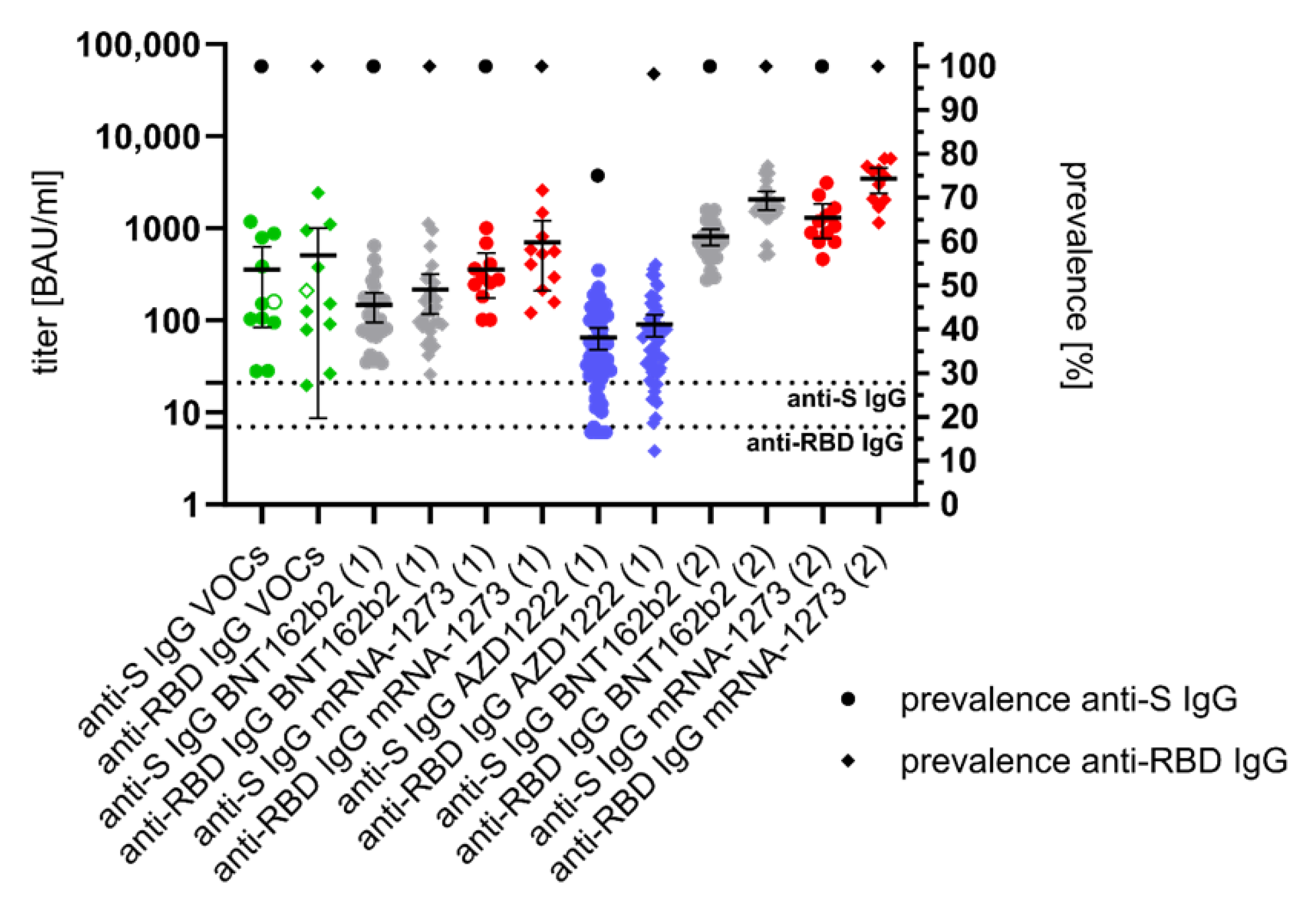
3.2. Anti-S- and Anti-RBD-Specific IgG Response after SARS-CoV-2 Infection and Vaccination
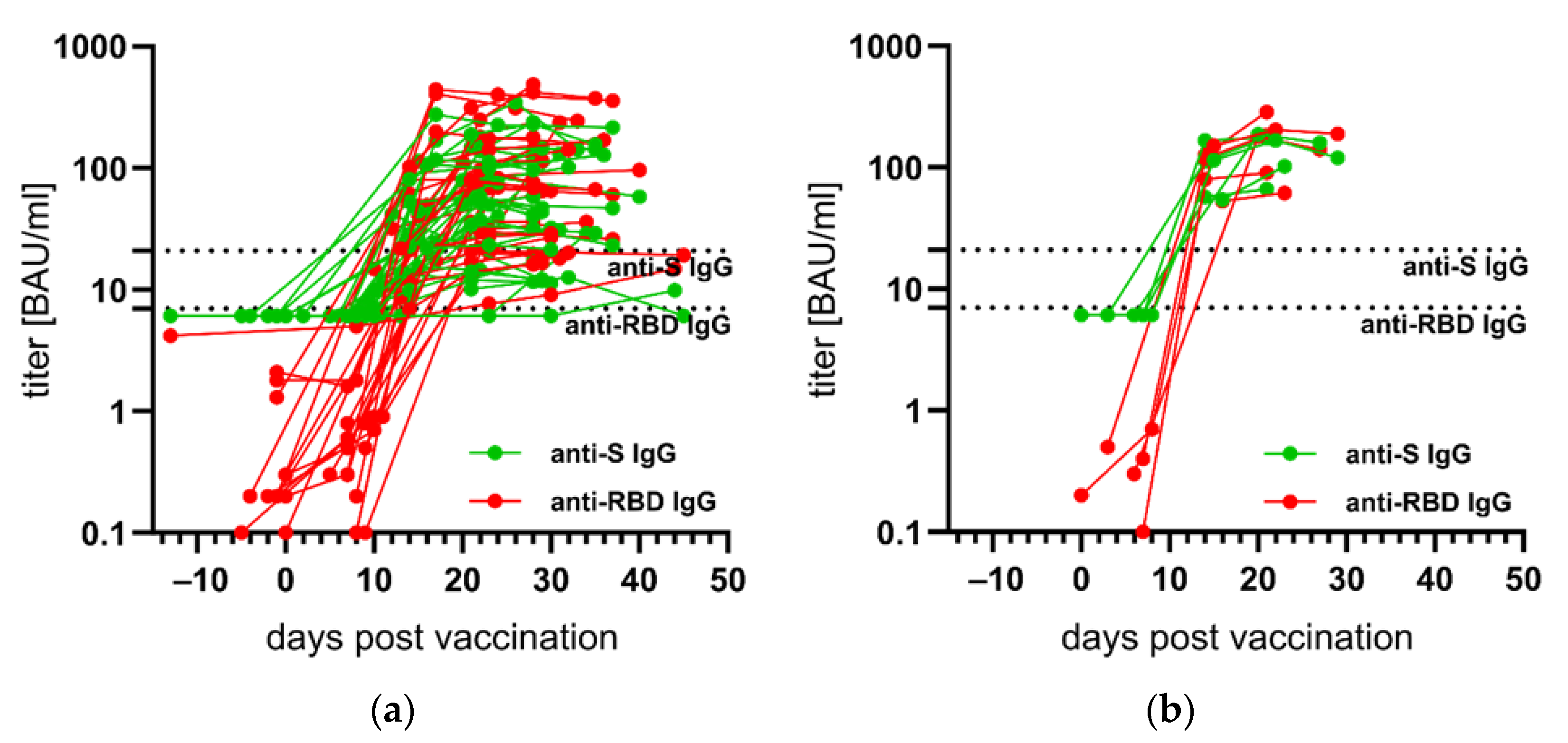
3.3. Kinetics of Anti-S- and Anti-RBD IgG after First SARS-CoV-2 Vaccination
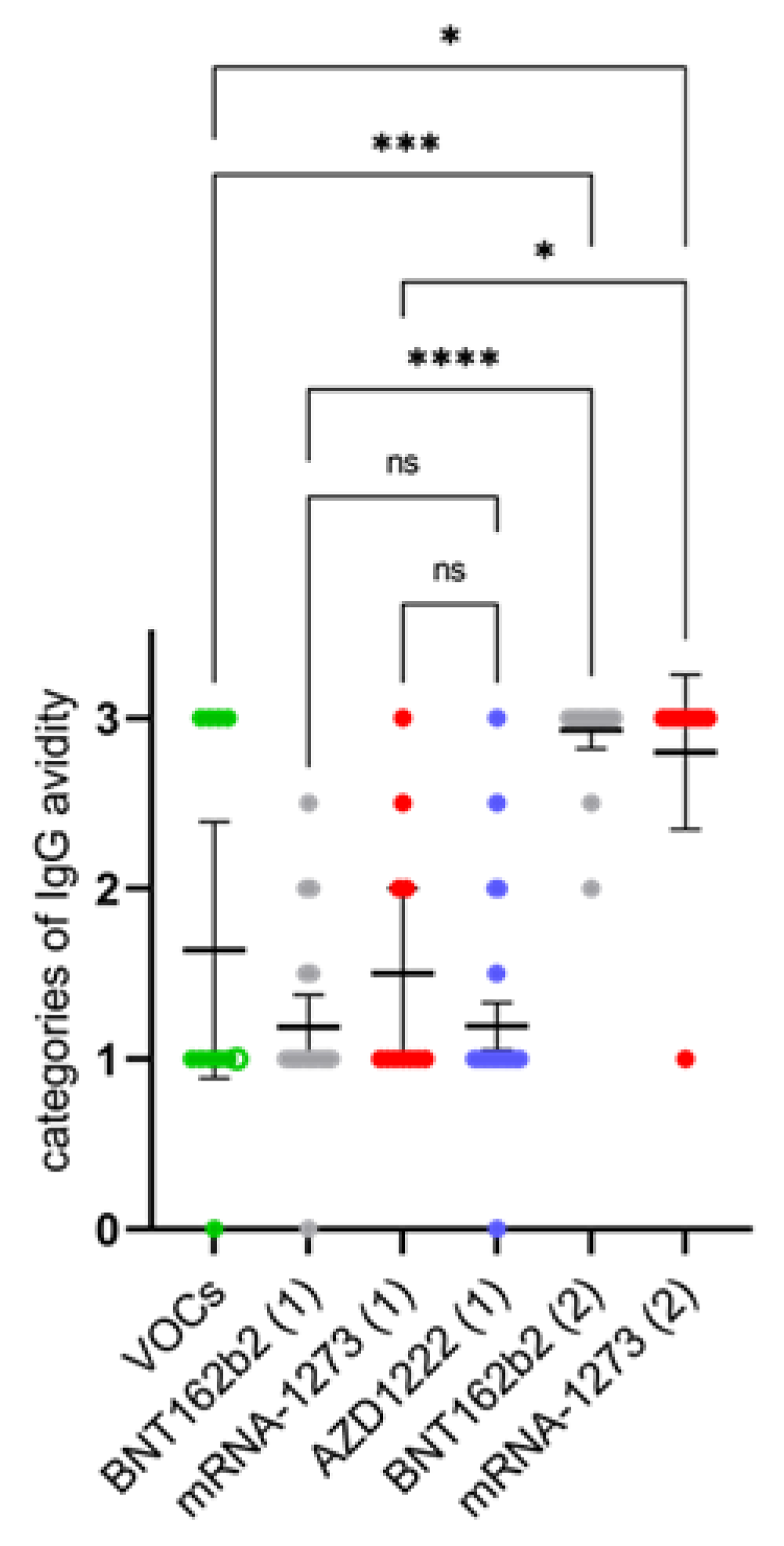
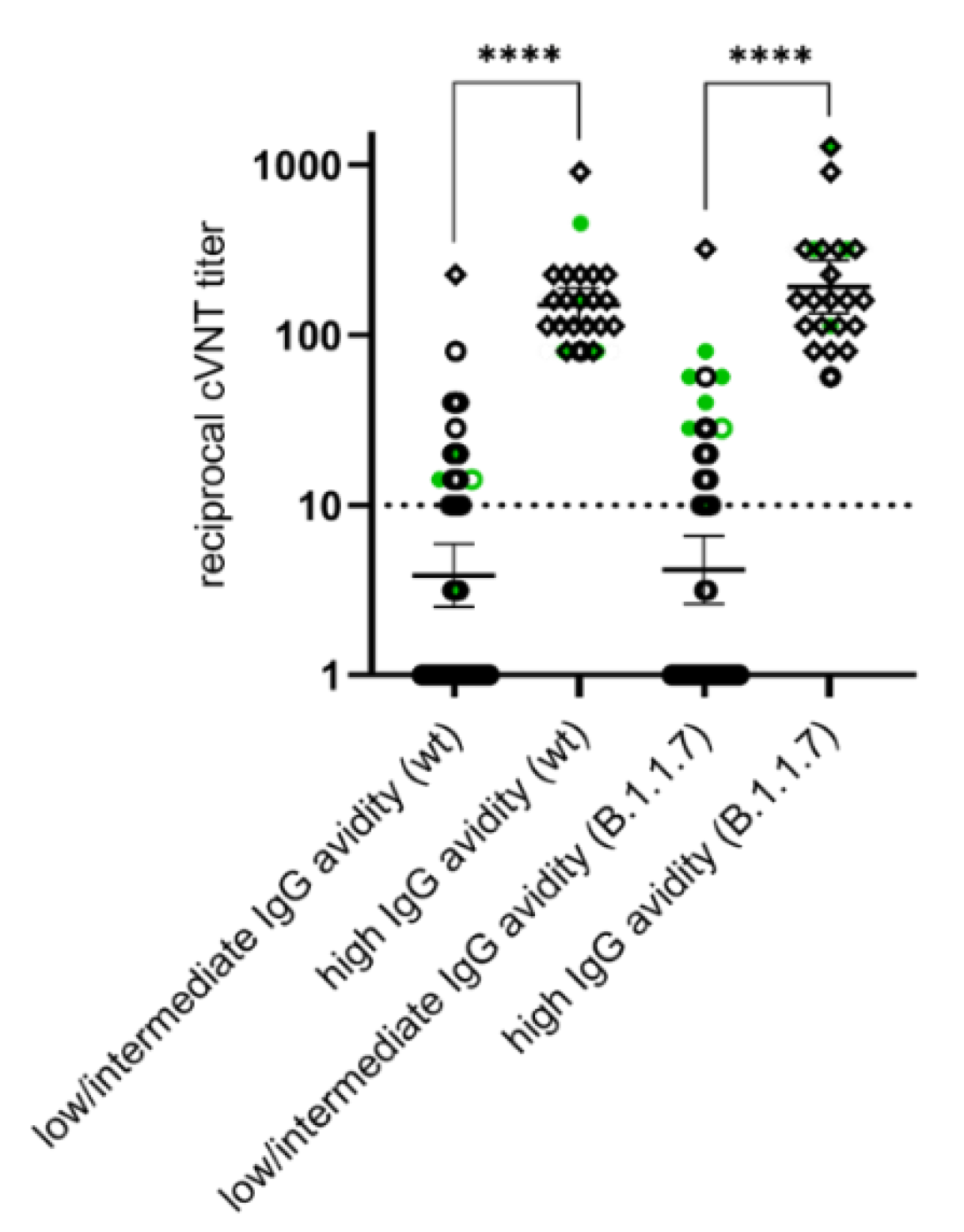
3.4. Differences in the Anti-N IgG Response between SARS-CoV-2 Infected and Vaccinated Individuals and Development of High Avidity SARS-CoV-2 IgGs after the Second Vaccination
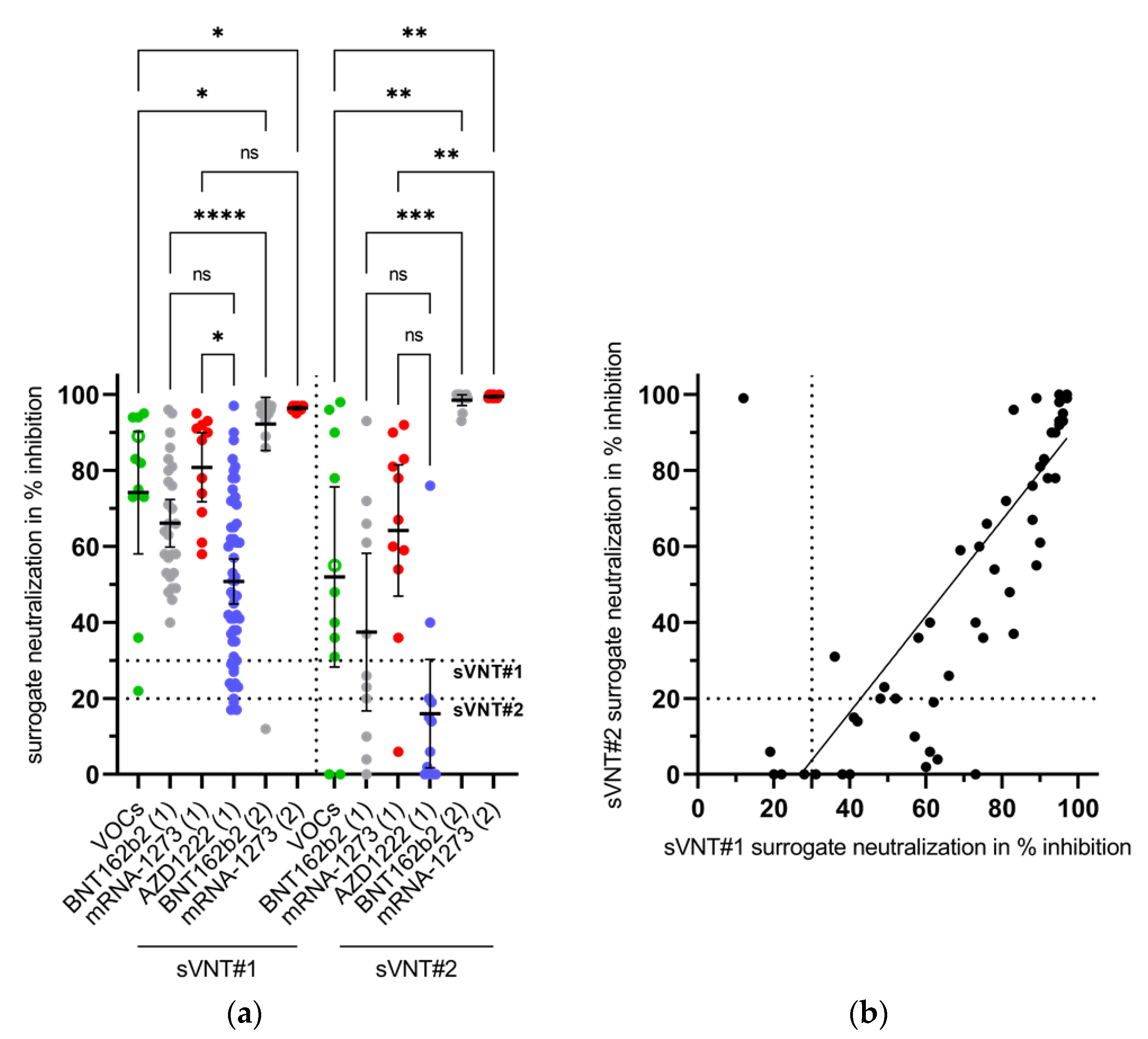
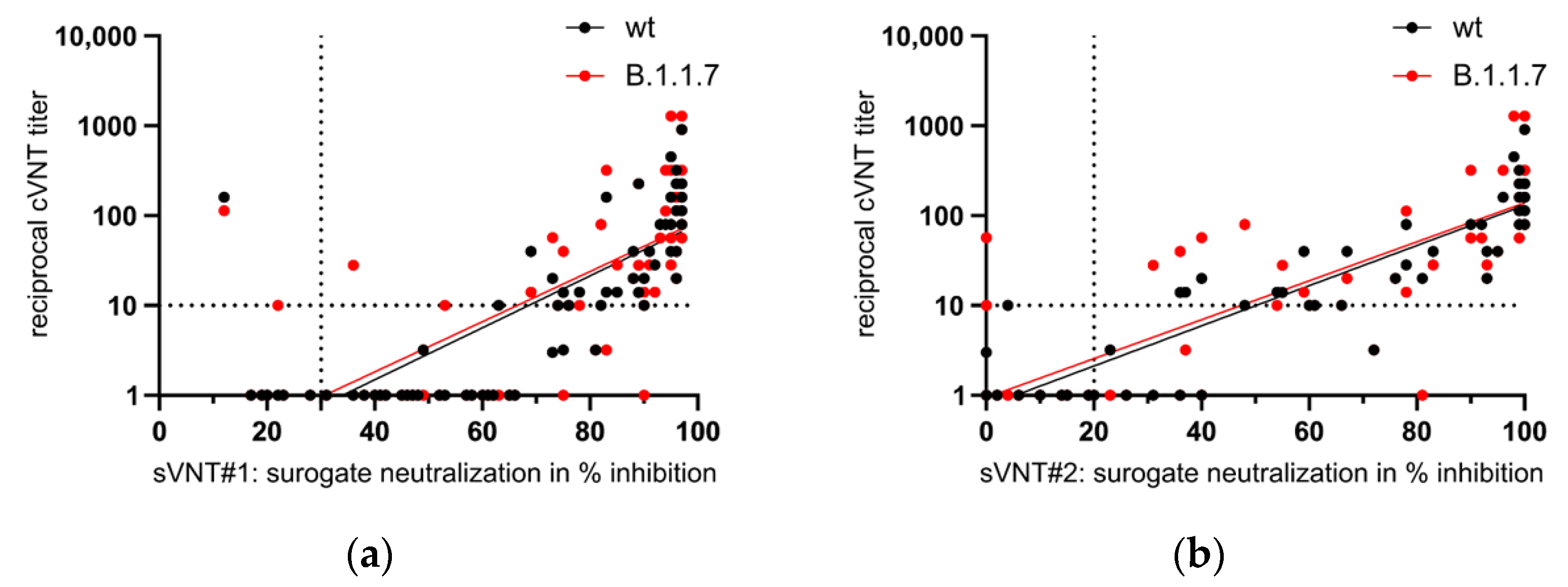
3.5. Appearance of Potent Virus-Neutralizing Antibodies after the Second Vaccination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 May 2021).
- Klasse, P.J.; Nixon, D.F.; Moore, J.P. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Sci. Adv. 2021, 7, eabe8065. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; Lopez-Cortes, A.; Gonzalez, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. Npj Vaccines 2021, 6, 28. [Google Scholar] [CrossRef]
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 Vaccines. JAMA 2021, 325, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- EU. Safe COVID-19 Vaccines for Europeans. Available online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/safe-covid-19-vaccines-europeans_en (accessed on 12 May 2021).
- STIKO. Stellungnahme Der Ständigen Impfkommission Zum Zeitpunkt der Gabe Eines mRNA-Impfstoffs Nach Erstimpfung Mit AstraZeneca Vaccine (Vaxzevria) bei <60-Jährigen. Available online: https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/Stellungnahme-Impfabstand.html (accessed on 12 May 2021).
- Rambaut, A.; Holmes, E.C.; O’Toole, Á.; Hill, V.; McCrone, J.T.; Ruis, C.; du Plessis, L.; Pybus, O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020, 5, 1403–1407. [Google Scholar] [CrossRef]
- Strömer, A.; Rose, R.; Schäfer, M.; Schön, F.; Vollersen, A.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Performance of a Point-of-Care Test for the Rapid Detection of SARS-CoV-2 Antigen. Microorganisms 2020, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Strömer, A.; Rose, R.; Grobe, O.; Neumann, F.; Fickenscher, H.; Lorentz, T.; Krumbholz, A. Kinetics of Nucleo- and Spike Protein-Specific Immunoglobulin G and of Virus-Neutralizing Antibodies after SARS-CoV-2 Infection. Microorganisms 2020, 8, 1572. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, P.A.; Page, M.; Bernasconi, V.; Mattiuzzo, G.; Dull, P.; Makar, K.; Plotkin, S.; Knezevic, I. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet 2021, 397, 1347–1348. [Google Scholar] [CrossRef]
- Bauer, G. The potential significance of high avidity immunoglobulin G (IgG) for protective immunity towards SARS-CoV-2. Int. J. Infect. Dis. 2021, 106, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.; Struck, F.; Schreiner, P.; Staschik, E.; Soutschek, E.; Motz, M. The challenge of avidity determination in SARS-CoV-2 serology. J. Med. Virol. 2021, 93, 3092–3104. [Google Scholar] [CrossRef]
- Bauer, G. The variability of the serological response to SARS-corona virus-2: Potential resolution of ambiguity through determination of avidity (functional affinity). J. Med. Virol. 2021, 93, 311–322. [Google Scholar] [CrossRef]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerg. Microbes Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- von Rhein, C.; Scholz, T.; Henss, L.; Kronstein-Wiedemann, R.; Schwarz, T.; Rodionov, R.N.; Corman, V.M.; Tonn, T.; Schnierle, B.S. Comparison of potency assays to assess SARS-CoV-2 neutralizing antibody capacity in COVID-19 convalescent plasma. J. Virol. Methods 2021, 288, 114031. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lou, F.; Fan, H. SARS-CoV-2 variants: A new challenge to convalescent serum and mRNA vaccine neutralization efficiency. Signal. Transduct. Target. Ther. 2021, 6, 151. [Google Scholar] [CrossRef]
- Bradley, T.; Grundberg, E.; Selvarangan, R.; LeMaster, C.; Fraley, E.; Banerjee, D.; Belden, B.; Louiselle, D.; Nolte, N.; Biswell, R.; et al. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1959–1961. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, G. Covid-19: Infections fell by 65% after first dose of AstraZeneca or Pfizer vaccine, data show. BMJ 2021, 373, n1068. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; Rourke, E.; et al. The impact of SARS-CoV-2 vaccines on antibody responses in the general population in the United Kingdom. medRxiv 2021. [Google Scholar] [CrossRef]
- Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Gethings, O.; Vihta, K.-D.; Jones, J.; House, T.; Van Steen House, H.; Bell, I.; et al. Impact of vaccination on SARS-CoV-2 cases in the community: A population-based study using the UK’s COVID-19 Infection Survey. medRxiv 2021. [Google Scholar] [CrossRef]
- Torjesen, I. Covid-19: First doses of vaccines in Scotland led to a substantial fall in hospital admissions. BMJ 2021, 372, n523. [Google Scholar] [CrossRef]
- Vasileiou, E.; Simpson, C.R.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; Chuter, A.; et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet 2021, 397, 1646–1657. [Google Scholar] [CrossRef]
- Hedman, K.; Lappalainen, M.; Söderlund, M.; Hedman, L. Avidity of IgG in serodiagnosis of infectious diseases. Rev. Med. Microbiol. 1993, 4, 123–129. [Google Scholar] [CrossRef]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Embregts, C.W.E.; Verstrepen, B.; Langermans, J.A.M.; Böszörményi, K.P.; Sikkema, R.S.; de Vries, R.D.; Hoffmann, D.; Wernike, K.; Smit, L.A.M.; Zhao, S.; et al. Evaluation of a multi-species SARS-CoV-2 surrogate virus neutralization test. medRxiv 2021. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A.; National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine againstf the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]

| Groups | Number of Individuals | Median Age in Years | Age Span in Years | Gender (Female/Male) | Time Span from RT-PCR or the First/Second Vaccination to Serum Sampling in Days |
|---|---|---|---|---|---|
| SARS-CoV-2 VOC infection | 11 | 52 | 18 to 69 | 6/5 | 28 to 41 |
| SARS-CoV-2 vaccination | 100 | 41 | 18 to 62 | 77/23 | −13 to 65/15 to 49 |
| mRNA vaccines | |||||
| Pfizer/BioNTech (BNT162b2) | 33 | 45 | 23 to 62 | 25/8 | 0 to 37/15 to 49 |
| Moderna (mRNA-1273) | 11 | 48 | 33 to 61 | 7/4 | 22 to 34/20 to 22 |
| vector vaccine | |||||
| AstraZeneca (ChAdOx1 nCoV-19/AZD1222) | 56 | 31 | 18 to 60 | 45/11 | −13 to 65/ no data available yet |
| Groups | Anti-N IgG | Anti-RBD IgG | Anti-S1 IgG | High IgG Avidity (Index ≥ 2.5) | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | n | % | n | % | n | % | n | % | |
| VOCs | 11 | 10 | 90.9 | 9 | 81.8 | 9 | 81.8 | 4 | 36.4 |
| BNT162b2 (1) | 27 | 0 | 0 | 23 | 85.2 | 26 | 96.3 | 2 | 7.4 |
| BNT162b2 (2) | 21 | 0 | 0 | 21 | 100 | 21 | 100 | 20 | 95.2 |
| m-1273 (1) | 11 | 0 | 0 | 11 | 100 | 11 | 100 | 2 | 18.2 |
| m-1273 (2) | 10 | 0 | 0 | 10 | 100 | 10 | 100 | 9 | 90 |
| AZD1222 (1) | 56 | 1 | 1.8 | 50 | 89.3 | 54 | 96.4 | 3 | 5.4 |
| sVNT#2 (Decision Limit ≥ 20%) | |||||
|---|---|---|---|---|---|
| No evidence for VNA | Evidence for VNA | n | % | ||
| sVNT#1 (decision limit ≥ 30%) | No evidence for VNA | 4 | 1 | 5 | 7.4 |
| Evidence for VNA | 11 | 52 | 63 | 92.6 | |
| n | 15 | 53 | 68 | ||
| % | 22.1 | 77.9 | |||
| IgG Avidity | n | % | |||
|---|---|---|---|---|---|
| Low/Intermediate | High | ||||
| cVNT (wt) | No evidence for VNA | 37 | 0 | 37 | 47.4 |
| Evidence for VNA | 16 | 25 | 41 | 52.6 | |
| n | 53 | 25 | 78 | ||
| % | 67.9 | 32.1 | |||
| cVNT (B.1.1.7) | No evidence for VNA | 36 | 0 | 36 | 46.2 |
| Evidence for VNA | 17 | 25 | 42 | 53.8 | |
| n | 53 | 25 | 78 | ||
| % | 67.9 | 32.1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, F.; Rose, R.; Römpke, J.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Development of SARS-CoV-2 Specific IgG and Virus-Neutralizing Antibodies after Infection with Variants of Concern or Vaccination. Vaccines 2021, 9, 700. https://doi.org/10.3390/vaccines9070700
Neumann F, Rose R, Römpke J, Grobe O, Lorentz T, Fickenscher H, Krumbholz A. Development of SARS-CoV-2 Specific IgG and Virus-Neutralizing Antibodies after Infection with Variants of Concern or Vaccination. Vaccines. 2021; 9(7):700. https://doi.org/10.3390/vaccines9070700
Chicago/Turabian StyleNeumann, Franziska, Ruben Rose, Janine Römpke, Olaf Grobe, Thomas Lorentz, Helmut Fickenscher, and Andi Krumbholz. 2021. "Development of SARS-CoV-2 Specific IgG and Virus-Neutralizing Antibodies after Infection with Variants of Concern or Vaccination" Vaccines 9, no. 7: 700. https://doi.org/10.3390/vaccines9070700
APA StyleNeumann, F., Rose, R., Römpke, J., Grobe, O., Lorentz, T., Fickenscher, H., & Krumbholz, A. (2021). Development of SARS-CoV-2 Specific IgG and Virus-Neutralizing Antibodies after Infection with Variants of Concern or Vaccination. Vaccines, 9(7), 700. https://doi.org/10.3390/vaccines9070700







