Comparison and Analysis of Neutralizing Antibody Levels in Serum after Inoculating with SARS-CoV-2, MERS-CoV, or SARS-CoV Vaccines in Humans
Abstract
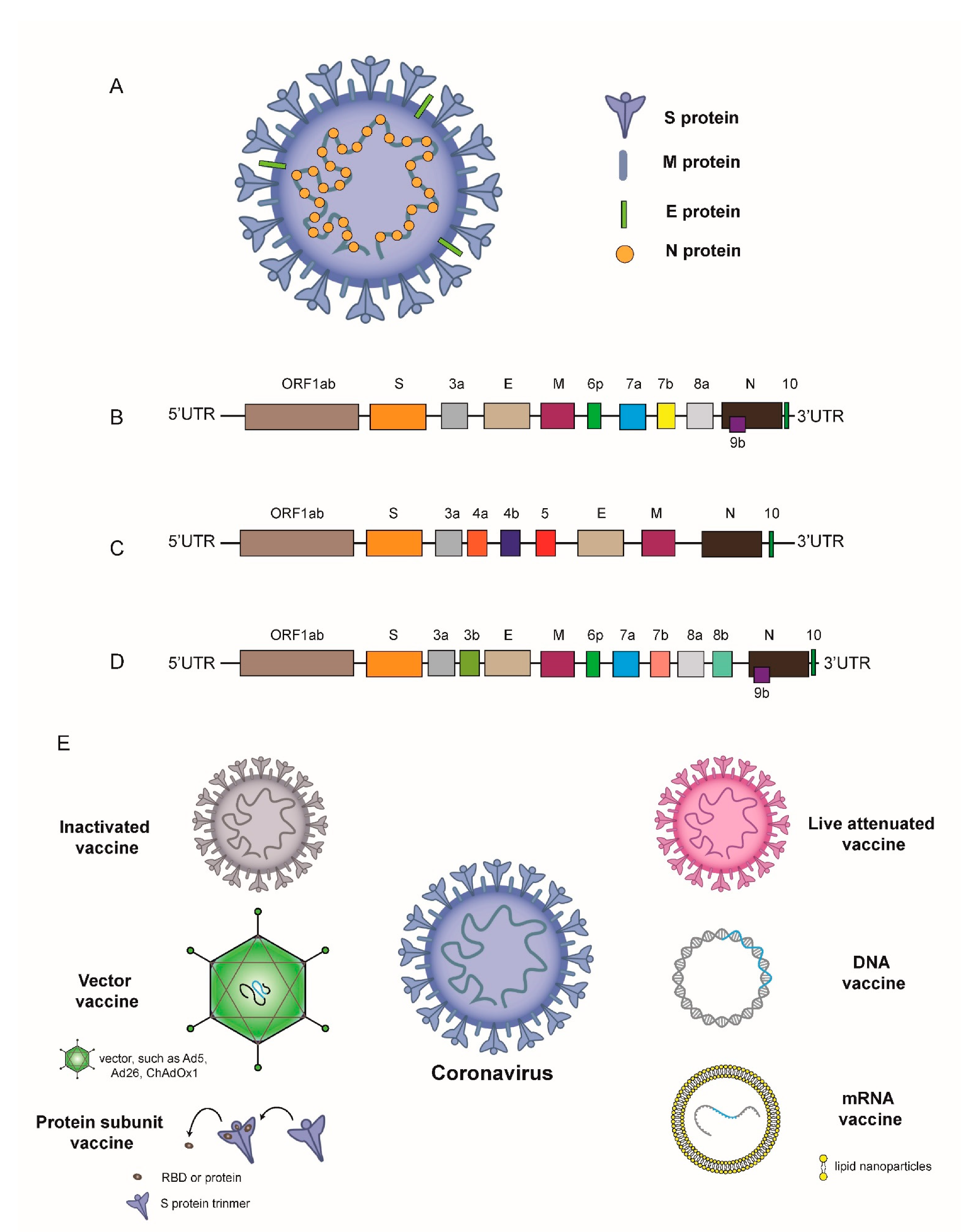
1. Introduction
2. Clinical Trials on the Ability of Different Types of SARS-CoV-2 Vaccines to Induce Neutralizing Antibody Levels after Vaccination
2.1. SARS-CoV-2 Inactivated Vaccine
2.2. SARS-CoV-2 Vector Vaccines
2.3. SARS-CoV-2 DNA Vaccines
2.4. SARS-CoV-2 mRNA Vaccines
2.5. SARS-CoV-2 Subunit Vaccines
3. Clinical Trials on the Ability of Different Types of MERS-CoV Vaccines to Induce Neutralizing Antibody Levels after Vaccination
3.1. MERS-CoV Vector Vaccines
3.2. MERS-CoV DNA Vaccines
4. Clinical Trials on the Ability of Different Types of SARS-CoV Vaccines to Induce Neutralizing Antibody Levels after Vaccination
4.1. SARS-CoV DNA Vaccines
4.2. SARS-CoV Inactivated Vaccines
5. Methods to Detect Neutralizing Antibodies
5.1. Plaque Reduction Neutralization Test, PRNT
5.2. Fluorescent Neutralization Assay, FNA
5.3. Pseudovirus Neutralization, PsVNA
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gene Med. 2021, 23, e3303. [Google Scholar] [CrossRef]
- Neerukonda, S.N.; Katneni, U. A Review on SARS-CoV-2 virology, pathophysiology, animal models, and anti-viral interventions. Pathogens 2020, 9, 426. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Khailany, R.A.; Safdar, M.; Ozaslan, M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020, 19, 100682. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Voss, D.; Pfefferle, S.; Drosten, C.; Stevermann, L.; Traggiai, E.; Lanzavecchia, A.; Becker, S. Studies on membrane topology, N-glycosylation and functionality of SARS-CoV membrane protein. Virol. J. 2009, 6, 79. [Google Scholar] [CrossRef]
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 15 May 2021).
- MERS Situation Update. Available online: http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html (accessed on 30 April 2021).
- WHO. Summary of Possible SARS Cases from 1 November 2002, to 31 July 2003. Available online: http://www.who.int/csr/sars/country/table2004_04_21/en/ (accessed on 31 December 2003).
- Graham, B.S. Rapid COVID-19 vaccine development. Science 2020, 368, 945–946. [Google Scholar] [CrossRef]
- Amanat, F.; Krammer, F. SARS-CoV-2 vaccines: Status report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Interim Report: Safety and Immunogenicity of an Inactivated Vaccine against SARS-CoV-2 in Healthy Chilean Adults in a Phase 3 Clinical Trial. Available online: https://www.medrxiv.org/content/10.1101/2021.03.31.21254494v1 (accessed on 1 April 2021).
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021, 21, 00070. [Google Scholar] [CrossRef]
- Neutralization of UK-Variant VUI-202012/01 with COVAXIN Vaccinated Human Serum. Available online: https://doi.org/10.1101/2021.01.26.426986 (accessed on 27 January 2021).
- Van, D.N.; Lambe, T.; Spencer, A.; Belij, R.S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij, R.S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W.; et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Custers, J.; Kim, D.; Leyssen, M.; Gurwith, M.; Tomaka, F.; Robertson, J.; Heijnen, E.; Condit, R.; Shukarev, G.; Heerwegh, D.; et al. Vaccines based on replication incompetent Ad26 viral vectors: Standardized template with key considerations for a risk/benefit assessment. Vaccine 2020, 39, 3081–3101. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le, G.M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van, D.W.; Leroux, R.I.; et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Solforosi, L.; Kuipers, H.; Jongeneelen, M.; Rosendahl Huber, S.K.; van der Lubbe, J.E.M.; Dekking, L.; Czapska-Casey, D.N.; Izquierdo, G.A.; Baert, M.R.M. Immunogenicity and efficacy of one and two doses of Ad26.COV2.S COVID vaccine in adult and aged NHP. J. Exp. Med. 2021, 218, e20202756. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, 2 March to 21 April 2021. JAMA 2021, e217517. [Google Scholar] [CrossRef]
- MacNeil, J.R.; Su, J.R.; Broder, K.R.; Guh, A.Y.; Gargano, J.W.; Wallace, M.; Hadler, S.C.; Scobie, H.M.; Blain, A.E.; Moulia, D.; et al. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 651–656. [Google Scholar] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- The Sputnik V Vaccine’s Efficacy is Confirmed at 91.4% Based on Data Analysis of the Final Control Point of Clinical Trials. Available online: https://www.prnewswire.co.uk/news-releases/the-sputnik-v-vaccine-s-efficacy-is-confirmed-at-91-4-based-on-data-analysis-of-the-final-control-point-of-clinical-trials-859568853.html (accessed on 14 December 2020).
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- Tebas, P.; Yang, S.; Boyer, J.D.; Reuschel, E.L.; Patel, A.; Christensen, Q.A.; Andrade, V.M.; Morrow, M.P.; Kraynyak, K.; Agnes, J.; et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine 2021, 31, 100689. [Google Scholar] [CrossRef]
- South Korea’s Genexine Begins Phase I/IIa Trials for COVID-19 Vaccine. Available online: https://www.bioworld.com/articles/435995-south-koreas-genexine-begins-phase-iiia-trials-for-covid-19-vaccine (accessed on 23 June 2020).
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu, B.S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Bayart, J.L.; Mullier, F.; Dogné, J.M.; Closset, M.; Douxfils, J. Early antibody response in healthcare professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2). Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M.; Salvagno, G.L.; Peserico, D.; Pighi, L.; De Nitto, S.; Henry, B.M.; Porru, S.; Lippi, G. Comprehensive as-sessment of humoral response after Pfizer BNT162b2 mRNA Covid-19 vaccination: A three-case series. Clin. Chem. Lab. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 vaccine: First approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines 2021, 6, 57. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney, C.S.; Zhou, H.; et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Goepfert, P.A.; Fu, B.; Chabanon, A.L.; Bonaparte, M.I.; Davis, M.G.; Essink, B.J.; Frank, I.; Haney, O.; Janosczyk, H.; Keefer, M.C.; et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: Interim results of a randomised, placebo-controlled, phase 1-2, dose-ranging study. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Bittaye, M.; Flaxman, A.; Lopez, F.R.; Bellamy, D.; Kupke, A.; Mair, C.; Makinson, R.; Sheridan, J.; Rohde, C.; et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: A dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect. Dis. 2020, 20, 816–826. [Google Scholar] [CrossRef]
- Koch, T.; Dahlke, C.; Fathi, A.; Kupke, A.; Krähling, V.; Okba, N.M.A.; Halwe, S.; Rohde, C.; Eickmann, M.; Volz, A.; et al. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: An open-label, phase 1 trial. Lancet Infect. Dis. 2020, 20, 827–838. [Google Scholar] [CrossRef]
- Modjarrad, K.; Roberts, C.C.; Mills, K.T.; Castellano, A.R.; Paolino, K.; Muthumani, K.; Reuschel, E.L.; Robb, M.L.; Racine, T.; Oh, M.D.; et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: A phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019, 19, 1013–1022. [Google Scholar] [CrossRef]
- Martin, J.E.; Louder, M.K.; Holman, L.A.; Gordon, I.J.; Enama, M.E.; Larkin, B.D.; Andrews, C.A.; Vogel, L.; Koup, R.A.; Roederer, M.; et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine 2008, 26, 6338–6343. [Google Scholar] [CrossRef]
- Lin, J.T.; Zhang, J.S.; Su, N.; Xu, J.G.; Wang, N.; Chen, J.T.; Chen, X.; Liu, Y.X.; Gao, H.; Jia, Y.P.; et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir. Ther. 2007, 12, 1107–1113. [Google Scholar]
- Perera, R.A.; Mok, C.K.; Tsang, O.T.; Lv, H.; Ko, R.L.; Wu, N.C.; Yuan, M.; Leung, W.S.; Chan, J.M.; Chik, T.S.; et al. Serological assays for severe acute respiratory syndrome coro-navirus 2 (SARS-CoV-2), March 2020. Eurosurveillance 2020, 25, 2000421. [Google Scholar] [CrossRef]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.Y. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat. Commun. 2020, 11, 4059. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.H.D.; Eguia, R.; Dingens, A.S.; Loes, A.N.; Malone, K.D.; Wolf, C.R.; Chu, H.Y.; Tortorici, M.A.; Veesler, D.; Murphy, M.; et al. Protocol and reagents for pseudotyping lentiviral particles with SARS-CoV-2 spike protein for neutralization assays. Viruses 2020, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Structural and functional analysis of a potent sarbecovirus neutralizing antibody. bioRxiv 2020, 3. [Google Scholar] [CrossRef]
- Xiong, H.L.; Wu, Y.T.; Cao, J.L.; Yang, R.; Liu, Y.X.; Ma, J.; Qiao, X.Y.; Yao, X.Y.; Zhang, B.H.; Zhang, Y.L.; et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg. Microbes Infect. 2020, 9, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Neerukonda, S.N.; Vassell, R.; Herrup, R.; Liu, S.; Wang, T.; Takeda, K.; Yang, Y.; Lin, T.L.; Wang, W.; Weiss, C.D. Establishment of a well-characterized SARS-CoV-2 lentiviral pseudovirus neutralization assay using 293T cells with stable expression of ACE2 and TMPRSS2. PLoS ONE 2021, 16, e0248348. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody response to 2-Dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021. [Google Scholar] [CrossRef]
- Singh, J.; Samal, J.; Kumar, V.; Sharma, J.; Agrawal, U.; Ehtesham, N.Z.; Sundar, D.; Rahman, S.A.; Hira, S.; Hasnain, S.E. Structure-function analyses of new SARS-CoV-2 variants B.1.1.7, B.1.351 and B.1.1.28.1: Clinical, diagnostic, therapeutic and public health implications. Viruses 2021, 13, 439. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Y.; Liu, J.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Xia, H.; Swanson, K.A.; Cutler, M.; Cooper, D.; et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat. Med. 2021, 27, 620–621. [Google Scholar] [CrossRef]
| Developer | Vaccine Type | The Vaccine Dose | The Booster Dose | Number of Doses | Neutralizing Antibody GMT | The Control Group |
|---|---|---|---|---|---|---|
| Sinovac [14] | Inactivated vaccine (CoronaVac) | 3 μg, 6 μg | Day 14 or Day 28 | 2 | Day 42, MNA IC50 were 23.8 in the 3 μg group and 30.1 in the 6 μg group; Day 56, MNA IC50 were 44.1 in the 3 μg group, and 65.4 in the 6 μg group. | MNA IC50 were 163.7 (convalescent asymptomatic patients) and 76.1 (hospitalized patients) |
| Wuhan Institute of Biological Products/Sinopharm [16] | Inactivated vaccine | 5 μg | Day 14 or Day 21 | 2 | 14 days after the whole injection, PRNT IC50 were 121 on day 0 and 14 group, and 247 on day 0 and 21 group. | / |
| Beijing Institute of Biological Products/Sinopharm [17] | Inactivated vaccine (BBIBP-CorV) | 4 μg, 8 μg | Day 14 or Day21 or Day 28 (except the 8 μg group) | 2 or 1 | 28 days after the injection, in the 4 μg group, MNA IC50 were 169.5 on day 0 and 14; 282.7 on day 0 and 21; and 218.0 on day 0 and 28; 14.7 in the 8 μg group. | / |
| Bharat Biotech [20] | Inactivated vaccine (BBV152) | 3 μg, 6 μg | Day 28 | 2 | Day 56, PRNT IC50 were 100.9 in the 3 μg group and 197.0 in the 6 μg group; MNA IC50 were 92.5 in the 3 μg group and 160.1 in the 6 μg group. | / |
| CanSino Biological/Beijing Institute of Biotechnology [27] | Adenovirus Type 5 Vector vaccine | 5 × 1010, 1 × 1011 Virus particles | / | 1 | Day 28, LTIA [27] were 19.5 in the high dose group and 18.3 in the low dose group; PBNA EC50 was 61.4 in the high dose group and 55.3 in the low dose group. | / |
| Janssen/Johnson & Johnson [29] | Adenovirus Type 26 Vector vaccine (Ad26.COV2.S) | 5 × 1010, 1 × 1011 Virus particles | Day 28 (a part of every dose group) | 2 or 1 | Day 29, MNA IC50 were 224 (5 × 1010 VP, one dose), 224 (5 × 1010 VP, two-dose), 215 (1 × 1011 VP, one dose), 354 (1 × 1011 VP, two-dose). Day 56, MNA IC50 were 310 (5 × 1010 VP, one dose), 288 (5 × 1010 VP, two-dose), 370 (1 × 1011 VP, one dose), 488 (1 × 1011 VP, two-dose) | / |
| University of Oxford/AstraZeneca [23] | Vector vaccine (ChAdOx1 nCoV-19) | 5 × 1010 Virus particles | Day 28 (10 volunteers received) | 2 or 1 | Day 28, PRNT IC50 was 218, MNA IC80 was 51, and VN IC100 was 29; the MNA IC80 of receiving booster dose was 136. | / |
| Inovio [37] | DNA vaccine (INO-4800) | 1.0 mg, 2.0 mg | Week 4 | 2 | Week 6, PRNT IC50 were 102.3 in the 1.0 mg group and 63.5 in the 2.0 mg group. | PRNT IC50: 160 |
| Moderna/NIAID [40] | mRNA vaccine (mRNA-1273) | 25 μg, 100 μg, 250 μg | Day 28 | 2 | Day 43, PsVNA ID50 were 112.3 in the 25 μg group, 343.8 in the 100 μg group, 332.2 in the 250 μg group. PRNT IC80 were 339.7 in the 25 μg group, 654.3 in the 100 μg group, and 332.2 in the 250 μg group. | PsVNA ID50: 109.2, PRNT IC80: 158.3 |
| BioNTech/Pfizer [42] | mRNA vaccine (BNT162b1) | 10 μg, 30 μg, 100 μg | Day 21 (except the 100 μg group) | 2 or 1 | Day 28, FNA IC50 were 168 in the 10 μg group and 267 in the 30 μg group. Day 35, FNA IC50 were 180 in the 10 μg group, 437 in the 30 μg group; Day 21, FNA IC50 was 33 in the 100 μg group. | FNA IC50: 94 |
| Novavax [48] | Protein Subunit (NVX-CoV2373) | 5 μg, 25 μg (with or without adjuvant) | Day 21 | 2 | Day 35, MN IC>99% were 3906 in the 5 μg group (with adjuvant), 3305 in 25 μg group (with adjuvant), 41 in 25 μg group (without adjuvant), and 128 in 25 μg group (with adjuvant in the first dose and without adjuvant in the second dose). | MN IC>99% were 254 (asymptomatic patients) and 837 (symptomatic patients) |
| Sanofi Pesteur/GSK [49] | Protein Subunit (CoV2 preS dTM) | 1.3 μg (with adjuvant), 2.6 μg (with adjuvant), 2.6 μg (without adjuvant) | Day 22 (a part of per dose group) | 2 or 1 | Day 36, MNA IC50 were 13.1 in the 1.3 μg group (AF03-adjuvant), 20.5 in the 1.3 μg group (AS03-adjuvant), 43.2 in the 2.6 μg group (AF03-adjuvant), and 75.1 in the 2.6 μg group (AS03-adjuvant). NAb titer was not deteced in the 2.6 μg group (wtihout adjuvant). | / |
| Developer | Vaccine Type | The Vaccine Dose | The Booster Dose | Number of Doses | Neutralizing Antibody GMT | The Control Group |
|---|---|---|---|---|---|---|
| University of Oxford/AstraZeneca [50] | Vector vaccine (ChAdOx1 MERS) | 5 × 109 VP, 2.5 × 1010 VP, 5 × 1010 VP | / | 1 | PsVNA ID50 was 12.5 in the low dose group, 25 in the middle dose group, and 50 in the high dose group | / |
| German Center for Infection Research [51] | Vaccinia virus Ankara vector vaccine | 1 × 107 PFU, 1 × 108 PFU | Week 4, Week 12 | 3 | Week 14, MNA NT50 was 10 | MNA NT50 was 70 in the acute phase and 40 in the convalescent phase |
| GeneOne Life Science/Inovio [52] | DNA vaccine (GLS-5300/INO-4700) | 0.67 mg, 2 mg, 6 mg | Day 28 | 2 | PRNT IC80 was positive in 75% of the low-dose group and 82% of the high-dose group | / |
| Developer | Vaccine Type | The Vaccine Dose | The Booster Dose | Number of Doses | Neutralizing Antibody GMT | The Control Group |
|---|---|---|---|---|---|---|
| NIAID [53] | DNA vaccine (VRC) | 4 mg | Day 28, Day 56 | 3 | No neutralizing antibodies were detected in PRNT IC80, neutralizing antibodies detected in PsVNA ID50 peaked at 8 to 12 weeks | / |
| Sinovac Biotech [54] | Inactivated vaccines (ISCV) | 16 SU, 32 SU | Day 28 | 2 | The neutralizing antibody peaked at 2 weeks after the second immunization and decreased at 4 weeks after immunization | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Chen, K.; Fang, L.; Mao, H.; Lou, X.; Li, C.; Zhang, Y. Comparison and Analysis of Neutralizing Antibody Levels in Serum after Inoculating with SARS-CoV-2, MERS-CoV, or SARS-CoV Vaccines in Humans. Vaccines 2021, 9, 588. https://doi.org/10.3390/vaccines9060588
Yu S, Chen K, Fang L, Mao H, Lou X, Li C, Zhang Y. Comparison and Analysis of Neutralizing Antibody Levels in Serum after Inoculating with SARS-CoV-2, MERS-CoV, or SARS-CoV Vaccines in Humans. Vaccines. 2021; 9(6):588. https://doi.org/10.3390/vaccines9060588
Chicago/Turabian StyleYu, Sicong, Keda Chen, Lei Fang, Haiyan Mao, Xiuyu Lou, Chaonan Li, and Yanjun Zhang. 2021. "Comparison and Analysis of Neutralizing Antibody Levels in Serum after Inoculating with SARS-CoV-2, MERS-CoV, or SARS-CoV Vaccines in Humans" Vaccines 9, no. 6: 588. https://doi.org/10.3390/vaccines9060588
APA StyleYu, S., Chen, K., Fang, L., Mao, H., Lou, X., Li, C., & Zhang, Y. (2021). Comparison and Analysis of Neutralizing Antibody Levels in Serum after Inoculating with SARS-CoV-2, MERS-CoV, or SARS-CoV Vaccines in Humans. Vaccines, 9(6), 588. https://doi.org/10.3390/vaccines9060588






