Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Protocol
2.2. Eligibility Criteria
- Population—subjects participated in clinical trials related to COVID-19 vaccines;
- Intervention—COVID-19 vaccination;
- Comparator(s)—COVID-19 vaccine or placebo;
- Outcomes—vaccine efficacy for prevention of COVID-19 (primary outcome) was assessed on the basis of incidences from randomized controlled trials. Secondary outcome was vaccine safety, including adverse events at the injection site (e.g., pain, swelling, induration, erythema), systemic adverse events (e.g., fever, headache, fatigue, muscle pain, joint pain, nausea and/or vomiting, chill) and serious adverse events (e.g., dehydration, syncope, atrial fibrillation, pulmonary embolism, acute kidney injury and so on). During day 0 to 7, 14, or 28 after injections, subjects were asked to record any adverse events.
- Study designs—randomized controlled trials were eligible for inclusion. Animal studies, case reports, reviews, editorials, letters and conference abstracts were excluded. Articles describing the results of phase III COVID-19 vaccine trials were included first. If there were no safety-related outcomes in the above articles, the results of phase I/II trials of the same vaccine would be included. Studies were excluded if there was an overlap in subjects with another study within the same analysis.
2.3. Data Extraction and Quality Assessment
2.4. Outcomes
2.5. Data Synthesis and Statistical Analysis
3. Results
3.1. Characteristics of the Studies
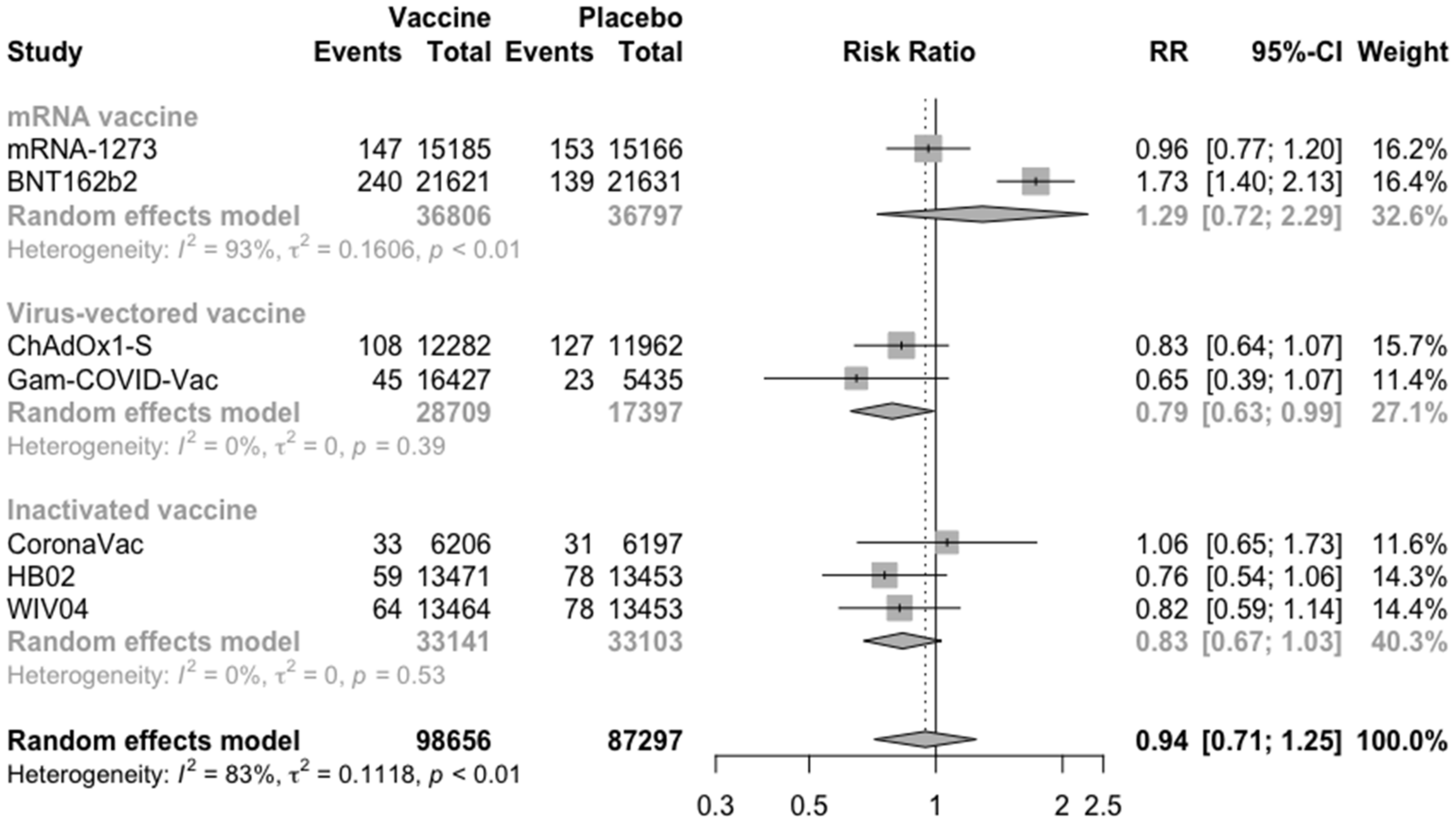
3.2. COVID-19 Vaccine Efficacy
3.3. COVID-19 Vaccine Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Biancolella, M.; Mehrian-Shai, R.; Erickson, C.; Pollitt, K.J.G.; Vasiliou, V.; Watt, J.; Reichardt, J.K.V. COVID-19 update: The first 6 months of the pandemic. Hum. Genom. 2020, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Luyten, J.; Beutels, P. The Social Value Of Vaccination Programs: Beyond Cost-Effectiveness. Health Aff. 2016, 35, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Chetta, A.; Cazzola, M.; Calzetta, L. SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines. Vaccines 2021, 9, 227. [Google Scholar] [CrossRef]
- Alturki, S.O.; Alturki, S.O.; Connors, J.; Cusimano, G.; Kutzler, M.A.; Izmirly, A.M.; Haddad, E.K. The 2020 Pandemic: Current SARS-CoV-2 Vaccine Development. Front. Immunol. 2020, 11, 1880. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Draft Landscape of COVID-19 Candidate Vaccines. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 28 May 2021).
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.; Mantovani, A.; Forni, G.; Mantovani, A.; Moretta, L.; Rappuoli, R.; Rezza, G.; Bagnasco, A.; Barsacchi, G.; Bussolati, G.; et al. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Up-To-Date Mapping of COVID-19 Treatment and Vaccine Development. Available online: https://covid19-help.org/ (accessed on 28 May 2021).
- Higgins, J.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available online: www.cochrane-handbook.org (accessed on 28 May 2021).
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Stone, P.W. Popping the (PICO) question in research and evidence-based practice. Appl. Nurs Res. 2002, 15, 197–198. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Yuan, P.; Ai, P.; Liu, Y.; Ai, Z.; Wang, Y.; Cao, W.; Xia, X.; Zheng, J.C. Safety, Tolerability, and Immunogenicity of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. medRxiv Prepr. Serv. Health Sci. 2020, 4998. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Palacios, R.; Batista, A.P.; Albuquerque, C.S.N.; Patiño, E.G.; Santos, J.D.P. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. SSRN 2021, 66. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 324, 951–960. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef]
- Parker, E.P.K.; Shrotri, M.; Kampmann, B. Keeping track of the SARS-CoV-2 vaccine pipeline. Nat. Rev. Immunol. 2020, 20, 650. [Google Scholar] [CrossRef]
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 Vaccines. JAMA 2021, 325, 1318–1320. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Yaghoubi, A. COVID-19 vaccine: Where are we now and where should we go? Expert Rev. Vaccines 2021, 20, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.K.L.; Kayarohanam, S.; Fuloria, S.; Fuloria, N.K.; Janakiraman, A.K.; Djearamane, S.; Wu, Y.S.; Chakravarthi, S.; Subramaniyan, V. Covid-19 vaccine candidates under clinical evaluation-a review. Int. J. Pharm. Res. 2021, 13, 4588–4598. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal. Transduct. Target. Ther. 2020, 5, 237. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kream, R.M.; Stefano, G.B. An Evidence Based Perspective on mRNA-SARS-CoV-2 Vaccine Development. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e924700. [Google Scholar] [CrossRef]
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef]
- Stone, C.A., Jr.; Rukasin, C.R.F.; Beachkofsky, T.M.; Phillips, E.J. Immune-mediated adverse reactions to vaccines. Br. J. Clin. Pharmacol. 2019, 85, 2694–2706. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.C.; Phillips, E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N. Engl. J. Med. 2020, 384, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir. Med. 2021, 9, e20–e21. [Google Scholar] [CrossRef]
- Tang, J.W.; Toovey, O.T.R.; Harvey, K.N.; Hui, D.D.S. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK. J. Infect. 2021, 82, e8–e10. [Google Scholar] [CrossRef] [PubMed]

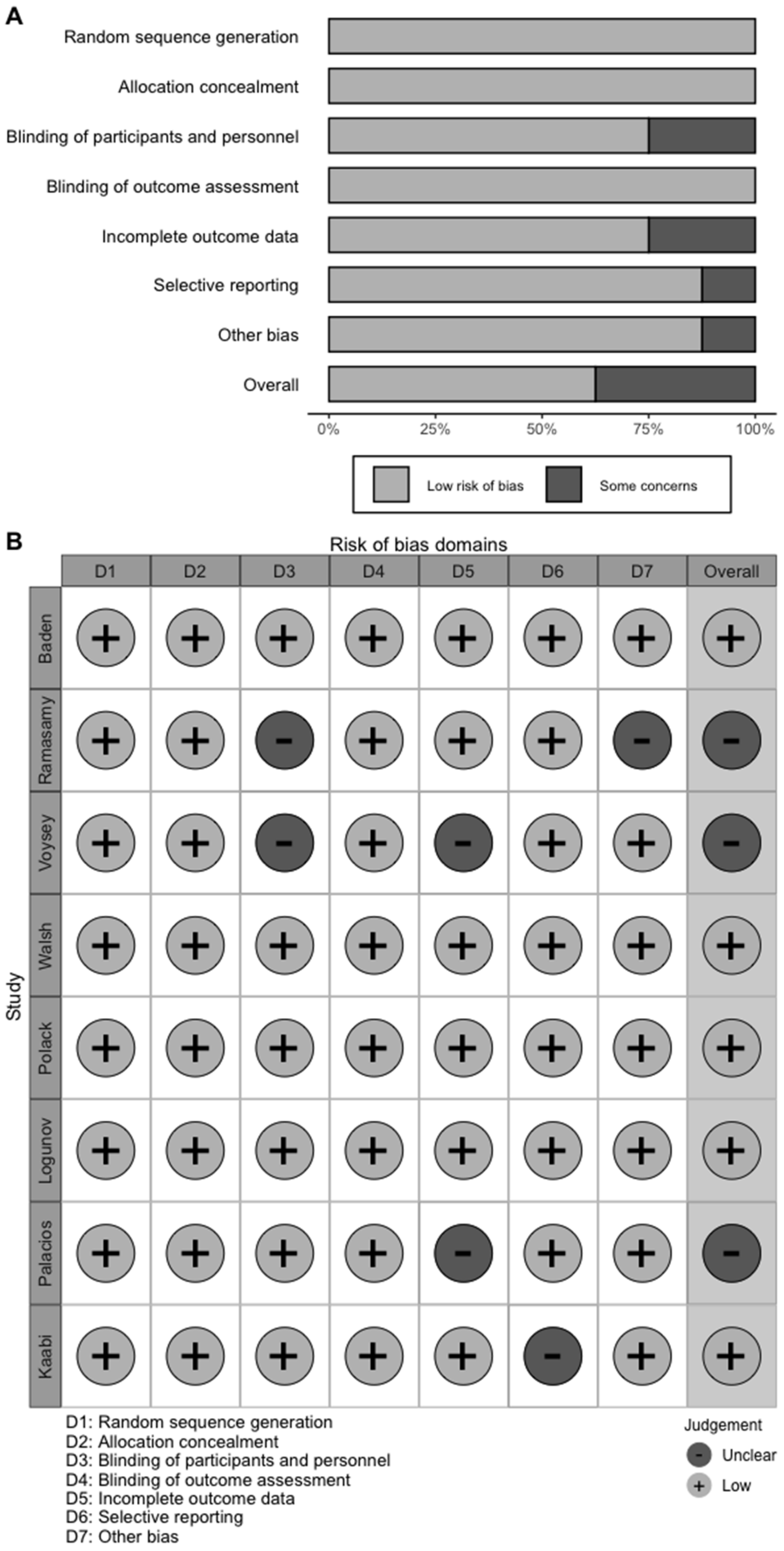
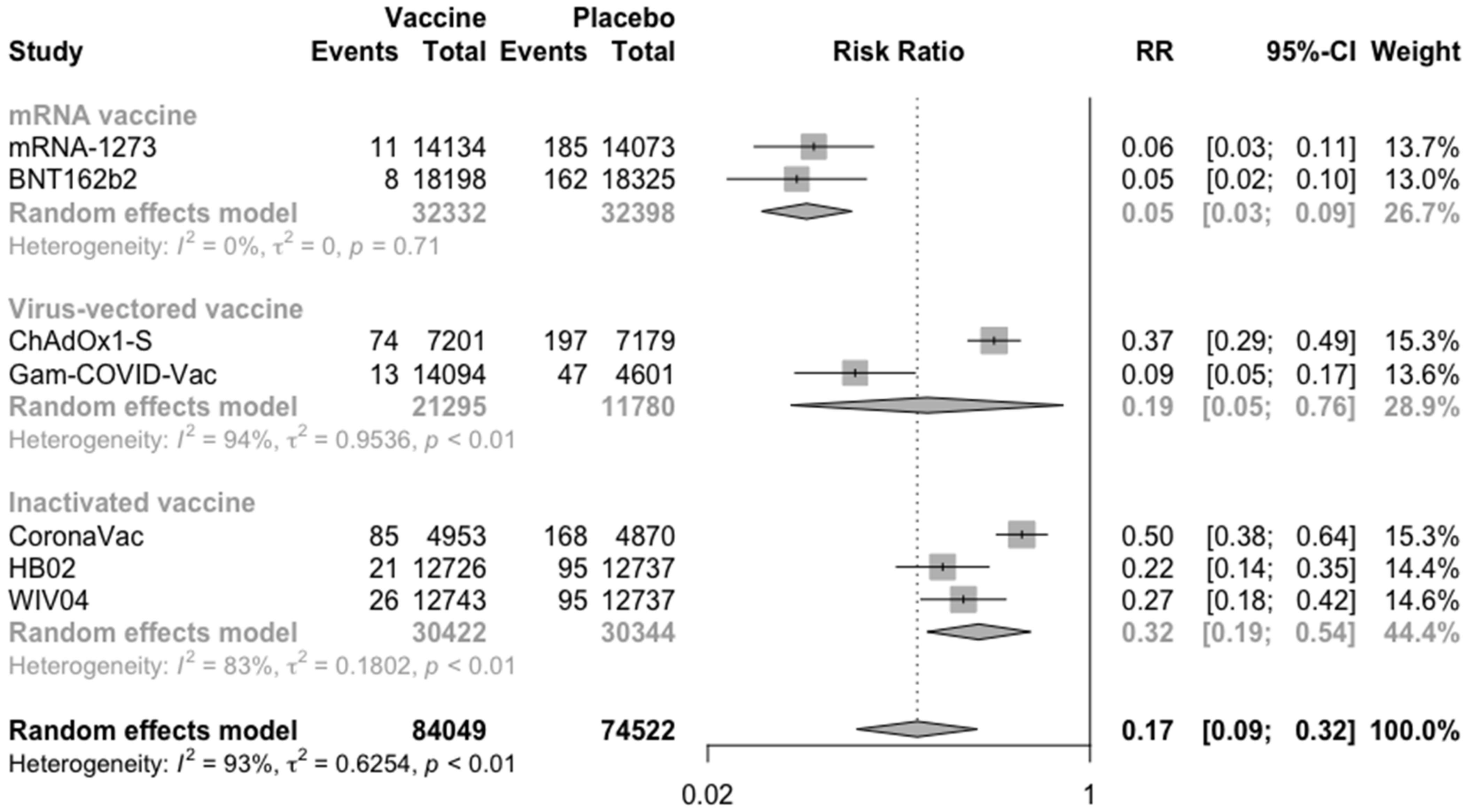

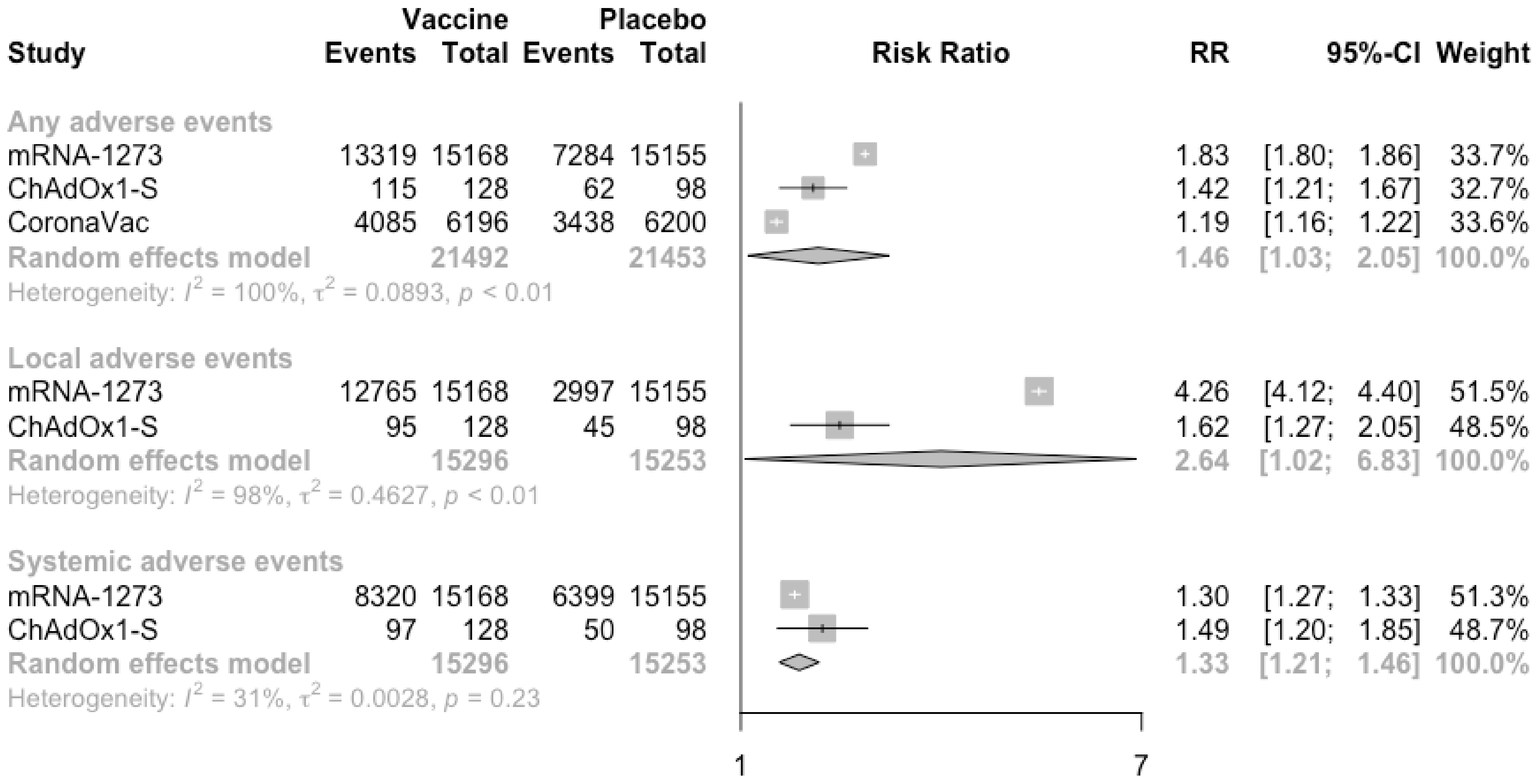
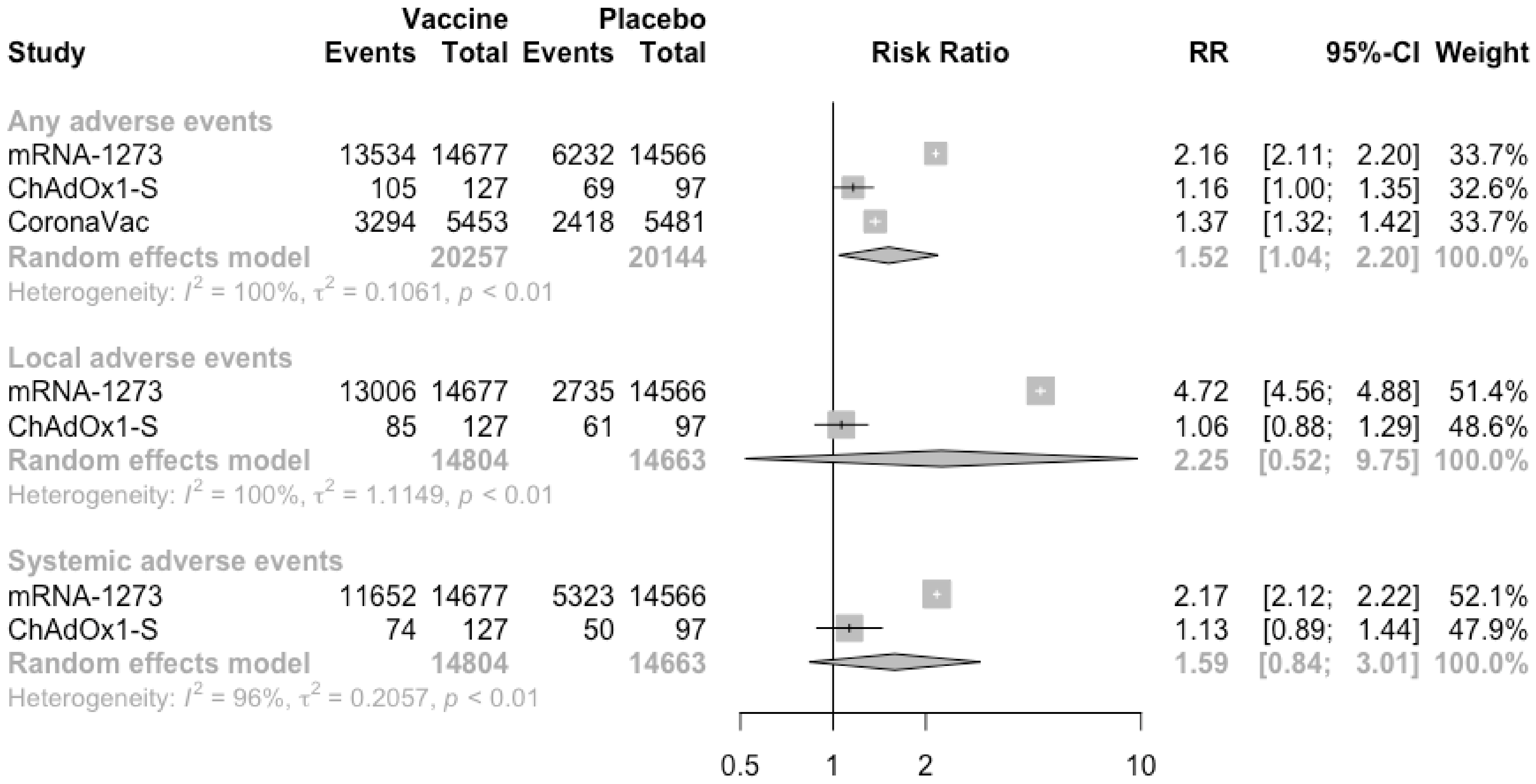
| Study and Year | Trial Number | Study Characteristics | Vaccine Developer | Dose and Route of Administration | Number of Scheduled Doses (Time of Inoculations) | Type of Candidate Vaccine | Study Duration | Characteristics of Vaccine Recipients | Participating Countries | Age (Mean/Median and Range/SD) | Male (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baden et al., 2021 | NCT04470427 | Phase III, multicenter, randomized, observer-blind, placebo-controlled | Moderna/National Institute of Allergy and Infectious Diseases’ Vaccine Research Center | mRNA-1273 (100 μg IM) | Prime and boost inoculation (0, 28 days) | mRNA vaccine | 27 July 2020 to 21 November 2020 | Persons 18 years of age or older with no known history of SARS-CoV-2 infection and with locations or circumstances that put them at an appreciable risk of SARS-CoV-2 infection | USA | 51.4 (18–95) | 52.7 |
| Ramasamy et al., 2020 | NCT04400838 | Phase II/III, multicenter, randomized, single-blind, placebo-controlled | University of Oxford/AstraZeneca | ChAdOx1-S (3.5–6.5 × 1010 viral particles IM) | Prime and boost inoculation (0, 28 days) | Virus-vectored vaccine | May 30, 2020 to 26 October 2020 | Adults aged 18–55 years, then adults aged 56–69 years, and then adults aged 70 years and older, without severe or uncontrolled medical comorbidities | UK | 18–55 years: 43.2 (22–55); 56–69 years: 60.4 (56–69.4); ≥70 years: 73 (70–82) | 18–55 years: 36.4; 56–69 years: 55.0; ≥70 years: 64.3 |
| Voysey et al., 2021 | NCT04324606, NCT04400838, NCT04444674, ISRCTN89951424 | Phase II/III, multicenter, randomized, single-blind, placebo-controlled | University of Oxford/AstraZeneca | ChAdOx1-S (3.5–6.5 × 1010 viral particles IM) | Prime and boost inoculation (0, 28 days) | Virus-vectored vaccine | 23 April 2020 to 6 November 2020 | Health adults aged 18 or older | UK Brazil South Africa | ≥18 | 43.6 |
| Walsh et al., 2020 | NCT04368728 | Phase I, multicenter, randomized, observed-blind, placebo-controlled | BioNTech/Fosun Pharma/Pfizer | BNT162b2 (30 µg IM) | Prime and boost inoculation (0, 21 days) | mRNA vaccine | 4 May 2020 to 22 June 2020 | Healthy adults 18 to 55 years of age or 65 to 85 years of age | USA | 18–55 years: 36.1 (19–54); 65–85 years: 69.1 (65–77) | 18–55 years: 38.1; 65–85 years: 47.6 |
| Polack et al., 2020 | NCT04368728 | Phase III, multicenter, randomized, observed-blind, placebo-controlled | BioNTech/Fosun Pharma/Pfizer | BNT162b2 (30 µg IM) | Prime and boost inoculation (0, 21 days) | mRNA vaccine | 27 July 2020 to 14 November 2020 | Adults 16 years of age or older who were healthy or had stable chronic medical conditions | USA Argentina Brazil South Africa | 52 (16–91) | 50.6 |
| Logunov et al., 2021 | NCT04530396 | Phase III, multicenter, randomized, double-blind, placebo-controlled | Gamaleya Research Institute | Gam-COVID-Vac (1 ± 0.5 × 1011 viral particles IM) | Prime and boost inoculation (0, 21 days) | Virus-vectored vaccine | 7 September 2020 to 24 November 2020 | Adults aged 18 or older with no known history of SARS-CoV-2 infection, and without severe or uncontrolled medical comorbidities | Russia | 45.3 (12.0) | 61.2 |
| Palacios et al., 2021 | NCT04456595 | Phase III, multicenter, randomized, double-blind, placebo-controlled | Sinovac | CoronaVac (3 μg IM) | Prime and boost inoculation (0, 14 days) | Inactivated vaccine | 21 July 2020 to 16 December 2020 | Participants aged 18 or older without previous SARS-CoV-2 infection | Brazil | 39.5 (10.8) | 35.8 |
| Kaabi et al., 2021 | NCT04510207 | Phase III, multicenter, randomized, double-blind, placebo-controlled | The Beijing Institute of Biological Products Co, Ltd., | HB02 (4 μg IM) or WIV04 (5 μg IM) | Prime and boost inoculation (0, 21 days) | Inactivated vaccine | 16 July 2020 to 20 December 2020 | Participants aged 18 or older without previous SARS-CoV-2 infection | United Arab Emirates (Abu Dhabi Sharjahand Bahrain) | 36.1 (9.3) | 84.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Peng, Z.; Luo, W.; Si, S.; Mo, M.; Zhou, H.; Xin, X.; Liu, H.; Yu, Y. Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis. Vaccines 2021, 9, 582. https://doi.org/10.3390/vaccines9060582
Cheng H, Peng Z, Luo W, Si S, Mo M, Zhou H, Xin X, Liu H, Yu Y. Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis. Vaccines. 2021; 9(6):582. https://doi.org/10.3390/vaccines9060582
Chicago/Turabian StyleCheng, Haoyue, Zhicheng Peng, Wenliang Luo, Shuting Si, Minjia Mo, Haibo Zhou, Xing Xin, Hui Liu, and Yunxian Yu. 2021. "Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis" Vaccines 9, no. 6: 582. https://doi.org/10.3390/vaccines9060582
APA StyleCheng, H., Peng, Z., Luo, W., Si, S., Mo, M., Zhou, H., Xin, X., Liu, H., & Yu, Y. (2021). Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis. Vaccines, 9(6), 582. https://doi.org/10.3390/vaccines9060582






