Effectiveness of the SA 14-14-2 Live-Attenuated Japanese Encephalitis Vaccine in Myanmar
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Vaccine
2.2. Determination of Anti-JEV Antibody by ELISA
2.3. Detection of Neutralizing Antibody to JEV
2.4. Statistical Analysis
3. Results
3.1. Anti-JEV IgG Titers
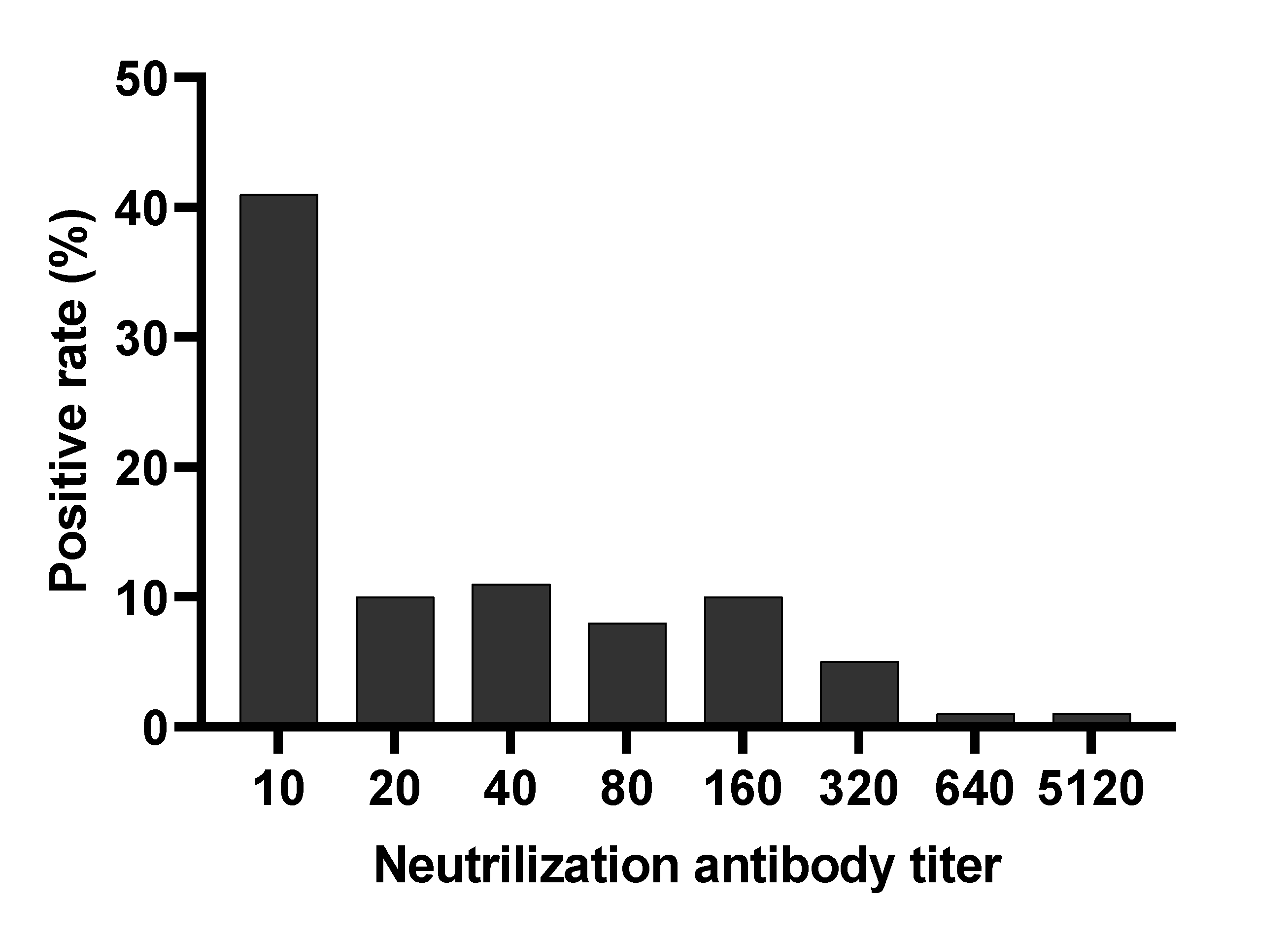
3.2. Neutralizing Antibody Titers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774; 774a–774e. [Google Scholar] [CrossRef]
- Solomon, T. Flavivirus Encephalitis. N. Engl. J. Med. 2004, 351, 370–378. [Google Scholar] [CrossRef]
- Swe, T.; Thein, S.; Myint, M.S. Pilot sero-epidemiological survey on Japanese encephalitis in north-western Burma. Biken J. 1979, 22, 125–129. [Google Scholar] [PubMed]
- Thein, S.; Aung, H.; Sebastian, A.A. Study of vector, amplifier, and human infection with Japanese encephalitis virus in a rangoon community. Am. J. Epidemiol. 1988, 128, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, A.K.; Tun, M.M.N.; Nabeshima, T.; Buerano, C.C.; Ando, T.; Inoue, S.; Hayasaka, D.; Lim, C.K.; Saijo, M.; Thu, H.M.; et al. Japanese Encephalitis- and Dengue-Associated Acute Encephalitis Syndrome Cases in Myanmar. Am. J. Trop. Med. Hyg. 2019, 100, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Win, A.Y.N.; Wai, K.T.; Harries, A.D.; Kyaw, N.T.T.; Oo, T.; Than, W.P.; Lin, H.H.; Lin, Z. The burden of Japanese encephalitis, the catch-up vaccination campaign, and health service providers’ perceptions in Myanmar: 2012–2017. Trop. Med. Health 2020, 48, 13. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, P.P.; Shewade, H.D.; Kyaw, N.T.T.; Hnin Phyo, K.; Lin, H.H.; Kyaw, A.M.M.; Mya, M.M.; Thaung, S.; Maung, Y.N. High vaccination coverage, inadequate knowledge and high vector density: Findings from a communi-ty-based cross-sectional study on Japanese Encephalitis in Yangon, Myanmar. F1000Res 2020, 9, 6. [Google Scholar] [CrossRef]
- Inoue, S.; Alonzo, M.T.G.; Kurosawa, Y.; Mapua, C.A.; Reyes, J.D.; Dimaano, E.M.; Alera, M.T.P.; Saito, M.; Oishi, K.; Hasebe, F.; et al. Evaluation of a Dengue IgG Indirect Enzyme-Linked Immunosorbent Assay and a Japanese Encephalitis IgG Indirect Enzyme-Linked Immunosorbent Assay for Diagnosis of Secondary Dengue Virus Infection. Vector Borne Zoonotic Dis. 2010, 10, 143–150. [Google Scholar] [CrossRef]
- Tun, M.M.N.; Thant, K.Z.; Inoue, S.; Kurosawa, Y.; Lwin, Y.Y.; Lin, S.; Aye, K.T.; Khin, P.T.; Myint, T.; Htwe, K.; et al. Serological characterization of dengue virus infections observed among dengue hemorrhagic fever/dengue shock syndrome cases in upper Myanmar. J. Med. Virol. 2013, 85, 1258–1266. [Google Scholar] [CrossRef]
- Markoff, L. Points to consider in the development of a surrogate for efficacy of novel Japanese encephalitis virus vaccines. Vaccine 2000, 18 (Suppl. S2), 26–32. [Google Scholar] [CrossRef]
- Sohn, Y.M. Japanese encephalitis immunization in South Korea: Past, present, and future. Emerg. Infect. Dis. 2000, 6, 17–24. [Google Scholar]
- Sohn, Y.M.; Park, M.S.; Rho, H.O.; Chandler, L.J.; Shope, R.E.; Tsai, T.F. Primary and booster immune responses to SA14-14-2 Japanese encephalitis vaccine in Korean infants. Vaccine 1999, 17, 2259–2264. [Google Scholar] [CrossRef]
- Liu, W.; Clemens, J.D.; Yang, J.-Y.; Xu, Z.Y. Immunization against Japanese encephalitis in China: A policy analysis. Vaccine 2006, 24, 5178–5182. [Google Scholar] [CrossRef]
- Endy, T.P.; Nisalak, A. Japanese Encephalitis Virus: Ecology and Epidemiology. Curr. Top. Microbiol. Immunol. 2002, 267, 11–48. [Google Scholar] [CrossRef]
- Tseng, H.F.; Tan, H.F.; Chang, C.K.; Huang, W.L.; Ho, W.C. Seroepidemiology study of Japanese encephalitis neutralizing antibodies in southern Taiwan: A comparative study between urban city and country townships. Am. J. Infect. Control. 2003, 31, 435–440. [Google Scholar] [CrossRef]
- Ministry of Health and Sports, Department of Public Health. Myanmar. Monthly Epidemiology Bulletin. Available online: https://mohs.gov.mm/page/12769 (accessed on 30 June 2020).
- Latt, A.Z.; Win, K.M.A.H.M.; Aung, K.M.; Oo, K.K.; Thu, H.M.; Win, M.M.; Thu, H.M.; Yoksan, S. Isolation and Identification of Japanese Encephalitis Virus from Piglets in Thakata Township, Yangon. Myanmar Health Sci. Res. J. 2015, 27, 1–6. [Google Scholar]
- Hegde, N.R.; Gore, M.M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccin Immunother. 2017, 13, 1–18. [Google Scholar] [CrossRef]
- Van Gessel, Y.; Klade, C.S.; Putnak, R.; Formica, A.; Krasaesub, S.; Spruth, M.; Cena, B.; Tungtaeng, A.; Gettayacamin, M.; Dewasthaly, S. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO®) induced neutralizing antibody titers. Vaccine 2011, 29, 5925–5931. [Google Scholar] [CrossRef]
- Xin, Y.Y.; Jian, A.; Peng, G.Y.; Min, L.H.; Ming, Z.G. Safety of a Live-Attenuated Japanese Encephalitis Virus Vaccine (SA14-14-2) for Children. Am. J. Trop. Med. Hyg. 1988, 39, 214–217. [Google Scholar] [CrossRef]
- Bista, M.B.; Banerjee, M.; Shin, S.H.; Tandan, J.; Kim, M.H.; Sohn, Y.M.; Ohrr, H.C.; Tang, J.L.; Halstead, S.B. Efficacy of single-dose SA 14–14–2 vaccine against Japanese encephalitis: A case control study. Lancet 2001, 358, 791–795. [Google Scholar] [CrossRef]
- Vashishtha, V.M.; Kalra, A.; Bose, A.; Choudhury, P.; Yewale, V.N.; Bansal, C.P.; Gupta, S.G. Indian Academy of Pediatrics (IAP) recommended immunization schedule for children aged 0 through 18 years, India, 2013 and updates on immunization. Indian Pediatr. 2013, 50, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Maeki, T.; Tajima, S.; Ikeda, M.; Kato, F.; Taniguchi, S.; Nakayama, E.; Takasaki, T.; Lim, C.K.; Saijo, M. Analysis of cross-reactivity between flaviviruses with sera of patients with Japanese encephalitis showed the importance of neu-tralization tests for the diagnosis of Japanese encephalitis. J. Infect. Chemother. 2019, 25, 786–790. [Google Scholar] [CrossRef] [PubMed]
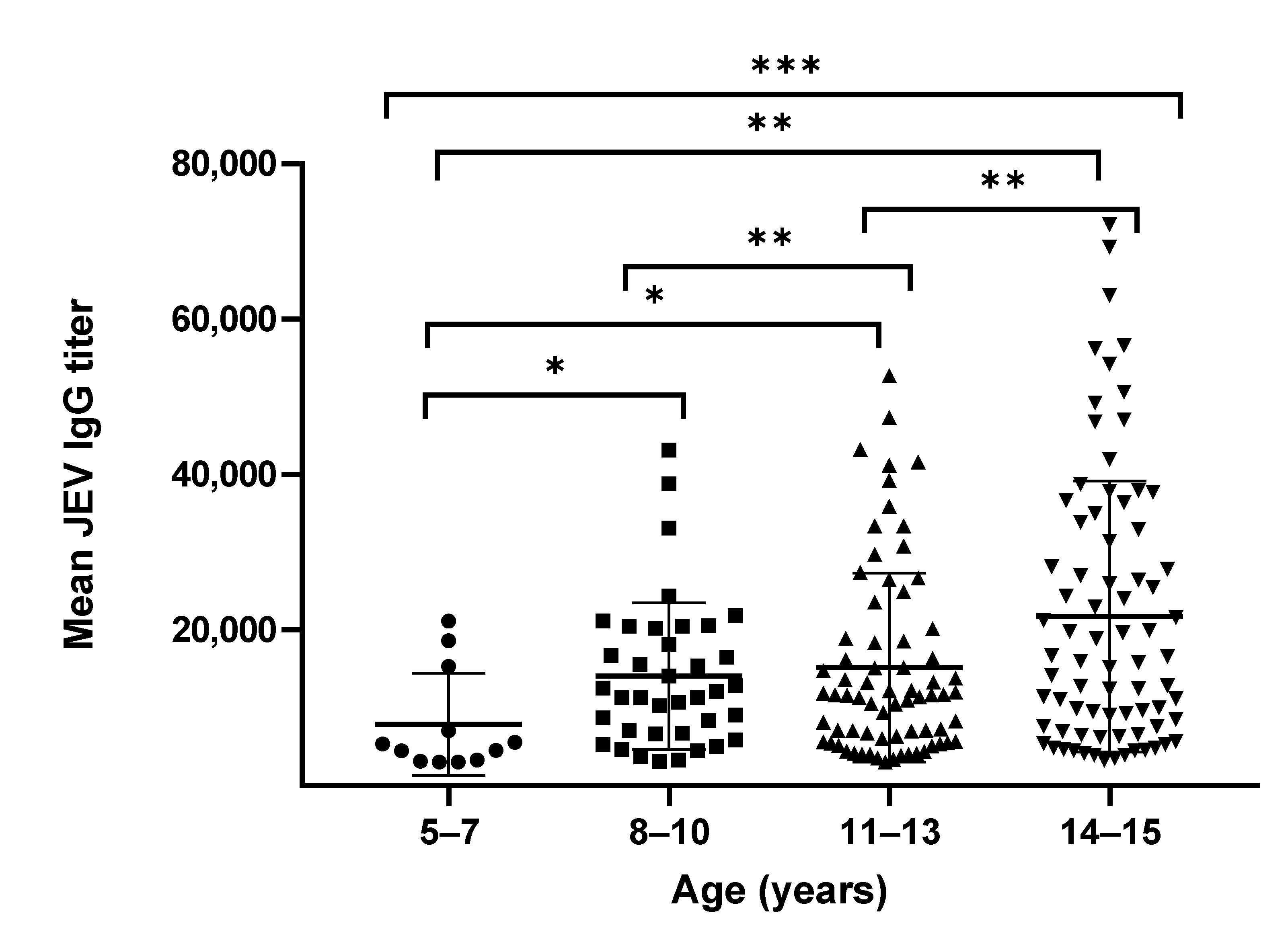
| Age Group (Years) | Gender (Female:Male) | Mean of JEV IgG Titer (95% CI) | GMT of JEV IgG Titer (95% CI) * | Mean of JEV NAb Titer (95% CI) | GMT of JEV NAb Titer (95% CI) |
|---|---|---|---|---|---|
| 5–7 | 5:7 | 7876 (3705–12,047) | 6046 (3826–9553) | 22 (0–52) | 14 (9–34) |
| 8–10 | 18:20 | 14,082 (10,982–17,182) | 11,401 (9133–14,233) | 37 (0–74) | 16 (12–22) |
| 11–13 | 39:32 | 15,158 (12,282–18,034) | 11,356 (9477–13,607) | 153 (0–324) | 30 (21–44) |
| 14–15 | 28:49 | 21,746 (17,784–25,709) | 15,468 (12,710–18,824) | 76 (53–98) | 37 (28–49) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngwe Tun, M.M.; Kyaw, A.K.; Nwe, K.M.; Inoue, S.; Thant, K.Z.; Morita, K. Effectiveness of the SA 14-14-2 Live-Attenuated Japanese Encephalitis Vaccine in Myanmar. Vaccines 2021, 9, 568. https://doi.org/10.3390/vaccines9060568
Ngwe Tun MM, Kyaw AK, Nwe KM, Inoue S, Thant KZ, Morita K. Effectiveness of the SA 14-14-2 Live-Attenuated Japanese Encephalitis Vaccine in Myanmar. Vaccines. 2021; 9(6):568. https://doi.org/10.3390/vaccines9060568
Chicago/Turabian StyleNgwe Tun, Mya Myat, Aung Kyaw Kyaw, Khine Mya Nwe, Shingo Inoue, Kyaw Zin Thant, and Kouichi Morita. 2021. "Effectiveness of the SA 14-14-2 Live-Attenuated Japanese Encephalitis Vaccine in Myanmar" Vaccines 9, no. 6: 568. https://doi.org/10.3390/vaccines9060568
APA StyleNgwe Tun, M. M., Kyaw, A. K., Nwe, K. M., Inoue, S., Thant, K. Z., & Morita, K. (2021). Effectiveness of the SA 14-14-2 Live-Attenuated Japanese Encephalitis Vaccine in Myanmar. Vaccines, 9(6), 568. https://doi.org/10.3390/vaccines9060568





