Chemical Conjugation Strategies for the Development of Protein-Based Subunit Nanovaccines
Abstract
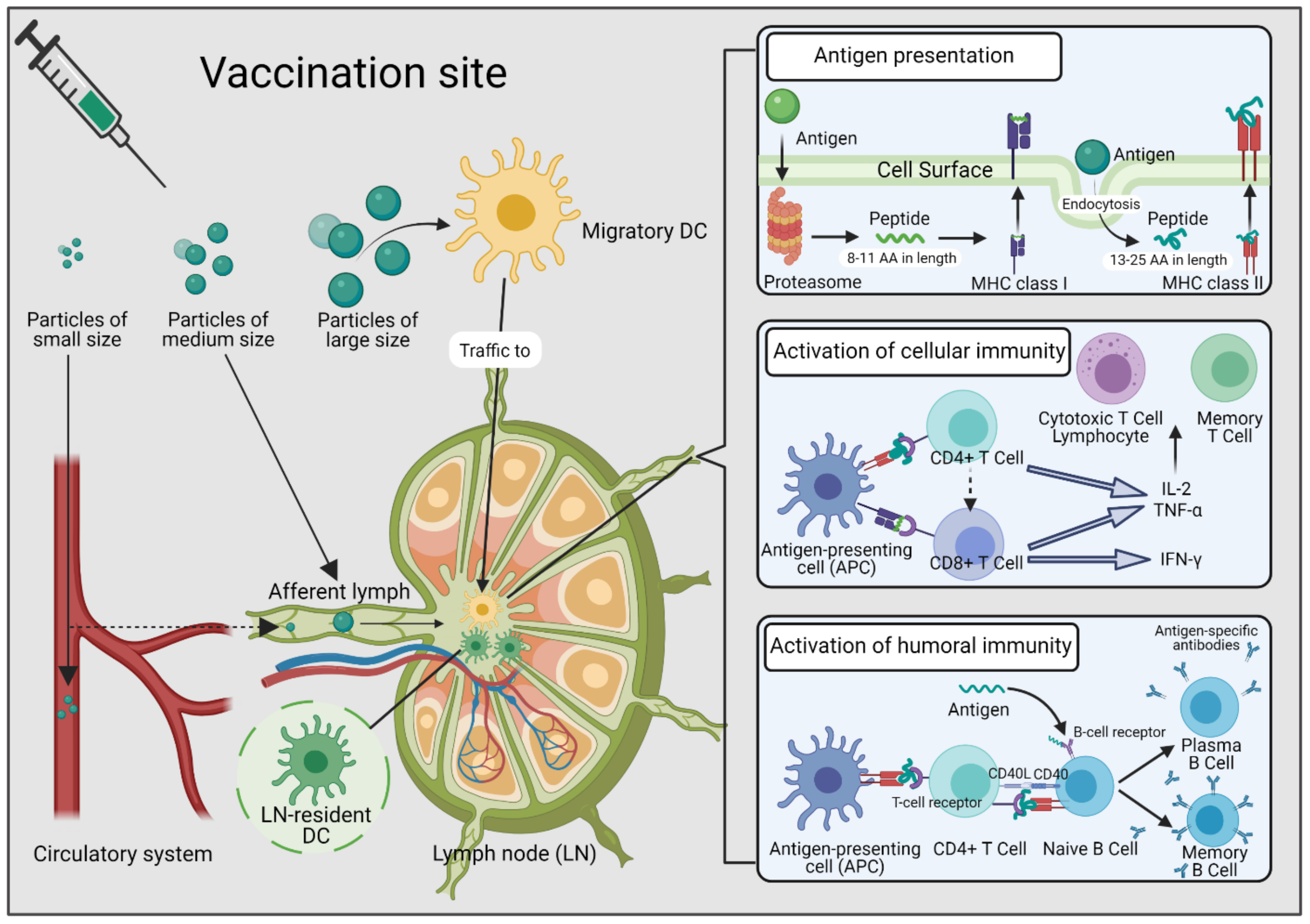
1. Introduction
2. Conjugation Strategies to Form Protein Nanoconjugates
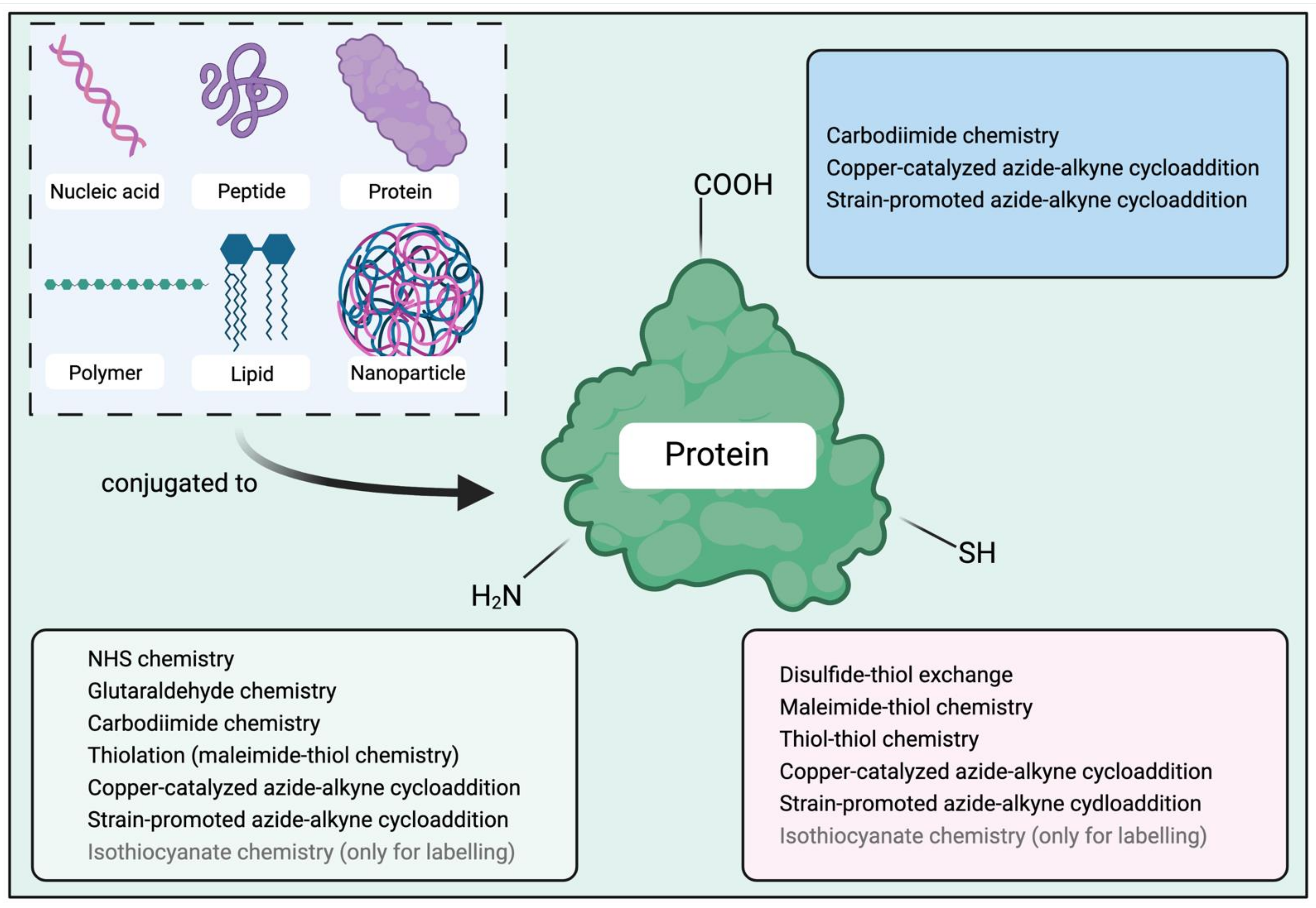
2.1. Conjugation Sites in Proteins
2.2. Conjugation Methods
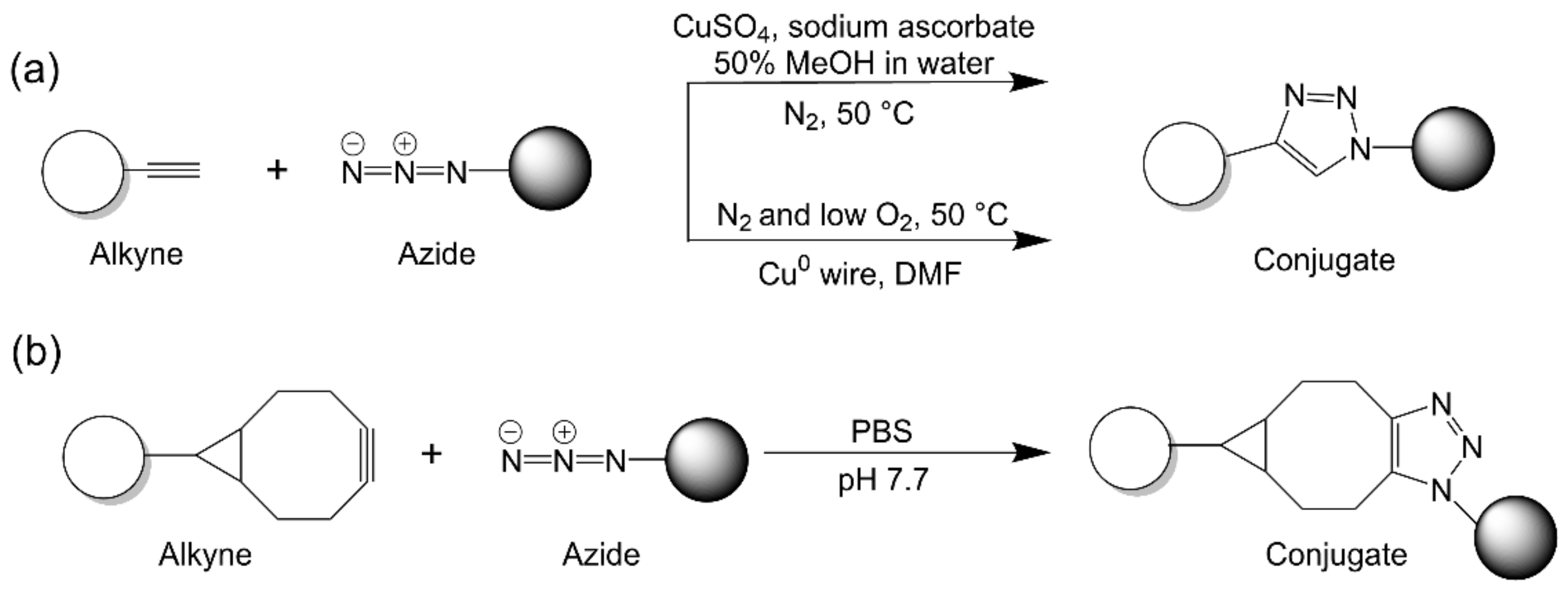
2.2.1. Strain-Promoted Azide–Alkyne Cycloaddition
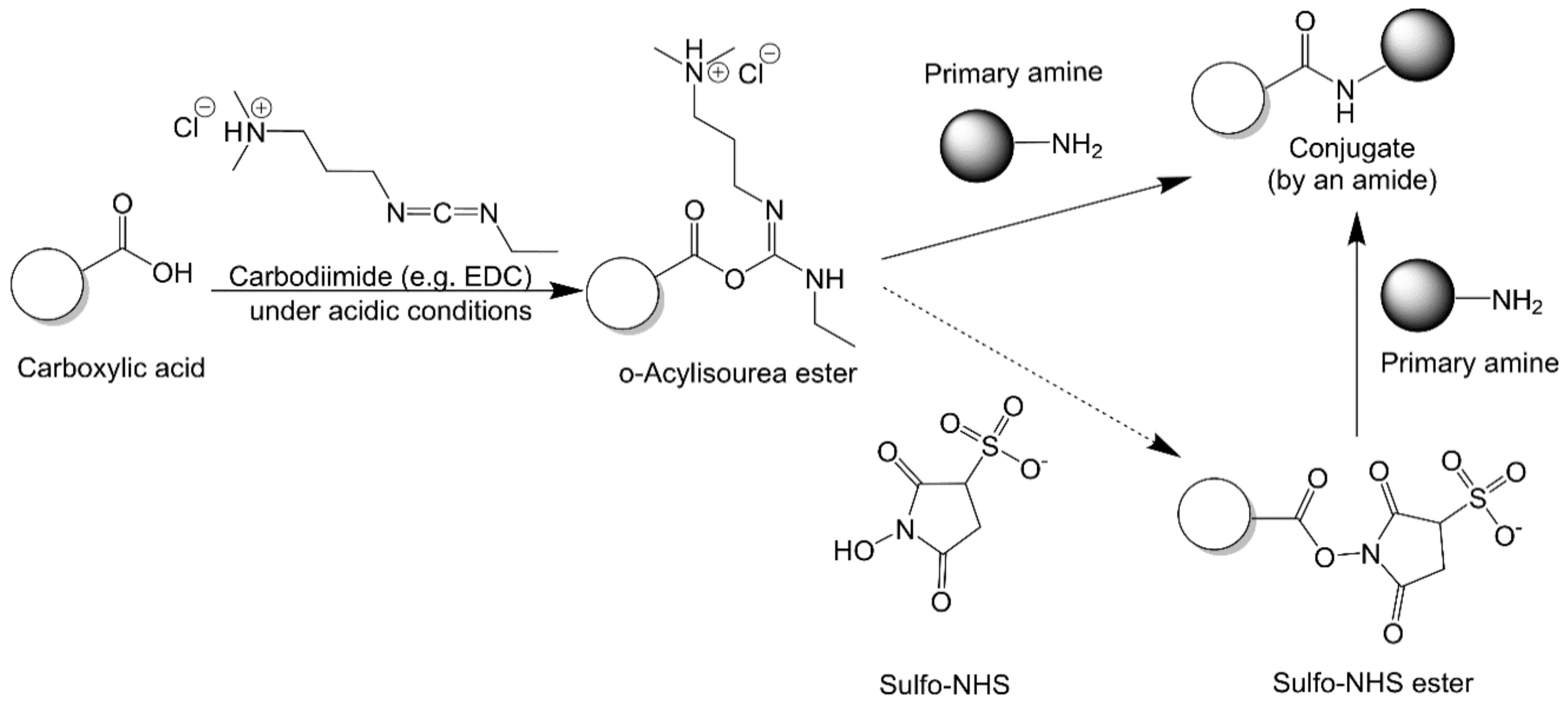
2.2.2. Carbodiimide Chemistry
2.2.3. N-Hydroxysuccinimide Chemistry
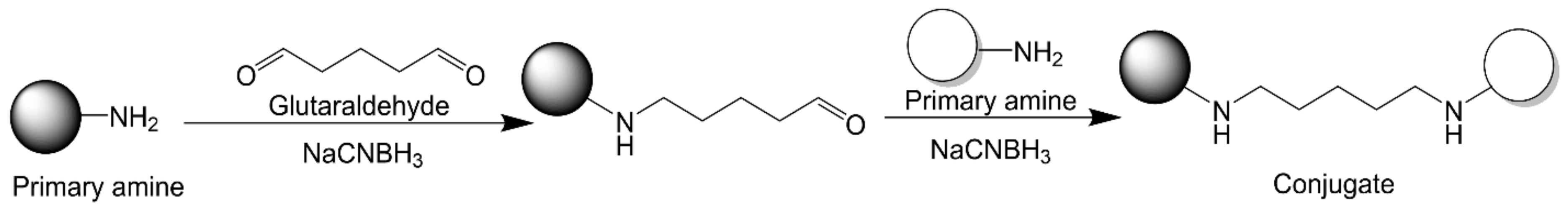
2.2.4. Glutaraldehyde Chemistry
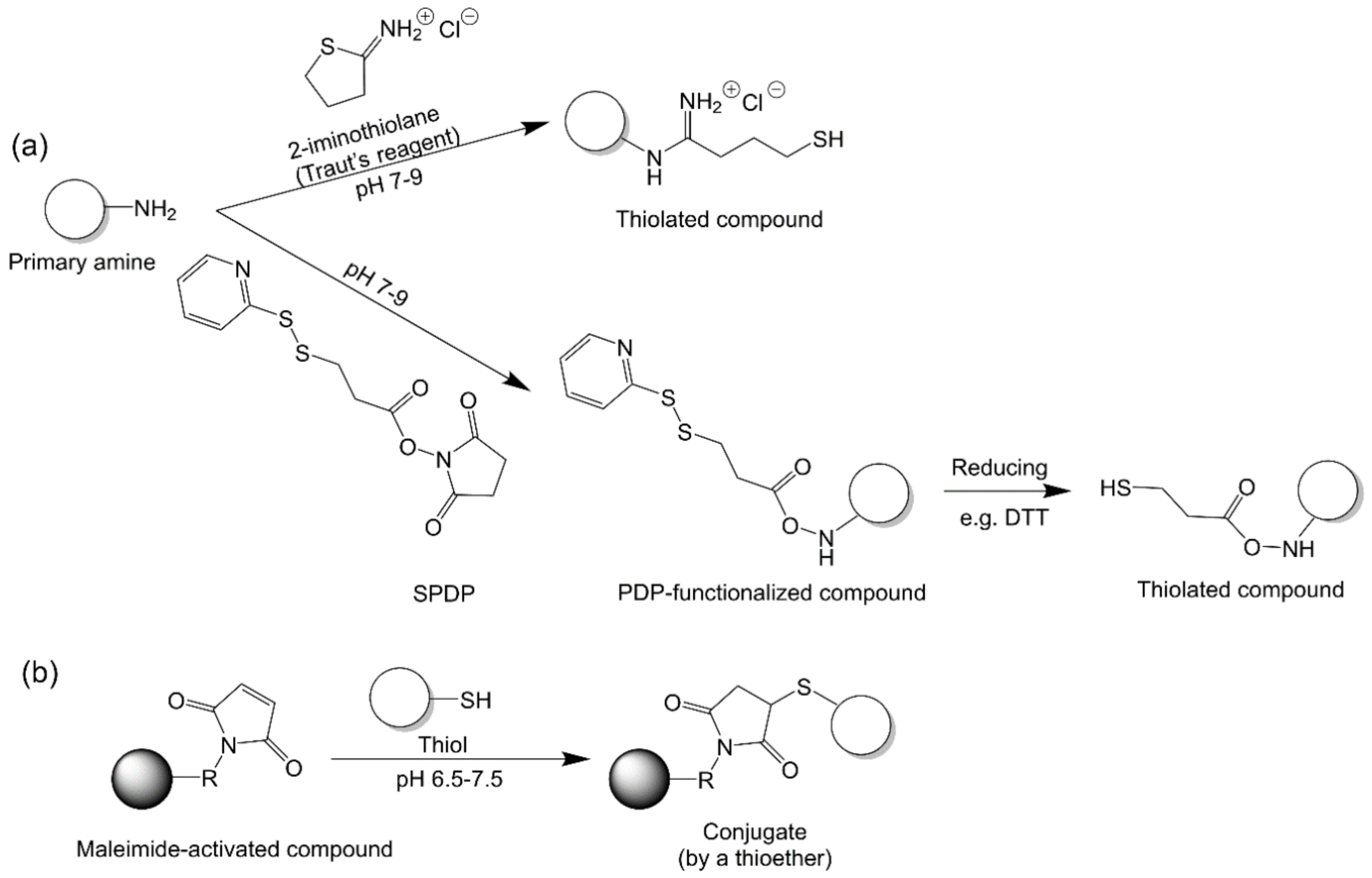
2.2.5. Maleimide–Thiol Chemistry
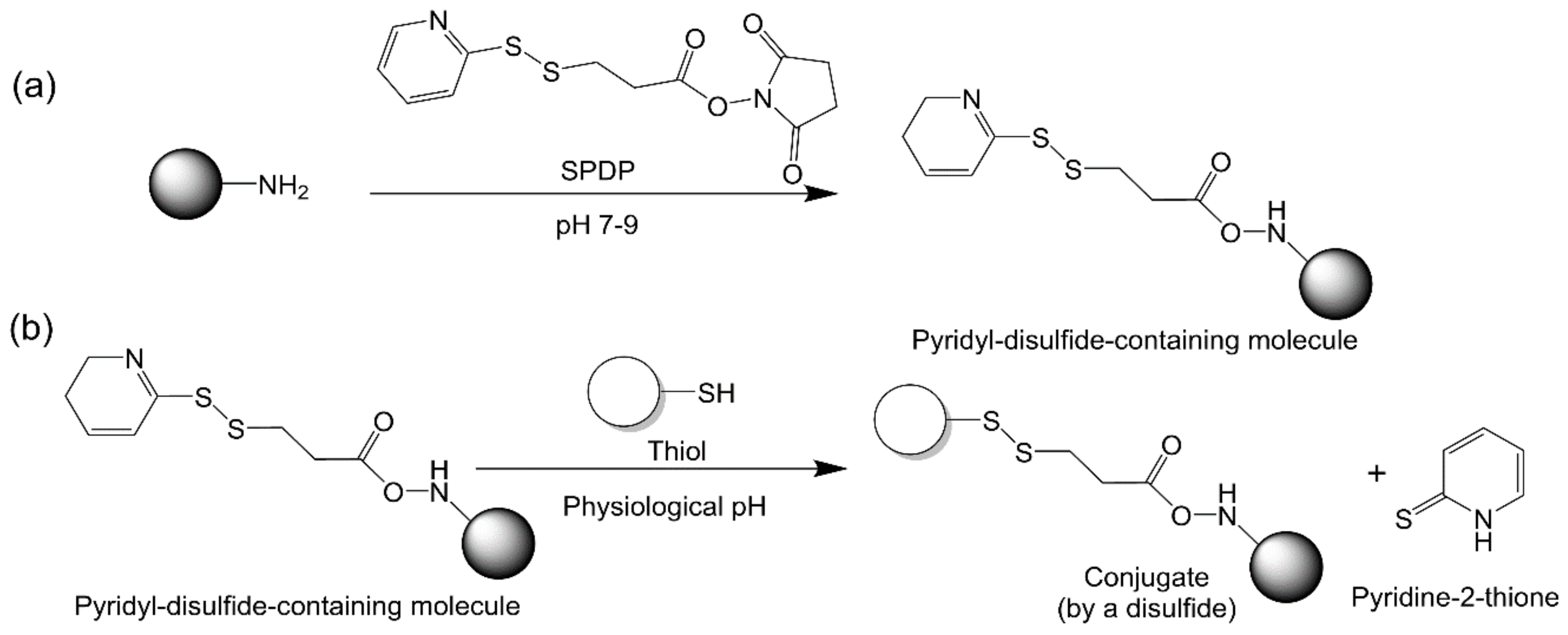
2.2.6. Thiol–Disulfide Exchange
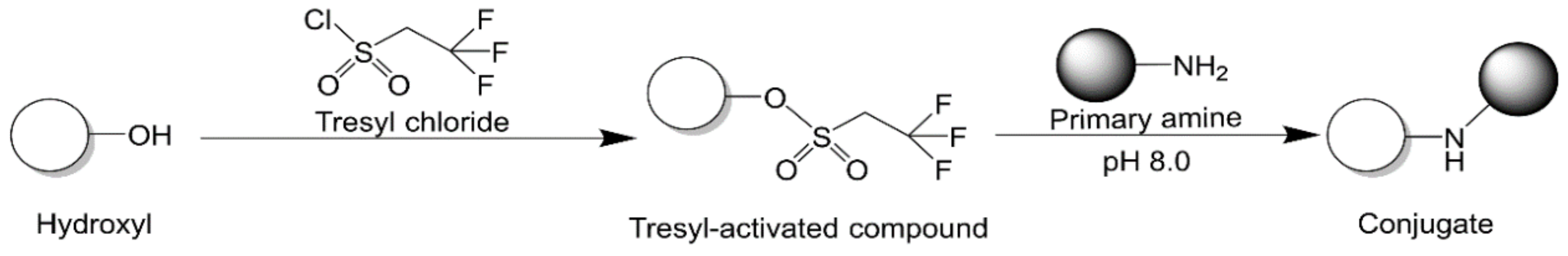
2.2.7. Tresyl Chloride Activation
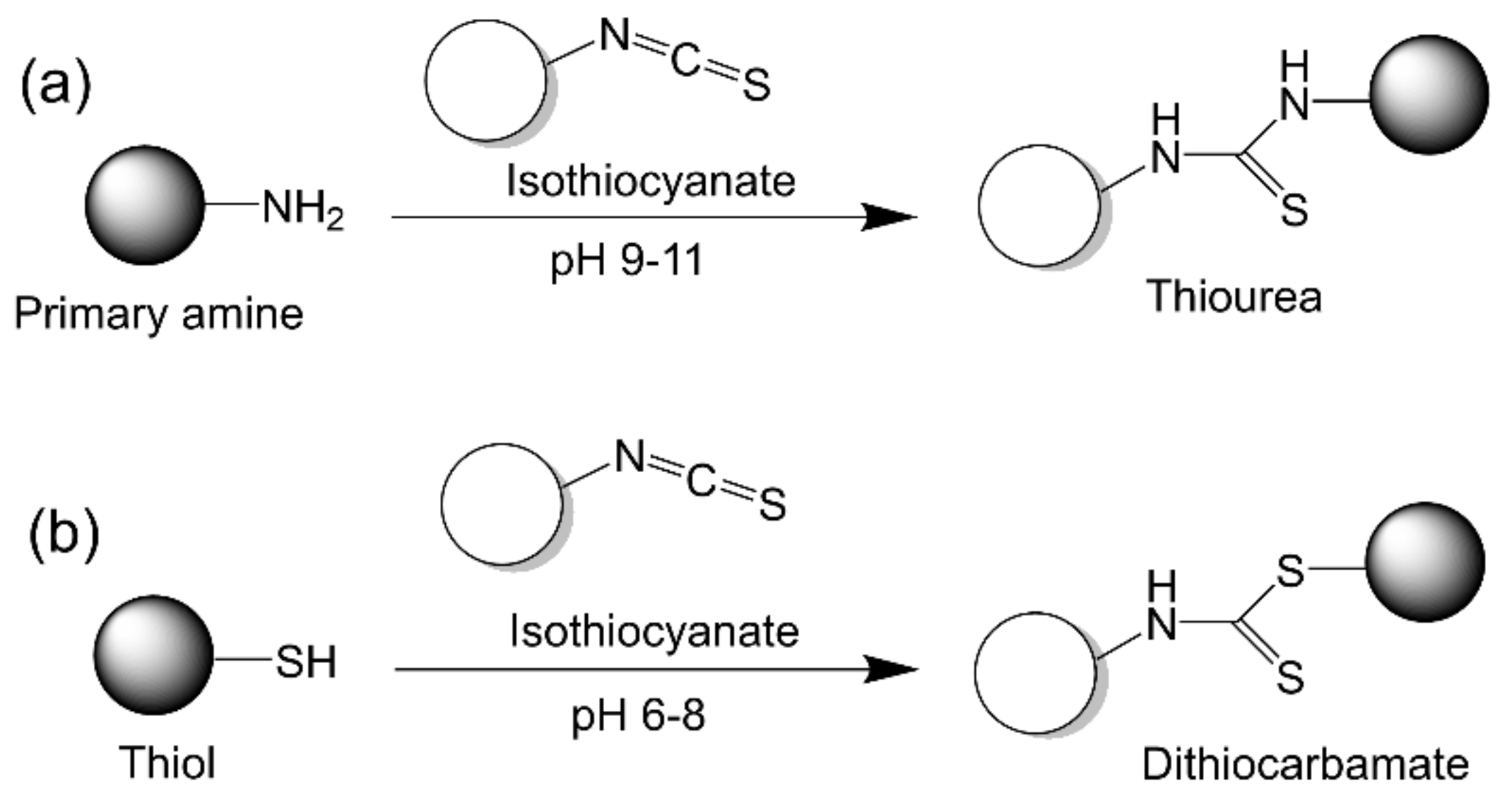
2.2.8. Isothiocyanate Chemistry
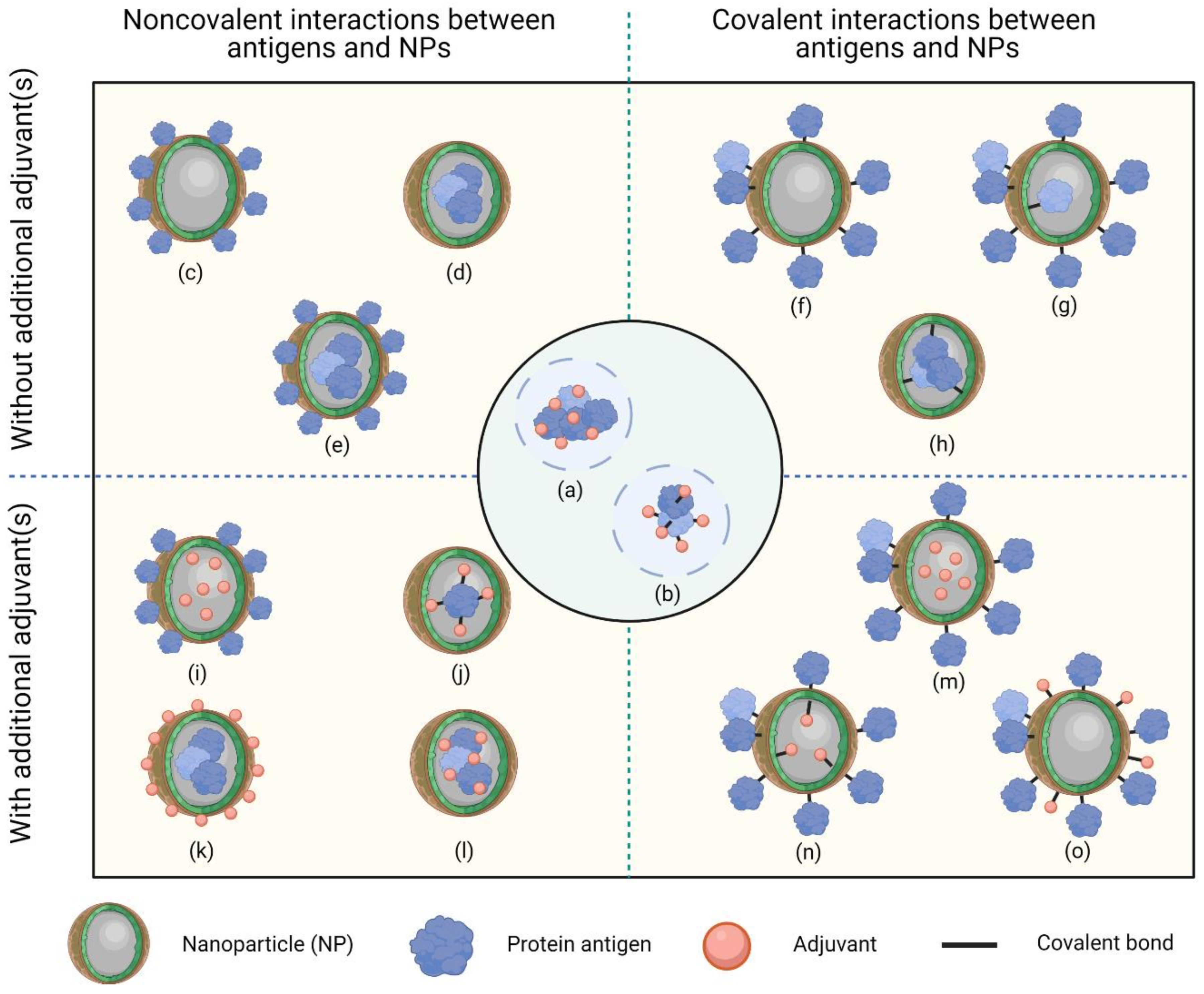
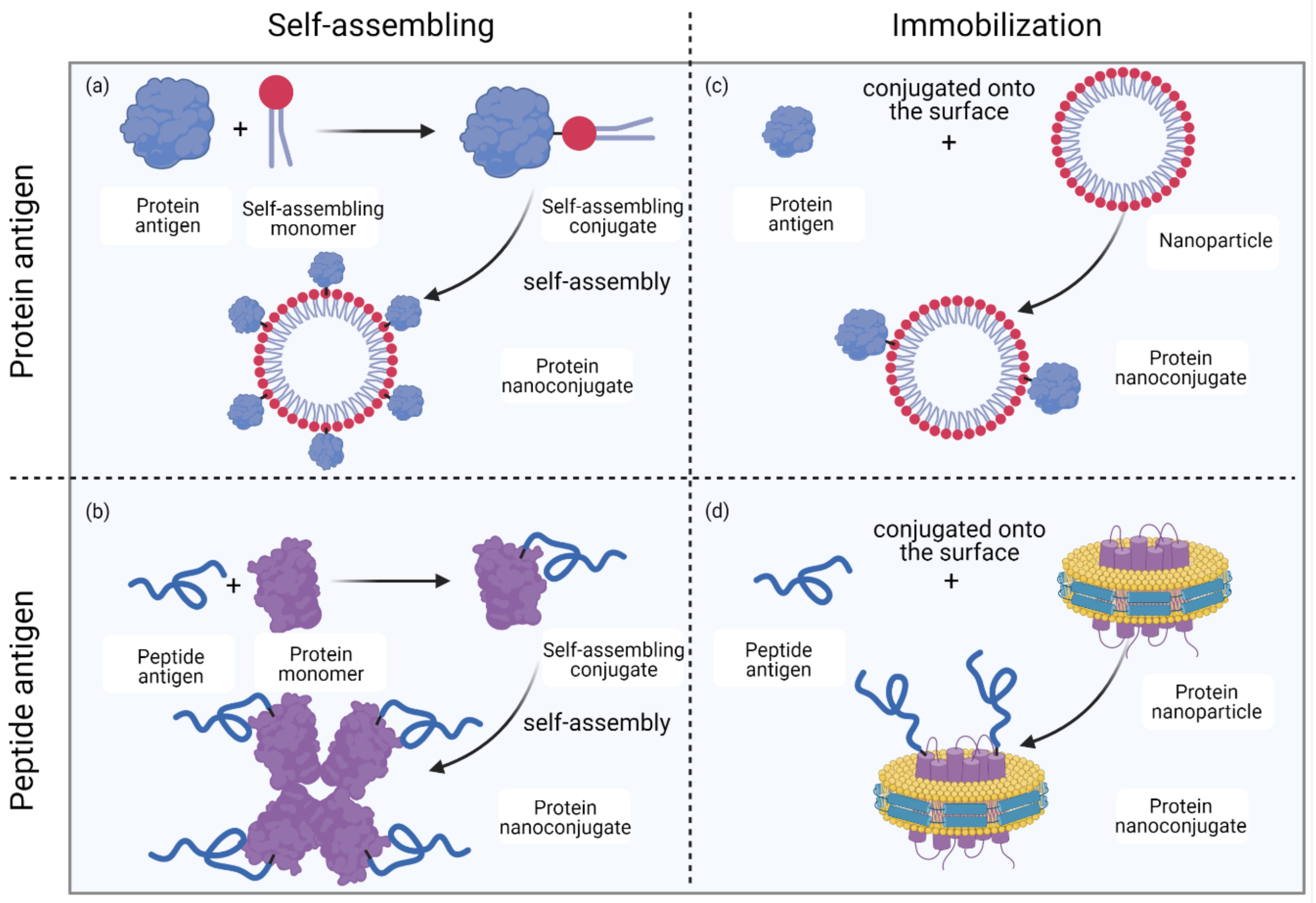
2.3. Formation of Protein Nanoconjugates
3. Protein Nanoconjugates as Vaccine Candidates
3.1. Protein–Lipid Nanoconjugates
3.2. Protein–Polymer Nanoconjugates
3.3. Protein–Protein or Protein–Peptide Nanoconjugates
3.4. Protein–Inorganic Substance Nanoconjugates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esparza, J. Early vaccine advocacy: Medals honoring Edward Jenner issued during the 19th century. Vaccine 2020, 38, 1450–1456. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.; Papneja, N.; Miller, W. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27 (Suppl. 2), S87–S97. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.A. The double-edged sword: How evolution can make or break a live-attenuated virus vaccine. Evolution 2011, 4, 635–643. [Google Scholar] [CrossRef]
- Lovalenti, P.M.; Anderl, J.; Yee, L.; Nguyen, V.; Ghavami, B.; Ohtake, S.; Saxena, A.; Voss, T.; Truong-Le, V. Stabilization of live attenuated influenza vaccines by freeze drying, spray drying, and foam drying. Pharm. Res. 2016, 33, 1144–1160. [Google Scholar] [CrossRef]
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Sander, V.A.; López, E.F.S.; Morales, L.M.; Duarte, V.A.R.; Corigliano, M.G.; Clemente, M. Use of veterinary vaccines for livestock as a strategy to control foodborne parasitic diseases. Front. Cell. Infect. Microbiol. 2020, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging concepts and technologies in vaccine development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.J. Recent advances in vaccine technologies. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Z.; Chen, H.; Liu, H.; Gao, Q.; Cong, F.; Gao, G.; Chen, Y. Subunit nanovaccine with potent cellular and mucosal immunity for COVID-19. ACS Appl. Bio Mater. 2020, 3, 5633–5638. [Google Scholar] [CrossRef]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef]
- Lee, S.; Nguyen, M.T. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015, 15, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Xu, J. Better adjuvants for better vaccines: Progress in adjuvant delivery systems, modifications, and adjuvant-antigen codelivery. Vaccines 2020, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Chandrudu, S.; Skwarczynski, M.; Toth, I. The application of self-assembled nanostructures in peptide-based subunit vaccine development. Eur. Polym. J. 2017, 93, 670–681. [Google Scholar] [CrossRef]
- Bashiri, S.; Koirala, P.; Toth, I.; Skwarczynski, M. Carbohydrate immune adjuvants in subunit vaccines. Pharmaceutics 2020, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Moyle, P.M. Bioconjugation approaches to producing subunit vaccines composed of protein or peptide antigens and covalently attached toll-like receptor ligands. Bioconjug. Chem. 2018, 29, 572–586. [Google Scholar] [CrossRef]
- Pashine, A.; Valiante, N.M.; Ulmer, J.B. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 2005, 11 (Suppl. 4), S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Skwarczynski, M.; Toth, I. Polymers for subunit vaccine delivery. Eur. Polym. J. 2019, 114, 397–410. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine 2014, 9, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, K.A.; Giddam, A.K.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposomes as nanovaccine delivery systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.K.; Dibi, M.; Mohammad, A.; Srouji, A.E. Nanovaccines formulation and applications-a review. J. Drug Deliv. Sci. Technol. 2018, 44, 380–387. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, P.; La Fauci, V.; Squeri, R.; Pellicanò, G.F.; Nunnari, G.; Trovato, M.; Di Pietro, A. The new era of vaccines: The “nanovaccinology”. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7163–7182. [Google Scholar] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Zhao, G.; Boer, J.C.; Ozberk, V.; Azuar, A.; Cruz, J.G.; Giddam, A.K.; Khalil, Z.G.; Pandey, M.; Shibu, M.A.; et al. Poly(amino acids) as a potent self-adjuvanting delivery system for peptide-based nanovaccines. Sci. Adv. 2020, 6, eaax2285. [Google Scholar] [CrossRef]
- Bartlett, S.; Skwarczynski, M.; Toth, I. Lipids as Activators of Innate Immunity in Peptide Vaccine Delivery. Curr. Med. Chem. 2020, 27, 2887–2901. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Y.; Shibu, M.A.; Toth, I.; Skwarczynskia, M.; Skwarczynski, M.; Skwarczyski, M. Cell-penetrating peptides: Efficient vectors for vaccine delivery. Curr. Drug Deliv. 2019, 16, 430–443. [Google Scholar] [CrossRef]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-based platforms for the delivery of peptides and proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Zaman, M.; Urbani, C.N.; Lin, I.-C.; Jia, Z.; Batzloff, M.R.; Good, M.F.; Monteiro, M.J.; Toth, I. Polyacrylate dendrimer nanoparticles: A self-adjuvanting vaccine delivery system. Angew. Chem. Int. Ed. 2010, 49, 5742–5745. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.-S.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci, F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008, 7, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Irvine, D.J. Guiding principles in the design of molecular bioconjugates for vaccine applications. Bioconjug. Chem. 2015, 26, 791–801. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Li, M.; Xi, J.; Liu, H. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl. Mater. Interfaces 2017, 9, 23466–23475. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwartz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Irvine, D.J.; Aung, A.; Silva, M. Controlling timing and location in vaccines. Adv. Drug Deliv. Rev. 2020, 158, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Lazarovits, J.; Poon, W.; Ouyang, B.; Nguyen, L.N.M.; Kingston, B.R.; Chan, W.C.W. Nanoparticle size influences antigen retention and presentation in lymph node follicles for humoral immunity. Nano Lett. 2019, 19, 7226–7235. [Google Scholar] [CrossRef]
- Yusuf, H.; Kett, V. Current prospects and future challenges for nasal vaccine delivery. Hum. Vaccines Immunother. 2017, 13, 34–45. [Google Scholar] [CrossRef]
- Ma, Y.; Zhuang, Y.; Xie, X.; Wang, C.; Wang, F.; Zhou, D.; Zeng, J.; Cai, L. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale 2011, 3, 2307–2314. [Google Scholar] [CrossRef]
- Kakami, Y.; Takeuchi, I.; Makino, K. Percutaneous immunization with 40-nm antigen-encapsulated elastic liposomes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 566, 128–133. [Google Scholar] [CrossRef]
- Courant, T.; Bayon, E.; Reynaud-Dougier, H.L.; Villiers, C.; Menneteau, M.; Marche, P.N.; Navarro, F.P. Tailoring nanostructured lipid carriers for the delivery of protein antigens: Physicochemical properties versus immunogenicity studies. Biomaterials 2017, 136, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.S.; Bitencourt, C.; Da Silveira, D.S.; Da Cruz, E.L.; A Pereira-Da-Silva, M.; Faccioli, L.H.; Lopez, R.F. Effective transcutaneous immunization using a combination of iontophoresis and nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2439–2448. [Google Scholar] [CrossRef]
- Yuba, E.; Tajima, N.; Yoshizaki, Y.; Harada, A.; Hayashi, H.; Kono, K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials 2014, 35, 3091–3101. [Google Scholar] [CrossRef]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef]
- Rezaei, M.; Hosseini, S.N.; Khavari-Nejad, R.A.; Najafi, F.; Mahdavi, M. HBs antigen and mannose loading on the surface of iron oxide nanoparticles in order to immuno-targeting: Fabrication, characterization, cellular and humoral immunoassay. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1543–1558. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Mannose-modified chitosan-nanoparticle-based Salmonella subunit oral vaccine-induced immune response and efficacy in a challenge trial in broilers. Vaccines 2020, 8, 299. [Google Scholar] [CrossRef]
- Kreutz, M.; Giquel, B.; Hu, Q.; Abuknesha, R.; Uematsu, S.; Akira, S.; Nestle, F.O.; Diebold, S.S. Antibody-antigen-adjuvant conjugates enable co-delivery of antigen and adjuvant to dendritic cells in cis but only have partial targeting specificity. PLoS ONE 2012, 7, e40208. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, W.; Cruz, J.G.; Marasini, N.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; Skwarczynski, M.; Toth, I. Development of polyelectrolyte complexes for the delivery of peptide-based subunit vaccines against group a streptococcus. Nanomaterials 2020, 10, 823. [Google Scholar] [CrossRef]
- Bros, M.; Nuhn, L.; Simon, J.; Moll, L.; Mailänder, V.; Landfester, K.; Grabbe, S. The protein corona as a confounding variable of nanoparticle-mediated targeted vaccine delivery. Front. Immunol. 2018, 9, 1760. [Google Scholar] [CrossRef]
- Gargett, T.; Abbas, M.N.; Rolan, P.; Price, J.D.; Gosling, K.M.; Ferrante, A.; Ruszkiewicz, A.; Atmosukarto, I.I.C.; Altin, J.; Parish, C.R.; et al. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol. Immunother. 2018, 67, 1461–1472. [Google Scholar] [CrossRef]
- Karandikar, S.; Mirani, A.; Waybhase, V.; Patravale, V.B.; Patankar, S. Chapter 10—Nanovaccines for oral delivery-formulation strategies and challenges. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–293. [Google Scholar]
- Zhang, L.; Wu, S.; Qin, Y.; Fan, F.; Zhang, Z.; Huang, C.; Ji, W.; Lu, L.; Wang, C.; Sun, H.; et al. Targeted Codelivery of an Antigen and Dual Agonists by Hybrid Nanoparticles for Enhanced Cancer Immunotherapy. Nano Lett. 2019, 19, 4237–4249. [Google Scholar] [CrossRef]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Coullerez, G.; Hofmann-Amtenbrink, M.; Vries, M.; Motazacker, M.; Rezaee, F.; Hofmann, H. Significance of surface charge and shell material of superparamagnetic iron oxide nanoparticle (SPION) based core/shell nanoparticles on the composition of the protein corona. Biomater. Sci. 2015, 3, 265–278. [Google Scholar] [CrossRef]
- Kim, S.-H.; Moon, J.-H.; Jeong, S.-U.; Jung, H.-H.; Park, C.-S.; Hwang, B.Y.; Lee, C.-K. Induction of antigen-specific immune tolerance using biodegradable nanoparticles containing antigen and dexamethasone. Int. J. Nanomed. 2019, 14, 5229–5242. [Google Scholar] [CrossRef]
- Giddam, A.K.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposome-based delivery system for vaccine candidates: Constructing an effective formulation. Nanomedicine 2012, 7, 1877–1893. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving controlled biomolecule–biomaterial conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Slütter, B.; Bal, S.M.; Que, I.; Kaijzel, E.; Löwik, C.; Bouwstra, J.; Jiskoot, W. Antigen-adjuvant nanoconjugates for nasal vaccination: An improvement over the use of nanoparticles? Mol. Pharm. 2010, 7, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Vartak, A.; Sucheck, S.J.; Wall, K.A. Liposomal Fc domain conjugated to a cancer vaccine enhances both humoral and cellular immunity. ACS Omega 2019, 4, 5204–5208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, C.; Zheng, X.; Shao, X.; Zhang, X.; Zhang, Q.; Jiang, X. Preparation and evaluation of antigen/N-trimethylaminoethylmethacrylate chitosan conjugates for nasal immunization. Vaccine 2014, 32, 2582–2590. [Google Scholar] [CrossRef]
- Yanase, N.; Toyota, H.; Hata, K.; Yagyu, S.; Seki, T.; Harada, M.; Kato, Y.; Mizuguchi, J. OVA-bound nanoparticles induce OVA-specific IgG1, IgG2a, and IgG2b responses with low IgE synthesis. Vaccine 2014, 32, 5918–5924. [Google Scholar] [CrossRef]
- Sanchez-Villamil, J.I.; Tapia, D.; Torres, A.G. Development of a gold nanoparticle vaccine against enterohemorrhagic Escherichia coli O157:H7. mBio 2019, 10, e01869-19. [Google Scholar] [CrossRef]
- Pickens, C.J.; Johnson, S.; Pressnall, M.M.; Leon, M.A.; Berkland, C.J. Practical considerations, challenges, and limitations of bioconjugation via azide-alkyne cycloaddition. Bioconjug. Chem. 2018, 29, 686–701. [Google Scholar] [CrossRef]
- Hussein, W.M.; Liu, T.; Maruthayanar, P.; Mukaida, S.; Moyle, P.M.; Wells, J.W.; Toth, I.; Skwarczynski, M. Double conjugation strategy to incorporate lipid adjuvants into multiantigenic vaccines. Chem. Sci. 2016, 7, 2308–2321. [Google Scholar] [CrossRef]
- Ahmad Fuaad, A.A.H.; Azmi, F.; Skwarczynski, M.; Toth, I. Peptide conjugation via CuAAC ‘click’ chemistry. Molecules 2013, 18, 13148–13174. [Google Scholar] [CrossRef]
- Hussein, W.M.; Liu, T.-Y.; Jia, Z.; McMillan, N.A.; Monteiro, M.; Toth, I.; Skwarczynski, M. Multiantigenic peptide-polymer conjugates as therapeutic vaccines against cervical cancer. Bioorg. Med. Chem. 2016, 24, 4372–4380. [Google Scholar] [CrossRef]
- Alavi, S.E.; Cabot, P.J.; Yap, G.Y.; Moyle, P.M. Optimized methods for the production and bioconjugation of site-specific, alkyne-modified glucagon-like peptide-1 (GLP-1) analogs to azide-modified delivery platforms using copper-catalyzed alkyne–azide cycloaddition. Bioconjug. Chem. 2020, 31, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Stephenson, R.J.; Skwarczynski, M.; Toth, I. Application of Fmoc-SPPS, thiol-maleimide conjugation, and copper(i)-catalyzed alkyne-azide cycloaddition “click” reaction in the synthesis of a complex peptide-based vaccine candidate against Group A Streptococcus. In Peptide Synthesis: Methods and Protocols; Hussein, W.M., Skwarczynski, M., Toth, I., Eds.; Springer: New York, NY, USA, 2020; p. 1327. [Google Scholar]
- Faruck, M.O.; Zhao, L.; Hussein, W.M.; Khalil, Z.G.; Capon, R.J.; Skwarczynski, M.; Toth, I. Polyacrylate-peptide antigen conjugate as a single-dose oral vaccine against Group A Streptococcus. Vaccines 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Yu, F.; Zhu, Z.; Shobaki, D.; Chen, H.; Wang, M.; Wang, J.; Qin, G.; Erasquin, U.J.; et al. Copper-catalyzed click reaction on/in live cells. J. Chem. Sci. 2017, 8, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Li, K.; Wang, Y.; Gruebele, M.; Ferguson, A.L.; Zimmerman, S.C. Polymeric “clickase” accelerates the copper click reaction of small molecules, proteins, and cells. J. Am. Chem. Soc. 2019, 141, 9693–9700. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. Development and applications of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) as a bioorthogonal reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.C.; McKay, C.S.; Legault, M.C.B.; Danielson, D.C.; Blake, J.A.; Pegoraro, A.F.; Stolow, A.; Mester, Z.; Pezacki, J.P. Cellular consequences of copper complexes used to catalyze bioorthogonal click reactions. J. Am. Chem. Soc. 2011, 133, 17993–18001. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Heterobifunctional Crosslinkers. In Bioconjugate Techniques, 2nd ed.; Hermanson, G.T., Ed.; Academic Press: New York, NY, USA, 2008; Chapter 5; pp. 276–335. [Google Scholar]
- Dommerholt, J.; Rutjes, F.P.J.T.; van Delft, F.L. Strain-promoted 1,3-dipolar cycloaddition of cycloalkynes and organic azides. Top. Curr. Chem. 2016, 374, 16. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Baskin, J.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef]
- Nogueira, J.C.F.; Greene, M.K.; Richards, D.; Furby, A.O.; Steven, J.; Porter, A.; Barelle, C.; Scott, C.J.; Chudasama, V. Oriented attachment of VNAR proteins, via site-selective modification, on PLGA–PEG nanoparticles enhances nanoconjugate performance. Chem. Commun. 2019, 55, 7671–7674. [Google Scholar] [CrossRef]
- Greene, M.K.; Richards, D.A.; Nogueira, J.C.F.; Campbell, K.; Smyth, P.; Fernández, M.; Scott, C.J.; Chudasama, V. Forming next-generation antibody–nanoparticle conjugates through the oriented installation of non-engineered antibody fragments. Chem. Sci. 2018, 9, 79–87. [Google Scholar] [CrossRef]
- Liu, X.; Gong, P.; Song, P.; Xie, F.; Ii, A.L.M.; Chen, S.; Lu, L.; Miller, A.L. Rapid conjugation of nanoparticles, proteins and siRNAs to microbubbles by strain-promoted click chemistry for ultrasound imaging and drug delivery. Polym. Chem. 2019, 10, 705–717. [Google Scholar] [CrossRef]
- Zeng, D.; Zeglis, B.M.; Lewis, J.S.; Anderson, C.J. The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals. J. Nucl. Med. 2013, 54, 829–832. [Google Scholar] [CrossRef]
- Song, F.; Chan, W.C. Principles of conjugating quantum dots to proteins via carbodiimide chemistry. Nanotechnology 2011, 22, 494006. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kyratzis, I. Covalent immobilization of proteins on carbon nanotubes using the cross-linker 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide—A critical assessment. Bioconjugate Chem. 2008, 19, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Thorek, D.L.; Elias, D.R.; Tsourkas, A. Comparative analysis of nanoparticle-antibody conjugations: Carbodiimide versus click chemistry. Mol. Imaging 2009, 8, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, N.O.; Tobias, C.A.; Fischer, I.; Wheatley, M.A. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. Part A 2004, 71, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.P.; Hynd, M.R.; Shuler, M.L.; Shain, W. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed. Mater. 2011, 6, 015002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Aouidat, F.; Sacco, P.; Marsich, E.; Djaker, N.; Spadavecchia, J. Galectin-1 protein modified gold (III)-PEGylated complex-nanoparticles: Proof of concept of alternative probe in colorimetric glucose detection. Colloids Surf. B Biointerfaces 2020, 185, 110588. [Google Scholar] [CrossRef] [PubMed]
- Mädler, S.; Bich, C.; Touboul, D.; Zenobi, R. Chemical cross-linking with NHS esters: A systematic study on amino acid reactivities. J. Mass Spectrom. 2009, 44, 694–706. [Google Scholar] [CrossRef]
- Tang, X.; Bruce, J.E. Chemical cross-linking for protein-protein interaction studies. Methods Mol. Biol. 2009, 492, 283–293. [Google Scholar] [PubMed]
- Hermanson, G.T. Chapter 5—Homobifunctional crosslinkers. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 275–298. [Google Scholar]
- Staros, J.V. N-hydroxysulfosuccinimide active esters: Bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry 1982, 21, 3950–3955. [Google Scholar] [CrossRef]
- Hermanson, G.T. Chapter 22—Enzyme modification and conjugation. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 951–957. [Google Scholar]
- Carter, J.M. Conjugation of peptides to carrier proteins via glutaraldehyde. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 1996; pp. 679–687. [Google Scholar]
- Sahin, S.; Ozmen, I. Covalent immobilization of trypsin on polyvinyl alcohol-coated magnetic nanoparticles activated with glutaraldehyde. J. Pharm. Biomed. Anal. 2020, 184, 113195. [Google Scholar] [CrossRef]
- Ravasco, J.M.J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A useful tool for chemical biology. Chemistry 2019, 25, 43–59. [Google Scholar] [CrossRef]
- Altinbasak, I.; Arslan, M.; Sanyal, R.; Sanyal, A. Pyridyl disulfide-based thiol–disulfide exchange reaction: Shaping the design of redox-responsive polymeric materials. Polym. Chem. 2020, 11, 7603–7624. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Cosar, M.; Aydin, D.; Kaga, E.; Arslan, M.; Sanyal, R.; Sanyal, A. Facile fabrication of a modular “catch and release” hydrogel interface: Harnessing thiol–disulfide exchange for reversible protein capture and cell attachment. ACS Appl. Mater. Interfaces 2018, 10, 14399–14409. [Google Scholar] [CrossRef]
- Steenpaß, T.; Lung, A.; Schubert, R. Tresylated PEG-sterols for coupling of proteins to preformed plain or PEGylated liposomes. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 20–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jain, A.; Yan, W.; Miller, K.R.; O’Carra, R.; Woodward, J.G.; Mumper, R.J. Tresyl-based conjugation of protein antigen to lipid nanoparticles increases antigen immunogenicity. Int. J. Pharm. 2010, 401, 87–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delgado, C.; Francis, G.E.; Fisher, D. Coupling of PEG to proteins by activation with tresyl chloride. Applications in Immunoaffinity Cell Partitioning. In Separations Using Aqueous Phase Systems: Applications in Cell Biology and Biotechnology; Fisher, D., Sutherland, I.A., Eds.; Springer: Boston, MA, USA, 1989; pp. 211–213. [Google Scholar]
- Nilsson, K.; Mosbach, K. [2] Immobilization of ligands with organic sulfonyl chlorides. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; pp. 56–69. [Google Scholar]
- Lanciotti, J.; Song, A.; Doukas, J.; Sosnowski, B.; Pierce, G.; Gregory, R.; Wadsworth, S.; O’Riordan, C. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol. Ther. 2003, 8, 99–107. [Google Scholar] [CrossRef]
- Le, H.T.; Yu, Q.-C.; Wilson, J.M.; Croyle, M.A. Utility of PEGylated recombinant adeno-associated viruses for gene transfer. J. Control. Release 2005, 108, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Patel, J.N.; Francis, G.E.; Fisher, D. Coupling of poly(ethylene glycol) to albumin under very mild conditions by activation with tresyl chloride: Characterization of the conjugate by partitioning in aqueous two-phase systems. Biotechnol. Appl. Biochem. 1990, 12, 119–128. [Google Scholar] [PubMed]
- Petri, L.; Szijj, P.A.; Kelemen, Á.; Imre, T.; Gömöry, Á.; Lee, M.; Hegedűs, K.; Ábrányi-Balogh, P.; Chudasama, V.; Keserű, G.M. Cysteine specific bioconjugation with benzyl isothiocyanates. RSC Adv. 2020, 10, 14928–14936. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, D.; Salmain, M.; Liedberg, B.; Boujday, S. Direct quantification of surface coverage of antibody in IgG-Gold nanoparticles conjugates. Talanta 2019, 204, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Oltolina, F.; Gregoletto, L.; Colangelo, D.; Gómez-Morales, J.; Delgado-López, J.M.; Prat, M. Monoclonal antibody-targeted fluorescein-5-isothiocyanate-labeled biomimetic nanoapatites: A promising fluorescent probe for imaging applications. Langmuir 2015, 31, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Caprifico, A.E.; Polycarpou, E.; Foot, P.J.S.; Calabrese, G. Biomedical and pharmacological uses of fluorescein isothiocyanate chitosan-based nanocarriers. Macromol. Biosci. 2021, 21, 2000312. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Hua, S.; De Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Personal. Ther. 2019, 34. [Google Scholar] [CrossRef]
- Pauthner, M.; Havenar-Daughton, C.; Sok, D.; Nkolola, J.P.; Bastidas, R.; Boopathy, A.V.; Carnathan, D.G.; Chandrashekar, A.; Cirelli, K.M.; Cottrell, C.A.; et al. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 2017, 46, 1073-1088.e6. [Google Scholar] [CrossRef]
- Bale, S.; Goebrecht, G.; Stano, A.; Wilson, R.; Ota, T.; Tran, K.; Ingale, J.; Zwick, M.B.; Wyatt, R.T. Covalent linkage of HIV-1 trimers to synthetic liposomes elicits improved B cell and antibody responses. J. Virol. 2017, 91, e00443-17. [Google Scholar] [CrossRef]
- Tokatlian, T.; Kulp, D.W.; Mutafyan, A.A.; Jones, C.A.; Menis, S.; Georgeson, E.; Kubitz, M.; Zhang, M.H.; Melo, M.B.; Silva, M.; et al. Enhancing humoral responses against HIV envelope trimers via nanoparticle delivery with stabilized synthetic liposomes. Sci. Rep. 2018, 8, 16527. [Google Scholar] [CrossRef]
- Toyota, H.; Yanase, N.; Yoshimoto, T.; Mitsunori, H.; Kato, Y.; Mizuguchi, J. Vaccination with OVA-bound nanoparticles encapsulating IL-7 inhibits the growth of OVA-expressing E. G7 tumor cells in vivo. Oncol. Rep. 2015, 33, 292–296. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, X.; Zhang, C.; Shao, X.; Zhang, X.; Zhang, Q.; Jiang, X. Conjugating influenza a (H1N1) antigen to n-trimethylaminoethylmethacrylate chitosan nanoparticles improves the immunogenicity of the antigen after nasal administration. J. Med. Virol. 2015, 87, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Hirosue, S.; Raczy, M.M.; Bonilla-Ramirez, L.; Jeanbart, L.; Wang, R.; Kwissa, M.; Franetich, J.-F.; Broggi, M.; Diaceri, G.; et al. Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nat. Mater. 2019, 18, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.L.; Pouniotis, D.; Hanley, J.; Xiang, S.D.; Ma, C.; Coppel, R.L.; Plebanski, M. A Synthetic nanoparticle based vaccine approach targeting MSP4/5 is immunogenic and induces moderate protection against murine blood-stage malaria. Front. Immunol. 2019, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Stano, A.; Scott, E.A.; Dane, K.Y.; Swartz, M.A.; Hubbell, J.A. Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials 2013, 34, 4339–4346. [Google Scholar] [CrossRef]
- van der Vlies, A.J.; O’Neil, C.P.; Hasegawa, U.; Hammond, N.; Hubbell, J.A. Synthesis of pyridyl disulfide-functionalized nanoparticles for conjugating thiol-containing small molecules, peptides, and proteins. Bioconjug. Chem. 2010, 21, 653–662. [Google Scholar] [CrossRef]
- Nembrini, C.; Stano, A.; Dane, K.Y.; Ballester, M.; van der Vlies, A.J.; Marsland, B.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc. Natl. Acad. Sci. USA 2011, 108, E989–E997. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kim, H.; Park, Y.; Kong, W.H.; Lee, S.W.; Kwok, S.J.J.; Hahn, S.K.; Yun, S.H. Noninvasive transdermal vaccination using hyaluronan nanocarriers and laser adjuvant. Adv. Funct. Mater. 2016, 26, 2512–2522. [Google Scholar] [CrossRef]
- Flanary, S.; Hoffman, A.S.; Stayton, P.S. Antigen delivery with poly(propylacrylic acid) conjugation enhances MHC-1 presentation and T-cell activation. Bioconjug. Chem. 2009, 20, 241–248. [Google Scholar] [CrossRef]
- Wilson, J.T.; Keller, S.; Manganiello, M.J.; Cheng, C.; Lee, C.-C.; Opara, C.; Convertine, A.; Stayton, P.S. pH-responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano 2013, 7, 3912–3925. [Google Scholar] [CrossRef]
- Neek, M.; Kim, T.I.; Wang, S.W. Protein-based nanoparticles in cancer vaccine development. Nanomedicine 2019, 15, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Molino, N.M.; Wang, S.W. Caged protein nanoparticles for drug delivery. Curr. Opin. Biotechnol. 2014, 28, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-A.; Kang, Y.J.; Shin, C.; Ra, J.-S.; Shin, H.-H.; Hong, S.Y.; Do, Y.; Kang, S. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomedicine 2014, 10, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-R.; Ko, H.K.; Ryu, J.H.; Ahn, K.Y.; Lee, Y.-H.; Oh, S.J.; Na, J.H.; Kim, T.W.; Byun, Y.; Kwon, I.C.; et al. Engineered human ferritin nanoparticles for direct delivery of tumor antigens to lymph node and cancer immunotherapy. Sci. Rep. 2016, 6, 35182. [Google Scholar] [CrossRef] [PubMed]
- Kar, U.K.; Srivastava, M.K.; Andersson, Å.; Baratelli, F.; Huang, M.; Kickhoefer, V.A.; Dubinett, S.M.; Rome, L.H.; Sharma, S. Novel CCL21-vault nanocapsule intratumoral delivery inhibits lung cancer growth. PLoS ONE 2011, 6, e18758. [Google Scholar] [CrossRef] [PubMed]
- Kar, U.K.; Jiang, J.; Champion, C.I.; Salehi, S.; Srivastava, M.; Sharma, S.; Rabizadeh, S.; Niazi, K.; Kickhoefer, V.; Rome, L.; et al. Vault nanocapsules as adjuvants favor cell-mediated over antibody-mediated immune responses following immunization of mice. PLoS ONE 2012, 7, e38553. [Google Scholar] [CrossRef] [PubMed]
- Neek, M.; Tucker, J.A.; Kim, T.I.; Molino, N.M.; Nelson, E.L.; Wang, S.-W. Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials 2018, 156, 194–203. [Google Scholar] [CrossRef]
- Molino, N.M.; Neek, M.; Tucker, J.A.; Nelson, E.L.; Wang, S.-W. Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials 2016, 86, 83–91. [Google Scholar] [CrossRef]
- Molino, N.M.; Anderson, A.K.L.; Nelson, E.L.; Wang, S.-W. Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano 2013, 7, 9743–9752. [Google Scholar] [CrossRef]
- Jones, D.S.; Rowe, C.G.; Chen, B.; Reiter, K.; Rausch, K.M.; Narum, D.L.; Wu, Y.; Duffy, P.E. A method for producing protein nanoparticles with applications in vaccines. PLoS ONE 2016, 11, e0138761. [Google Scholar] [CrossRef]
- Scaria, P.V.; Chen, B.; Rowe, C.G.; Jones, D.S.; Barnafo, E.; Fischer, E.R.; Anderson, C.; Macdonald, N.J.; Lambert, L.; Rausch, K.M.; et al. Protein-protein conjugate nanoparticles for malaria antigen delivery and enhanced immunogenicity. PLoS ONE 2017, 12, e0190312. [Google Scholar] [CrossRef]
- Kuai, R.; Sun, X.; Yuan, W.; Xu, Y.; Schwendeman, A.; Moon, J.J. Subcutaneous nanodisc vaccination with neoantigens for combination cancer immunotherapy. Bioconjug. Chem. 2018, 29, 771–775. [Google Scholar] [CrossRef]
- Scheetz, L.; Kadiyala, P.; Sun, X.; Son, S.; Najafabadi, A.H.; Aikins, M.; Lowenstein, P.R.; Schwendeman, A.; Castro, M.G.; Moon, J.J. Synthetic high-density lipoprotein nanodiscs for personalized immunotherapy against gliomas. Clin. Cancer Res. 2020, 26, 4369–4380. [Google Scholar] [CrossRef] [PubMed]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef]
- Kuai, R.; Yuan, W.; Son, S.; Nam, J.; Xu, Y.; Fan, Y.; Schwendeman, A.; Moon, J.J. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 2018, 4, eaao1736. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Powell, J.; Calcutt, A.; Zaman, M.; Phillips, Z.N.; Ho, M.F.; Batzloff, M.R.; Good, M.F. Physicochemical characterisation, immunogenicity and protective efficacy of a lead streptococcal vaccine: Progress towards Phase I trial. Sci. Rep. 2017, 7, 13786. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.-L.S.; Jayashi Flores, C.M.; Reynolds, S.; Wun, C.; Calcutt, A.; Baker, S.B.; Murugappan, S.; Depelsenaire, A.C.I.; Dooley, J.; Fahey, P.V.; et al. M-protein based vaccine induces immunogenicity and protection from Streptococcus pyogenes when delivered on a high-density microarray patch (HD-MAP). NPJ Vaccines 2020, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Sekuloski, S.; Batzloff, M.R.; Griffin, P.; Parsonage, W.; Elliott, S.; Hartas, J.; O’Rourke, P.; Marquart, L.; Pandey, M.; Rubin, F.A.; et al. Evaluation of safety and immunogenicity of a group A streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLoS ONE 2018, 13, e0198658. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Ozberk, V.; Eskandari, S.; Shalash, A.O.; Joyce, M.A.; Saffran, H.A.; Day, C.J.; Lepletier, A.; Spillings, L.; Mills, J.-L.; et al. Antibodies to neutralising epitopes synergistically block the interaction of the receptor-binding domain of SARS-CoV-2 to ACE 2. Clin. Transl. Immunol. 2021, 10, e1260. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Balbin, Y.; Santana-Mederos, D.; Quintero, L.; Fernandez, S.; Rodriguez, L.; Sanchez Ramirez, B.; Perez, R.; Acosta, C.; Mendez, Y.; Ricardo, M.G.; et al. SARS-CoV-2 RBD-Tetanus toxoid conjugate vaccine induces a strong neutralizing immunity in preclinical studies. bioRxiv 2021. [Google Scholar] [CrossRef]
- Gregory, A.; Williamson, D.; Titball, R. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, W.; Luo, Y.; Wang, B.-Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomedicine 2018, 14, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, W.; Wang, B.Z. Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro. Int. J. Nanomed. 2017, 12, 4747–4762. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.W.; Thornburg, N.J.; Blum, D.L.; Kuhn, S.J.; Wright, D.W.; Crowe, J.E., Jr. Gold nanorod vaccine for respiratory syncytial virus. Nanotechnology 2013, 24, 295102. [Google Scholar] [CrossRef] [PubMed]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020, 64, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ahn, S.; Lee, J.; Kim, J.Y.; Choi, M.; Gujrati, V.; Kim, H.; Kim, J.; Shin, E.-C.; Jon, S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control. Release 2017, 256, 56–67. [Google Scholar] [CrossRef]
- Gregory, A.; Williamson, E.; Prior, J.; Butcher, W.; Thompson, I.; Shaw, A.; Titball, R. Conjugation of Y. pestis F1-antigen to gold nanoparticles improves immunogenicity. Vaccine 2012, 30, 6777–6782. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, M.K.; Nguyen, T.L.; Kim, J. Hollow mesoporous silica nanoparticles with extra-large mesopores for enhanced cancer vaccine. ACS Appl. Mater. Interfaces 2020, 12, 34658–34666. [Google Scholar] [CrossRef]
- Thalhauser, S.; Peterhoff, D.; Wagner, R.; Breunig, M. Presentation of HIV-1 envelope trimers on the surface of silica nanoparticles. J. Pharm. Sci. 2020, 109, 911–921. [Google Scholar] [CrossRef]
- Giri, S.S.; Kim, S.G.; Kang, J.W.; Kwon, J.; Bin Lee, S.; Jung, W.J.; Park, S.C. Applications of carbon nanotubes and polymeric micro-/nanoparticles in fish vaccine delivery: Progress and future perspectives. Rev. Aquac. 2021. [Google Scholar] [CrossRef]
- Hu, F.; Li, Y.; Wang, Q.; Wang, G.; Zhu, B.; Wang, Y.; Zeng, W.; Yin, J.; Liu, C.; Bergmann, S.M.; et al. Carbon nanotube-based DNA vaccine against koi herpesvirus given by intramuscular injection. Fish Shellfish Immunol. 2020, 98, 810–818. [Google Scholar] [CrossRef]
- Qiu, D.-K.; Jia, Y.-J.; Gong, Y.-M.; Zheng, Y.-Y.; Wang, G.-X.; Zhu, B. Optimizing the immunization procedure of single-walled carbon nanotubes based vaccine against grass carp reovirus for grass carp. Aquaculture 2021, 533, 736152. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Lin, Q.; Li, N.-Q.; Huang, Z.-B.; Zhao, M.; Fu, X.-Z.; Wang, G.-X.; Zhu, B. Single-walled carbon nanotubes as delivery vehicles enhance the immunoprotective effect of an immersion DNA vaccine against infectious spleen and kidney necrosis virus in mandarin fish. Fish Shellfish Immunol. 2020, 97, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-R.; Zhao, Z.; Zhang, C.; Jia, Y.-J.; Qiu, D.-K.; Zhu, B.; Wang, G.-X. Carbon nanotubes-loaded subunit vaccine can increase protective immunity against rhabdovirus infections of largemouth bass (Micropterus Salmoides). Fish Shellfish Immunol. 2020, 99, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-J.; Guo, Z.-R.; Qiu, D.-K.; Zhao, Z.; Wang, G.-X.; Zhu, B. Immune efficacy of carbon nanotubes recombinant subunit vaccine against largemouth bass ulcerative syndrome virus. Fish Shellfish Immunol. 2020, 100, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Duan, J.; Kong, H.; Li, L.; Wang, C.; Xie, S.; Chen, S.; Gu, N.; Xu, H.; Yang, X.-D. Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small 2008, 4, 1364–1370. [Google Scholar] [CrossRef]

| Technique | Advantages | Limitations |
|---|---|---|
| Copper-catalyzed azide-alkyne cycloaddition |
|
|
| Strain-promoted azide-alkyne cycloaddition |
|
|
| Carbodiimide chemistry |
|
|
| N-hydroxysuccinimide (NHS) chemistry |
|
|
| Glutaraldehyde chemistry |
|
|
| Maleimide–thiol chemistry |
|
|
| Thiol-disulfide exchange |
|
|
| Tresyl chloride activation |
|
|
| Isothiocyanate chemistry |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Duong, V.T.; Shalash, A.O.; Skwarczynski, M.; Toth, I. Chemical Conjugation Strategies for the Development of Protein-Based Subunit Nanovaccines. Vaccines 2021, 9, 563. https://doi.org/10.3390/vaccines9060563
Lu L, Duong VT, Shalash AO, Skwarczynski M, Toth I. Chemical Conjugation Strategies for the Development of Protein-Based Subunit Nanovaccines. Vaccines. 2021; 9(6):563. https://doi.org/10.3390/vaccines9060563
Chicago/Turabian StyleLu, Lantian, Viet Tram Duong, Ahmed O. Shalash, Mariusz Skwarczynski, and Istvan Toth. 2021. "Chemical Conjugation Strategies for the Development of Protein-Based Subunit Nanovaccines" Vaccines 9, no. 6: 563. https://doi.org/10.3390/vaccines9060563
APA StyleLu, L., Duong, V. T., Shalash, A. O., Skwarczynski, M., & Toth, I. (2021). Chemical Conjugation Strategies for the Development of Protein-Based Subunit Nanovaccines. Vaccines, 9(6), 563. https://doi.org/10.3390/vaccines9060563









