The Relevance of Monoclonal Antibodies in the Treatment of COVID-19
Abstract
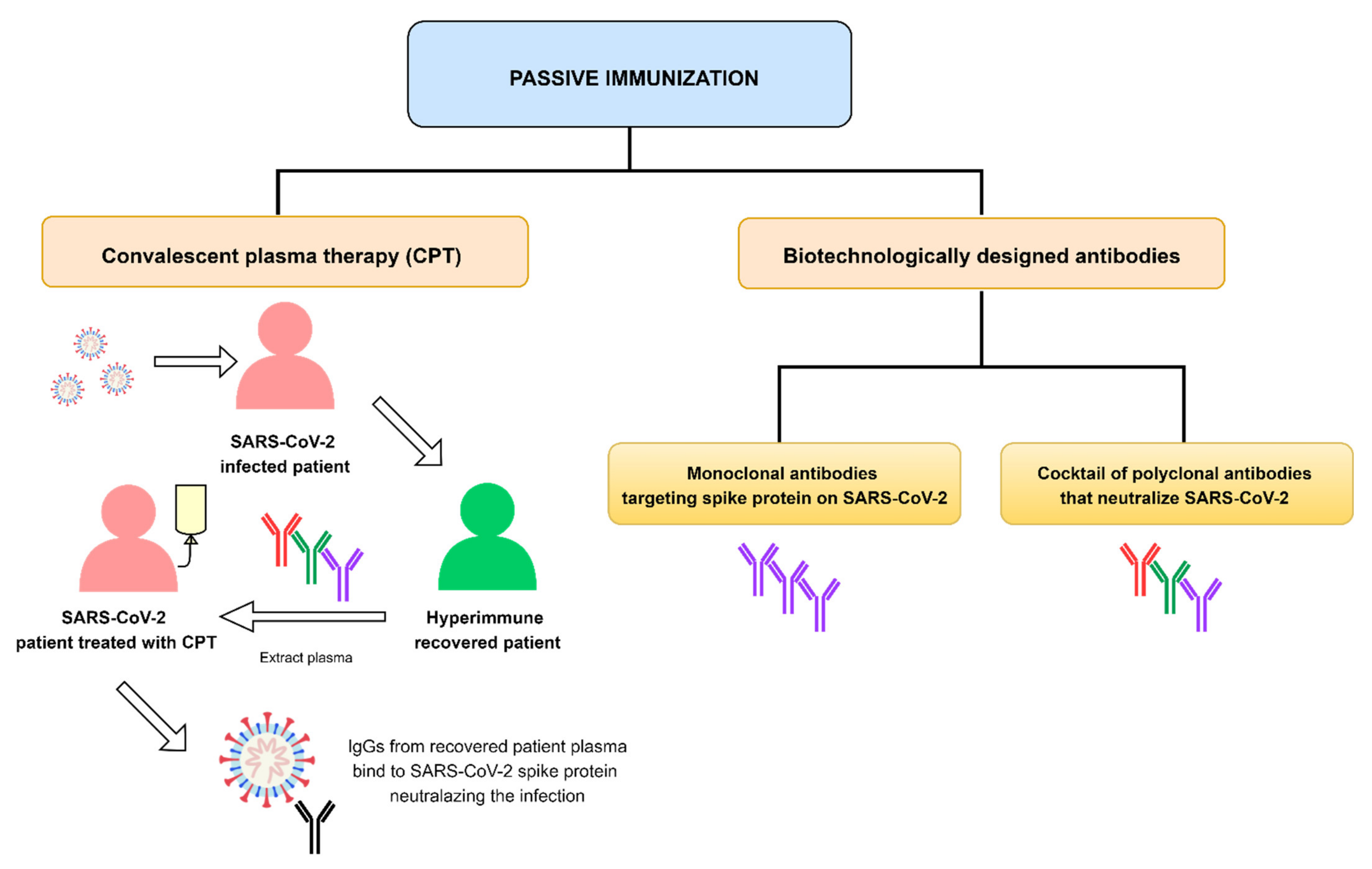
1. Introduction
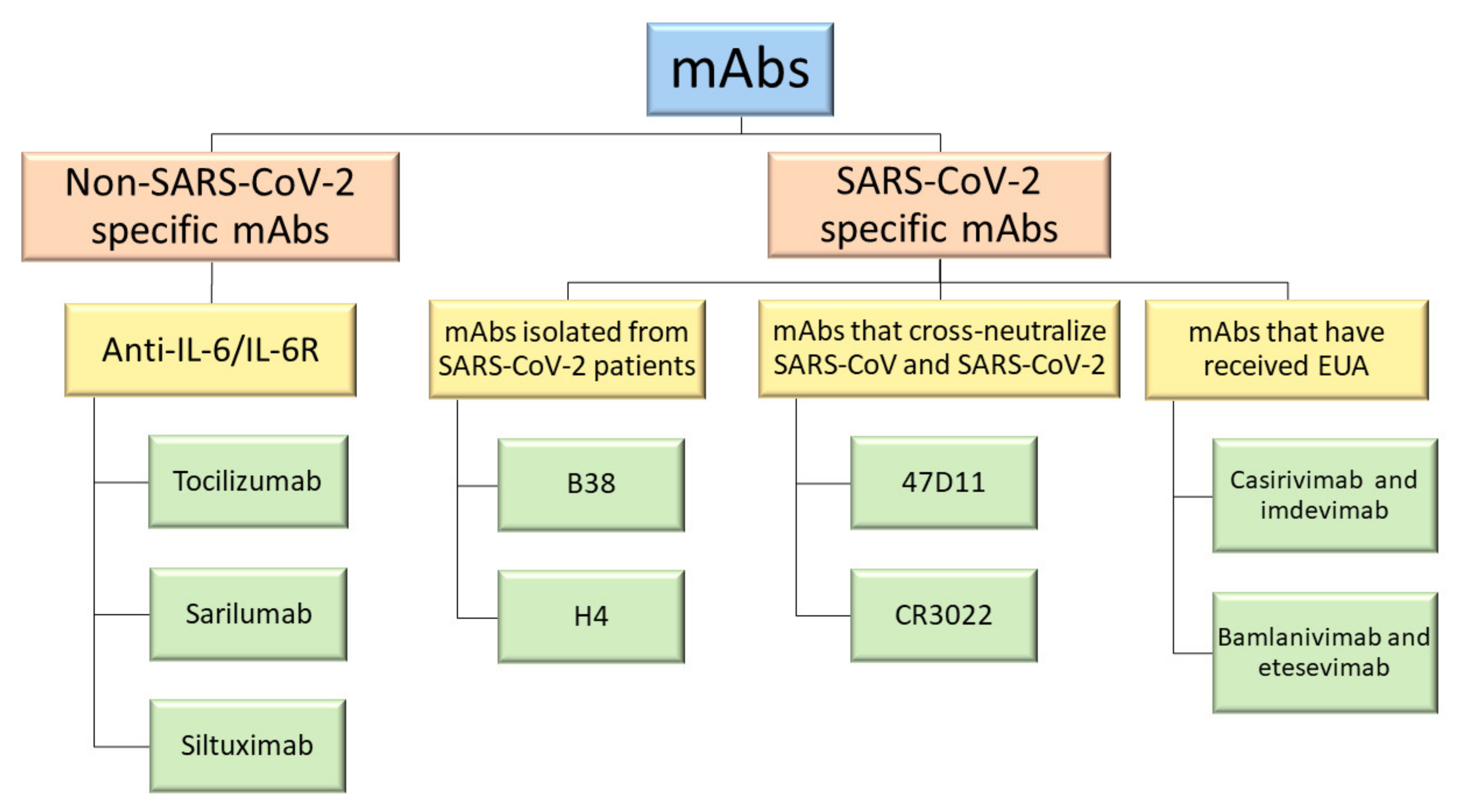
2. Non-SARS-CoV-2 Specific Monoclonal Antibodies
2.1. Tocilizumab (TCZ)
2.2. Sarilumab
2.3. Siltuximab
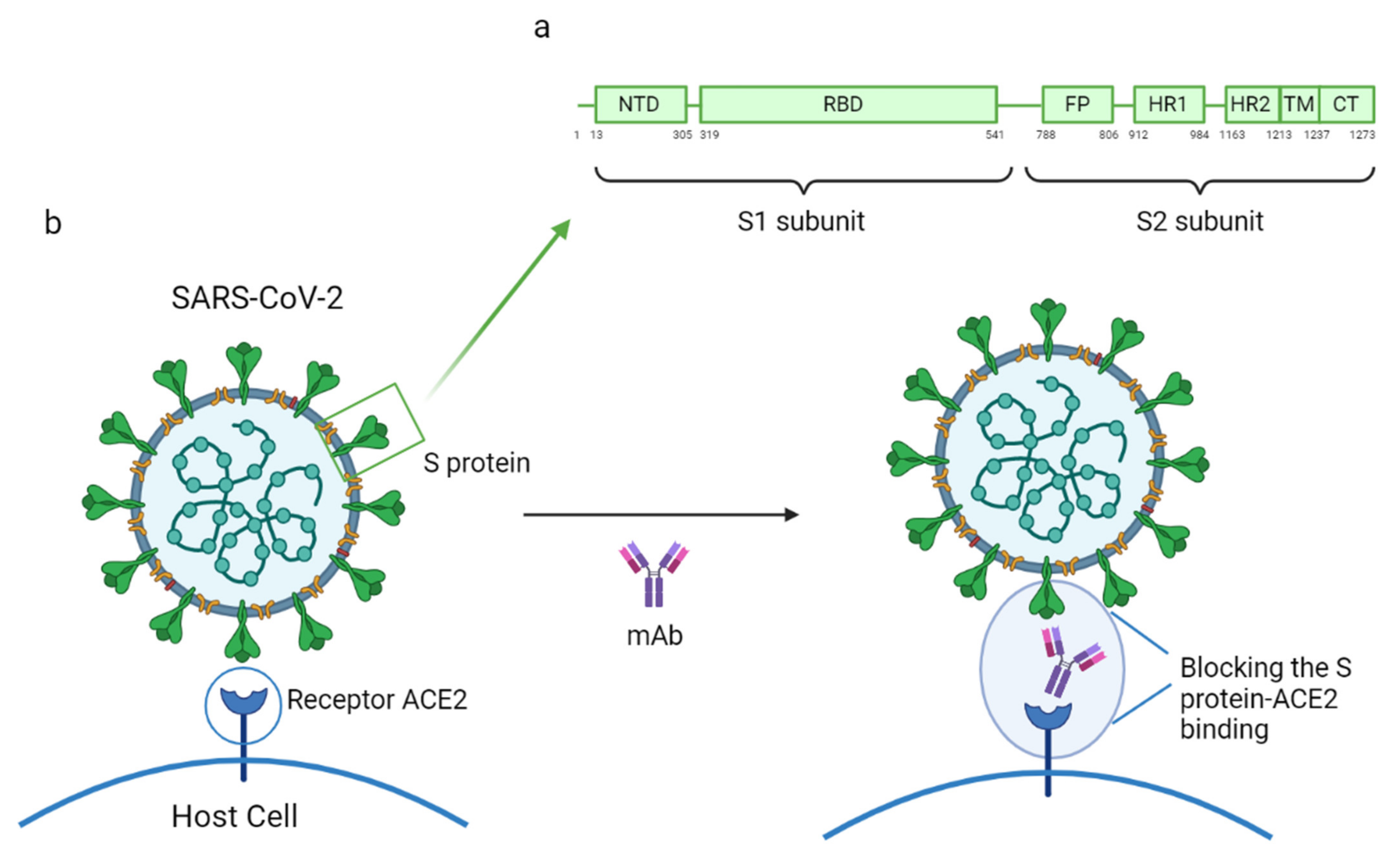
3. SARS-CoV-2 Specific Monoclonal Antibodies
3.1. mAbs Isolated from SARS-CoV-2 Patients
3.2. mAbs That Cross-Neutralize SARS-CoV and SARS-CoV-2
3.3. mAbs That Have Received Emergency Use Authorization (EUA)
3.4. How SARS-CoV-2 Mutations Could Affect the Efficacy of the Treatment with mAbs
4. Expert Opinion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Renn, A.; Fu, Y.; Hu, X.; Hall, M.D.; Simeonov, A. Fruitful Neutralizing Antibody Pipeline Brings Hope To Defeat SARS-Cov-2. Trends Pharmacol. Sci. 2020, 41, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Cantini, F.; Goletti, D.; Petrone, L.; Najafi Fard, S.; Niccoli, L.; Foti, R. Immune Therapy, or Antiviral Therapy, or Both for COVID-19: A Systematic Review. Drugs 2020, 80, 1929–1946. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.-G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Horby, P.W.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Emberson, J.; Palfreeman, A.; Raw, J.; Elmahi, E.; Prudon, B.; et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2020, 396, 1345–1352. [Google Scholar] [CrossRef]
- Owji, H.; Negahdaripour, M.; Hajighahramani, N. Immunotherapeutic approaches to curtail COVID-19. Int. Immunopharmacol. 2020, 88, 106924. [Google Scholar] [CrossRef]
- Jahanshahlu, L.; Rezaei, N. Monoclonal antibody as a potential anti-COVID-19. Biomed. Pharmacother. 2020, 129, 110337. [Google Scholar] [CrossRef]
- An, Z. Therapeutic Monoclonal Antibodies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 9780470485408. [Google Scholar]
- Zarkali, A.; Karageorgopoulos, D.E.; Rafailidis, P.I.; Falagas, M.E. Frequency of the off-label use of monoclonal antibodies in clinical practice: A systematic review of the literature. Curr. Med. Res. Opin. 2014, 30, 471–480. [Google Scholar] [CrossRef]
- Brechner, R.J.; Rosenfeld, P.J.; Babish, J.D.; Caplan, S. Pharmacotherapy for Neovascular Age-Related Macular Degeneration: An Analysis of the 100% 2008 Medicare Fee-For-Service Part B Claims File. Am. J. Ophthalmol. 2011, 151, 887–895. [Google Scholar] [CrossRef]
- DeFrancesco, L. COVID-19 antibodies on trial. Nat. Biotechnol. 2020, 38, 1242–1252. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- European Medicines Agency. Treatments and Vaccines for COVID-19—European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines-covid-19#research-and-development-section (accessed on 16 March 2021).
- Khan, F.A.; Stewart, I.; Fabbri, L.; Moss, S.; Robinson, K.; Smyth, A.R.; Jenkins, G. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021, 1–13. [Google Scholar] [CrossRef]
- European Medicines Agency. Tocilizumab: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/roactemra-epar-product-information_en.pdf (accessed on 17 March 2021).
- Fazakerley, I. COVID-19 Therapeutic Alert: Interleukin-6 Inhibitors (Tocilizumab or Sarilumab) for Patients Admitted to ICU with COVID-19 Pnuemonia (Adults). Available online: https://www.sehd.scot.nhs.uk/publications/DC20210108interleukin-6_inhibitors.pdf (accessed on 16 March 2021).
- ClinicalTrials.gov Home Page. Available online: https://clinicaltrials.gov/ (accessed on 16 March 2021).
- Boregowda, U.; Perisetti, A.; Nanjappa, A.; Gajendran, M.; Kutti Sridharan, G.; Goyal, H. Addition of Tocilizumab to the Standard of Care Reduces Mortality in Severe COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 586221. [Google Scholar] [CrossRef]
- Berardicurti, O.; Ruscitti, P.; Ursini, F.; D’Andrea, S.; Ciaffi, J.; Meliconi, R.; Iagnocco, A.; Cipriani, P.; Giacomelli, R. Mortality in tocilizumab-treated patients with COVID-19: A systematic review and meta-analysis. Clin. Exp. Rheumatol. 2020, 38, 1247–1254. [Google Scholar]
- Cortegiani, A.; Ippolito, M.; Greco, M.; Granone, V.; Protti, A.; Gregoretti, C.; Giarratano, A.; Einav, S.; Cecconi, M. Rationale and evidence on the use of tocilizumab in COVID-19: A systematic review. Pulmonology 2021, 27, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S. The effect of tocilizumab on mortality in hospitalized patients with COVID-19: A meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2021, 1–6. [Google Scholar] [CrossRef]
- COVID-19 Rapid Evidence Summary: Tocilizumab for COVID-19. Available online: https://www.nice.org.uk/advice/es33/chapter/Factors-for-decision-making (accessed on 16 March 2021).
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Hermine, O.; Mariette, X.; Tharaux, P.-L.; Resche-Rigon, M.; Porcher, R.; Ravaud, P.; Bureau, S.; Dougados, M.; Tibi, A.; Azoulay, E.; et al. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia. JAMA Intern. Med. 2021, 181, 32. [Google Scholar] [CrossRef]
- Stone, J.H.; Frigault, M.J.; Serling-Boyd, N.J.; Fernandes, A.D.; Harvey, L.; Foulkes, A.S.; Horick, N.K.; Healy, B.C.; Shah, R.; Bensaci, A.M.; et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N. Engl. J. Med. 2020, 383, 2333–2344. [Google Scholar] [CrossRef]
- Salvarani, C.; Dolci, G.; Massari, M.; Merlo, D.F.; Cavuto, S.; Savoldi, L.; Bruzzi, P.; Boni, F.; Braglia, L.; Turrà, C.; et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia. JAMA Intern. Med. 2021, 181, 24. [Google Scholar] [CrossRef]
- Veiga, V.C.; Prats, J.A.G.G.; Farias, D.L.C.; Rosa, R.G.; Dourado, L.K.; Zampieri, F.G.; Machado, F.R.; Lopes, R.D.; Berwanger, O.; Azevedo, L.C.P.; et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: Randomised controlled trial. BMJ 2021, 372, n84. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; Van Bentum-Puijk, W.; Berry, L.R.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.W.; Campbell, M.; Staplin, N.; Spata, E.; Emberson, J.; Pessoa-Amorim, G.; Peto, L. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): Preliminary results of a randomised, controlled, open-label, platform trial. medRxiv 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Soin, A.S.; Kumar, K.; Choudhary, N.S.; Sharma, P.; Mehta, Y.; Kataria, S.; Govil, D.; Deswal, V.; Chaudhry, D.; Singh, P.K.; et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): An open-label, multicenter, randomised, controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 511–521. [Google Scholar] [CrossRef]
- European Medicines Agency. Sarilumab: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/kevzara-epar-product-information_en.pdf (accessed on 17 March 2021).
- Ucciferri, C.; Vecchiet, J.; Falasca, K. Role of monoclonal antibody drugs in the treatment of COVID-19. World J. Clin. Cases 2020, 8, 4280–4285. [Google Scholar] [CrossRef] [PubMed]
- Khiali, S.; Rezagholizadeh, A.; Entezari-Maleki, T. A comprehensive review on sarilumab in COVID-19. Expert Opin. Biol. Ther. 2020, 21, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Giannasi, G.; Cecchini, P.; Gobbi, F.L.; Damiani, A.; Grossi, V.; Infantino, M.; Manfredi, M. COVID-19 pneumonia treated with Sarilumab: A clinical series of eight patients. J. Med. Virol. 2020, 92, 2368–2370. [Google Scholar] [CrossRef] [PubMed]
- Gremese, E.; Cingolani, A.; Bosello, S.L.; Alivernini, S.; Tolusso, B.; Perniola, S.; Landi, F.; Pompili, M.; Murri, R.; Santoliquido, A.; et al. Sarilumab use in severe SARS-CoV-2 pneumonia. medRxiv 2020, 27, 1–8. [Google Scholar] [CrossRef]
- Della-Torre, E.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Napolitano, A.; La Marca, S.; Boffini, N.; Da Prat, V.; Di Terlizzi, G.; Lanzillotta, M.; et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: An open-label cohort study. Ann. Rheum. Dis. 2020, 79, 1277–1285. [Google Scholar] [CrossRef]
- Montesarchio, V.; Parella, R.; Iommelli, C.; Bianco, A.; Manzillo, E.; Fraganza, F.; Palumbo, C.; Rea, G.; Murino, P.; De Rosa, R.; et al. Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy. J. Immunother. Cancer 2020, 8, e001089. [Google Scholar] [CrossRef]
- Castelnovo, L.; Tamburello, A.; Lurati, A.; Zaccara, E.; Marrazza, M.G.; Olivetti, M.; Mumoli, N.; Mastroiacovo, D.; Colombo, D.; Ricchiuti, E.; et al. Anti-IL6 treatment of serious COVID-19 disease. Medicine 2021, 100, e23582. [Google Scholar] [CrossRef]
- Kevzara Fails in PhIII COVID-19 Trial—PharmaTimes. Available online: http://www.pharmatimes.com/news/kevzara_fails_in_phiii_covid-19_trial_1347570 (accessed on 17 March 2021).
- COVID-19 Rapid Evidence Summary: Sarilumab for COVID-19. Available online: https://www.nice.org.uk/advice/es34/chapter/Factors-for-decision-making (accessed on 17 March 2021).
- Lescure, F.-X.; Honda, H.; Fowler, R.A.; Lazar, J.S.; Shi, G.; Wung, P.; Patel, N.; Hagino, O.; Bazzalo, I.J.; Casas, M.M.; et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 522–532. [Google Scholar] [CrossRef]
- European Medicines Agency. Siltuximab: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/sylvant-epar-product-information_en.pdf (accessed on 17 March 2021).
- Gritti, G.; Raimondi, F.; Ripamonti, D.; Riva, I.; Landi, F.; Alborghetti, L.; Frigeni, M.; Damiani, M.; Micò, C.; Fagiuoli, S.; et al. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: An observational cohort study. medRxiv 2020. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.; Xu, W.; Liu, S. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Valdez-Cruz, N.A.; García-Hernández, E.; Espitia, C.; Cobos-Marín, L.; Altamirano, C.; Bando-Campos, C.G.; Cofas-Vargas, L.F.; Coronado-Aceves, E.W.; González-Hernández, R.A.; Hernández-Peralta, P.; et al. Integrative overview of antibodies against SARS-CoV-2 and their possible applications in COVID-19 prophylaxis and treatment. Microb. Cell Fact. 2021, 20, 88. [Google Scholar] [CrossRef]
- Marovich, M.; Mascola, J.R.; Cohen, M.S. Monoclonal Antibodies for Prevention and Treatment of COVID-19. JAMA 2020, 324, 131. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef]
- Libster, R.; Pérez Marc, G.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Duyvesteyn, H.M.E.; Chen, C.-P.; Huang, C.-G.; Chen, T.-H.; Shih, S.-R.; Lin, Y.-C.; Cheng, C.-Y.; Cheng, S.-H.; Huang, Y.-C.; et al. Structural basis for the neutralization of SARS-CoV-2 by an antibody from a convalescent patient. Nat. Struct. Mol. Biol. 2020, 27, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Ho, M. Perspectives on the development of neutralizing antibodies against SARS-CoV-2. Antib. Ther. 2020, 3, 109–114. [Google Scholar] [CrossRef]
- Ku, Z.; Ye, X.; Salazar, G.T.; Zhang, N.; An, Z. Antibody therapies for the treatment of COVID-19. Antib. Ther. 2020, 3, 101–108. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; van Haperen, R.; Osterhaus, A.D.M.E.; van Kuppeveld, F.J.M.; Haagmans, B.L.; Grosveld, F.; Bosch, B.-J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020, 11, 2251. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, C.; Huang, A.; Xia, S.; Lu, S.; Shi, Z.; Lu, L.; Jiang, S.; Yang, Z.; Wu, Y.; et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020, 9, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.-C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, A.G.; Benton, D.J.; Hussain, S.; Harvey, R.; Martin, S.R.; Roustan, C.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Antibody-mediated disruption of the SARS-CoV-2 spike glycoprotein. Nat. Commun. 2020, 11, 5337. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Yuan, M.; Bangaru, S.; Huang, D.; Zhu, X.; Lee, C.-C.D.; Turner, H.L.; Peng, L.; Yang, L.; Burton, D.R.; et al. A natural mutation between SARS-CoV-2 and SARS-CoV determines neutralization by a cross-reactive antibody. PLoS Pathog. 2020, 16, e1009089. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; Slein, M.D.; Fischinger, S.; Burke, J.; Schäfer, A.; Leist, S.R.; Kuzmina, N.A.; Mire, C.; Honko, A.; Johnson, R.; et al. Dissecting strategies to tune the therapeutic potential of SARS-CoV-2–specific monoclonal antibody CR3022. JCI Insight 2021, 6, e143129. [Google Scholar] [CrossRef]
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 (accessed on 17 March 2021).
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 (accessed on 17 March 2021).
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. 2021. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0 (accessed on 17 March 2021).
- Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab Authorized Use. Available online: https://www.fda.gov/media/143603/download (accessed on 17 March 2021).
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 229–237. [Google Scholar] [CrossRef]
- Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab Authorized Use. Available online: https://www.fda.gov/media/143892/download (accessed on 17 March 2021).
- Baum, A.; Ajithdoss, D.; Copin, R.; Zhou, A.; Lanza, K.; Negron, N.; Ni, M.; Wei, Y.; Mohammadi, K.; Musser, B.; et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 2020, 370, 1110–1115. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab Authorized Use. Available online: https://www.fda.gov/media/145802/download (accessed on 17 March 2021).
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef]
- Bamlanivimab Revocation Letter. Available online: https://www.fda.gov/media/147629/download (accessed on 19 April 2021).
- Frequently Asked Questions on the Emergency Use Authorization for Bamlanivimab. Available online: https://www.fda.gov/media/143605/download (accessed on 17 March 2021).
- Frequently Asked Questions on the Emergency Use Authorization of Casirivimab and Imdevimab. Available online: https://www.fda.gov/media/143894/download (accessed on 17 March 2021).
- Frequently Asked Questions on the Emergency Use Authorization for Bamlanivimab and Etesevimab. Available online: https://www.fda.gov/media/145808/download (accessed on 17 March 2021).
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; VanBlargan, L.A.; Bloyet, L.-M.; Rothlauf, P.W.; Chen, R.E.; Stumpf, S.; Zhao, H.; Errico, J.M.; Theel, E.S.; Liebeskind, M.J.; et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe 2021, 29, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; Graham, B.S.; et al. Increased Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7 to Antibody Neutralization. bioRxiv 2021. [Google Scholar] [CrossRef]
| Drug | Authors | Design | Patients Enrolled | Regimen | Primary Outcomes | Main Findings |
|---|---|---|---|---|---|---|
| TCZ | Stone et al. | Prospective, multicenter, randomized, double blind, placebo-controlled trial | 243 (162 TCZ group; 81 placebo group) | Standard care plus a single dose of either TCZ (8 mg/kg, IV, max 800 mg) or placebo | Intubation or death, assessed in a time-to-event analysis | TCZ was not effective in preventing intubation or death in moderately ill hospitalized patients with COVID-19 |
| Rosas et al. | Randomized, double-blind, placebo-controlled, multicenter study | 452 (294 TCZ group; 144 placebo group) | A single IV infusion of TCZ (8 mg/kg, max 800 mg) or placebo plus standard care. A second infusion of TCZ or placebo could be administered 8 to 24 h after the first dose | Clinical status at day 28 on an ordinal scale ranging from 1 (discharged or ready for discharge) to 7 (death) | Administration of TCZ did not result in significantly better clinical status or lower mortality than placebo at day 28 | |
| Salvarani et al. | Open-label randomized multicenter study | 126 (60 TCZ group; 66 control group) | IV TCZ (8 mg/kg infusion, Max 800 mg) within 8 h from randomization, followed by a second dose after 12 h | Admission to the intensive care unit with invasive mechanical ventilation, death from all causes, or clinical aggravation documented by the finding of a PaO2/FIO2 ratio of less than 150 mmHg | No benefits in terms of disease progression were observed compared with standard care | |
| Hermine et al. | Cohort-embedded, investigator-initiated, multicenter, open-label, bayesian randomized clinical trial | 131 (64 TCZ group; 67 usual care group) | IV TCZ (8 mg/kg infusion, max 800 mg) on day 1, with additional fixed dose of 400 mg as intravenous infusion on day 3 if required | Scores higher than 5 on the World Health Organization 10-point Clinical Progression Scale (WHO-CPS) on day 4 and survival without need for ventilation (including non-invasive ventilation) at day 14 | TCZ did not reduce WHO-CPS scores to less than 5 at day 4 but might have reduced the risk of NIV, MV, or death by day 14. No difference was found in mortality on day 28 | |
| Salama et al. | Randomized, double-blind, placebo-controlled, multicenter clinical trial | 389 (249 TCZ group; 128 placebo group) | IV TCZ (8 mg/kg infusion, max 800 mg), with a second dose 8–24 h later if required) | Mechanical ventilation (invasive mechanical ventilation or extracorporeal membrane oxygenation) or death at day 28 | TCZ reduced the likelihood of progression to the composite outcome of mechanical ventilation or death, but it did not improve survival | |
| Gordon et al. | Open-label, randomized, multifactorial, adaptive platform trial | 747 (350 TCZ group; 397 control group) | TCZ (8 mg/kg, max 800 mg), was administered as an IV infusion over one hour; this dose could be repeated 12–24 h later at the discretion of the treating clinician | The primary outcome was an ordinal scale combining in-hospital mortality (assigned−1) and days free of organ support to day 21 | In critically ill patients with COVID-19 receiving organ support in intensive care, treatment with TCZ improved outcome, including survival | |
| Veiga et al. | Multicenter, randomized, open label, parallel group, superiority trial | 129 (65 TCZ group; 64 standard care group) | TCZ was administered as a single IV infusion at a dose of 8 mg/kg (max 800 mg) | The primary outcome, clinical status measured at 15 days using a seven- level ordinal scale, was analyzed as a composite of death or mechanical ventilation because the assumption of odds proportionality was not met | In patients with severe or critical COVID-19, TCZ plus standard care did not achieve better results than standard care alone in clinical outcomes at 15 days, and it might increase mortality | |
| Soin et al. | Open-label, multicenter, randomized, controlled, phase 3 trial | 180 (90 TCZ group; 90 standard care group) | A single IV infusion at 6 mg/kg up to a maximum dose of 480 mg. An additional dose of 6 mg/kg (max 480 mg/kg) could be administered if required | The primary efficacy endpoint was the proportion of patients with progression of COVID-19 from moderate to severe or from severe to death up to day 14 | Routine use of TCZ in patients admitted to hospital with moderate to severe COVID-19 is not supported. However, post-hoc evidence from this study suggests TCZ might still be effective in patients with severe COVID-19 and so should be investigated further in future studies | |
| Sarilumab | Gordon et al. | Open-label, randomized, multifactorial, adaptive platform trial | 442 (45 sarilumab group; 397 control group) | Sarilumab (400 mg) was administered once only as an IV infusion | The primary outcome was an ordinal scale combining in-hospital mortality (assigned−1) and days free of organ support to day 21 | In critically ill patients with COVID-19 receiving organ support in intensive care, treatment with sarilumab, improved outcome, including survival |
| Lescure et al. | Multinational, randomized, adaptive, phase 3, double-blind, placebo-controlled trial | 416 (159 sarilumab 200 mg; 173 sarilumab 400 mg; 84 placebo) | Sarilumab 200 mg, 400 mg or placebo were administered as an IV infusion. A second dose could be administered within 24–48 h of the first dose if required | The primary endpoint was time to ≥2-point clinical improvement (7-point scale; range: 1 (death) to 7 (not hospitalized)) | The efficacy of sarilumab was not demonstrated in patients hospitalized with COVID-19 and receiving supplemental oxygen |
| Groups of Specific mAbs | Name | Binding Site and Mechanism of Action |
|---|---|---|
| MAbs isolated from SARS-CoV-2 patients | B5 | SARS-CoV-2 RBD; partial competition with ACE2 |
| B38 | SARS-CoV-2 RBD; complete competition with ACE2 | |
| H2 | SARS-CoV-2 RBD; no competition with ACE2 | |
| H4 | SARS-CoV-2 RBD; complete competition with ACE2 | |
| EY6A | SARS-CoV-2 RBD and SARS-CoV RBD with lower affinity; site spatially separate from that of ACE2 | |
| MAbs that cross-neutralize SARS-CoV and SARS-CoV-2 | 47D11 | SARS-CoV-2 and SARS-CoV RBD; conserved epitope in the RBD |
| CR3022 | SARS-CoV RBD and SARS-CoV-2 RBD with lower affinity; conserved epitope in the RBD. Do not neutralize SARS-CoV-2 | |
| MAbs that have received Emergency Use Authorization (EUA) | Bamlanivimab (LY-CoV555) | SARS-CoV-2 RBD; EUA revoked |
| Casirivimab (REGN10933) and imdevimab (REGN10987) in a combined therapy | Non-overlapping epitopes of the SARS-CoV-2 RBD | |
| Bamlanivimab (LY-CoV555) and etesevimab (LY-CoV016) in a combined therapy | Different, but overlapping, epitopes of the SARS-CoV-2 RBD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrente-López, A.; Hermosilla, J.; Navas, N.; Cuadros-Rodríguez, L.; Cabeza, J.; Salmerón-García, A. The Relevance of Monoclonal Antibodies in the Treatment of COVID-19. Vaccines 2021, 9, 557. https://doi.org/10.3390/vaccines9060557
Torrente-López A, Hermosilla J, Navas N, Cuadros-Rodríguez L, Cabeza J, Salmerón-García A. The Relevance of Monoclonal Antibodies in the Treatment of COVID-19. Vaccines. 2021; 9(6):557. https://doi.org/10.3390/vaccines9060557
Chicago/Turabian StyleTorrente-López, Anabel, Jesús Hermosilla, Natalia Navas, Luis Cuadros-Rodríguez, José Cabeza, and Antonio Salmerón-García. 2021. "The Relevance of Monoclonal Antibodies in the Treatment of COVID-19" Vaccines 9, no. 6: 557. https://doi.org/10.3390/vaccines9060557
APA StyleTorrente-López, A., Hermosilla, J., Navas, N., Cuadros-Rodríguez, L., Cabeza, J., & Salmerón-García, A. (2021). The Relevance of Monoclonal Antibodies in the Treatment of COVID-19. Vaccines, 9(6), 557. https://doi.org/10.3390/vaccines9060557






