1. Introduction
Glaesserella parasuis, formerly known as Haemophilus parasuis, is a rod shaped, Gram-negative proteobacteria from the Pasteurellaceae family that is commonly found in the nasal microbiota of pigs. G. parasuis is a highly heterogeneous species, comprising commensal and virulent strains. Virulent strains can cause polyserositis, polyarthritis and meningitis, a pathological disorder called Glässer’s disease that is more prevalent in young piglets, especially in the nursery period.
The most commonly used treatment for Glässer’s disease and other bacterial diseases affecting piglets are antimicrobials. However, increasing concern about antimicrobial resistance arising from the livestock industry is promoting the research of alternative control tools, where vaccines have a relevant role [
1].
G. parasuis is an extracellular pathogen, and the induction of opsonizing antibodies (for example, by vaccination) is essential for protection against disease [
2,
3]. Prevention of Glässer’s disease may be achieved with attenuated bacteria, inactivated bacterins and subunit vaccines [
4]. However, commercial bacterins do not provide protection against all
G. parasuis strains, promoting the search for alternative vaccines that could confer cross-protection among virulent strains of different serovars. Several vaccine candidates have been proposed in the literature [
4], including virulence factors such as the virulence-associated trimeric autotransporters (VtaA), which are involved in phagocytosis resistance [
5] and adhesion to extracellular matrix proteins [
6]. In a previous study from our group, immunization with a combination of six VtaA proteins provided partial protection against a lethal infection with a virulent
G. parasuis strain in colostrum deprived piglets [
7]. A detailed study of the sequences of these proteins identified a peptide (F4) that was present in the VtaAs associated with virulent strains and was exposed on the bacterial surface [
8], supporting its potential as a vaccine candidate.
In swine, maternal immunity is transferred to the litter via colostrum and progressively decreases with the age of the piglets, while the maturation of the immune system of the piglet is not yet complete [
8,
9]. Sow vaccination increases the level of colostrum antibodies and, subsequently, the period when maternal antibodies are maintained at a detectable level in their offspring [
10,
11]. For this reason, together with the short window available for piglet vaccination and their immature immune system, maternal immunization can be an option to prevent diseases early in life [
12,
13,
14,
15].
Here, we evaluate the effect on the offspring of sow vaccination using adjuvanted F4 peptide as an immunogen and the protection observed in the piglets after an intranasal challenge with two different virulent strains of G. parasuis.
2. Materials and Methods
2.1. Vaccine and Challenge Strains
The protein fragment F4, consisting of amino acids 701 to 874 from the trimeric autotransporter VtaA9 from
G. parasuis [
8], was used as an immunogen in combination with a carbomer-based adjuvant. Vaccine formulation was composed of the purified F4 fragment mixed with Carbopol 5984 EP Polymer (1:9) (Lubrizol, Cleveland, OH, USA) to achieve a final concentration of 100 µg/mL of F4 peptide (#F4 API VACSUIS, WorldPathol, Zaragoza, Spain).
G. parasuis strains Nagasaki (virulent reference strain serovar 5 [SV5]) and P555/04 (clinical isolate from pericardium, serovar 13 [SV13], provided by Dr. Zielinski from INTA-Argentina), were grown overnight on chocolate agar plates (Biomérieux, Marcy-L’Étoile, France) at 37 °C 5% CO2 and were used for the intranasal inoculation of piglets.
2.2. Animal Study
Animal experimentation was performed in the BSL3 facilities of IRTA-CReSA (Bellaterra, Spain) following proper veterinary practices, in accordance with European (Directive 2010/63/EU) and Spanish (Real Decreto 53/2013) regulation and with the approval of the Ethics Commission in Animal Experimentation of the Generalitat de Catalunya (Protocol number 9211). The experimental procedure is represented in
Figure 1. Four pregnant sows were selected on the basis of low antibody levels against
G. parasuis using the commercial ELISA Ingezim–Haemophilus (Ingenasa, Madrid, Spain). Two sows were vaccinated at 32 and 12 days before farrowing with 2.5 mL of the F4 vaccine, while the other two sows remained unvaccinated as controls. Serum samples were taken from the sows at each time of vaccination and at delivery. In addition, colostrum samples were taken at delivery. After birth, piglets suckled from their mother for 1 day and then the litters were cross-fostered within each experimental group. One vaccinated sow (with five of its own piglets and six from the other vaccinated sow) and one nonvaccinated control sow (with nine of its own piglets and eight from the other control sow) remained in the study. Sows were removed from the study at day 18 (D18). At 1, 2 and 3 weeks of age, nasal samples were taken from the piglets for
G. parasuis detection. At 1, 2 and 3 weeks, serum samples were also obtained. One day later (D22), piglets were divided into two groups for the challenge, with piglets from the different biological sows in each group. Thus, nine piglets from nonvaccinated sows and six from vaccinated sows were intranasally inoculated using an atomizer with 6 × 10
9 colony forming units (CFU) of Nagasaki (SV5) with a total inoculation volume of 1.5 mL per piglet, while eight piglets from nonvaccinated sows and five from vaccinated sows were intranasally inoculated with 3 × 10
9 CFU of P555/04 (SV13) in 1.5 mL (
Figure 1). After 16 (P555/04 group) and 17 (Nagasaki group) days of challenge, final sampling and necropsies were performed for pathological examination and samples from different tissues were taken for analysis. Serum and bronchoalveolar lavage fluid (BALF) were also taken for immunological analysis.
2.3. Clinical Signs and Pathological Assessment
Rectal temperatures, weight and clinical signs (particularly those compatible with Glässer’s disease) were evaluated after the challenge with G. parasuis. Fever was considered to be a rectal temperature above 40 °C. Clinical signs were scored daily for each piglet, and later, a global clinical score was used for analysis: 0 = no symptoms; 1 = clinical signs on 1 day; 2 = clinical signs on more than 1 day; 3 = severe clinical signs (euthanasia).
Gross lesions were assessed in necropsy and the presence of G. parasuis in different organs was determined by culturing samples from body cavities (pericardic, thoracic and abdominal), four joints and blood.
Samples from the lung, trachea and nasal turbinate were taken for histopathological examination. Formalin fixed tissues were embedded in paraffin and 3–5 µm sections were cut for haematoxylin-eosin staining. Assessment of lymphoplasmacytic inflammation was scored as: 0, absence; 1, mild; 2, moderate; 3, severe.
2.4. DNA Extraction and G. parasuis Detection by PCR
Nasal swabs were resuspended in 500 µL of PBS and 200 µL of the suspensions were processed using the Nucleospin Blood kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. For pure bacterial cultures, DNA was extracted using a Chelex based Instagene Matrix (Bio-Rad Laboratories, Hercules, CA, USA) following the manufacturer’s instructions.
G. parasuis was detected and differentiated into virulent and nonvirulent using a specific
vtaA leader sequence PCR (LS-PCR) [
16]. In some cases, additional molecular serotyping PCR was performed for serovars 5/12 and 13 [
17]. Four µL of the DNA extracted from the nasal swabs or two µL of the purified DNA from pure cultures were used as template for the reactions. DNA from strains Nagasaki (serovar 5, virulent), P555/04 (serovar 13, virulent) and SW114 (nonvirulent) were included as controls for the PCRs. Primers used in the PCRs can be found in
Table S1.
2.5. Antibody Response
Antibodies were measured using ELISA as follows: High binding plates were coated overnight at 4 °C with F4 protein (500 ng/well) in a carbonate–bicarbonate buffer. After washing, wells were blocked with 1% casein in phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBS-Tw). Sera and colostrum were diluted 1:1000 in blocking solution and BALF samples were used undiluted. After an incubation of 1h at 37 °C, a goat antiporcine IgG HRP-conjugated antibody (Sigma-Aldrich, Madrid, Spain) was used for IgG detection at a 1:10,000 dilution in blocking solution. For IgA detection in BALF, samples were tested undiluted and detected with an HRP-conjugated goat anti-porcine IgA antibody (AbD Serotec, Oxford, UK) diluted 1:10,000. Finally, positive reactions were developed using the 3,3,3,5-tetramethylbenzidine (TMB) substrate (Sigma-Aldrich, Madrid, Spain) and the reaction was stopped with 1N sulfuric acid. Plates were then read in a Power Wave XS spectrophotometer (Biotech, Winooski, VT, USA) at 450 nm.
2.6. Interleukin Determination
Serum levels of IL-8, IL-10, TNF-α and TGF-β were evaluated via ELISA using different products. Two matched antibody pairs for swine IL-10 and TNF-α from Kingfisher Biotech (Saint Paul, MN, USA) and an ELISA kit from the same manufacturer for porcine IL-8 were used. TGF-β was evaluated using a TGF-β Human Matched Antibody Pair kit from Invitrogen (Carlsbad, CA, USA), following the manufacturer’s instructions in all cases.
2.7. Surfactant Protein D (SP-D) Monoclonal Antibodies Production and SP-D Determination by ELISA
Surfactant protein D (SP-D) was determined via an in-house sandwich ELISA using a monoclonal antibody pair (capture and detection) from hybridoma supernatants, whose production was performed as follows. Briefly, lung SP-D was extracted and purified from the BALF of healthy piglets following previously described protocol [
18]. Hybridomas were generated by immunizing BALB/c mice with the purified SP-D using standard procedures described before [
19] and with the approval of the Ethics Commission in Animal Experimentation from the Generalitat de Catalunya (Approved protocol 5767). Two monoclonal antibodies were selected according to their strong signal against the SP-D antigen via indirect ELISA, and their isotype was determined using a mouse typer isotyping kit (Bio-Rad, Hercules, CA, USA), following the manufacturer’s indications. Selected clones were R-133 (IgG2b) (used as a capture antibody) and R-123 (IgG1) (used for detection).
For ELISA, high binding 96 well plates were coated with 50 µL of the capture antibody hybridoma supernatant R-133 diluted 1:1 in carbonate bicarbonate buffer overnight at 4 °C. After coating, plates were washed and blocked with blocking solution as above for 1h at 37 °C. After an incubation of 50 µL of undiluted BALF for 1 h at 37 °C, the plates were washed, and a detection antibody mixture, which consisted of a mixture 1:1 of the hybridoma supernatant R-123 with blocking buffer, was added to the wells. After incubation for 1 h at 37 °C and washes, ELISA plates were finally incubated for 1 h at 37 °C with an HRP-conjugated goat anti-mouse IgG1 antibody (Invitrogen, Carlsbad, CA, USA) diluted 1:1000. Finally, reaction was developed with TMB for 5–10 min, and stopped with 1N sulfuric acid before reading at 450 nm.
2.8. Statistical Analysis and Modelling
At first, the collected data were screened for unlikely or missing values before running any valid statistical analysis. No data were excluded on this basis. Subsequently, a descriptive statistical analysis was performed to the potential predictors related to piglets and sows in the experiment with the main variables of interest: clinical signs, biological sows, sow vaccination, challenge strain and colonization with virulent strains of G. parasuis (GP.VIR) or nonvirulent strains (GP.NVIR) at the end of the study.
Different statistical models of multilevel multivariable linear regression with repeated measurements were run for the following continuous outcome variables: IL-8, IL-10, TGF-β, antibody levels against F4, SP-D levels in BALF, rectal temperature, weight after challenge, IgG against F4 in BALF, total IgG in BALF and ratio of α-F4 IgG/total IgG. Sow-ID was accounted as a random effect in the different models. The outcome variable that showed skew pattern was, therefore, transformed by taking their natural logarithm or log10.
A univariable model analysis was carried out to test the unconditional associations between dependents and different independent variables of interest. Only independent variables with
p ≤ 0.25 in this initial screening were included in multivariable linear regression models [
20]. Before proceeding with building our multivariable model analysis, we checked for correlations between the retained independent variables. If two variables were highly correlated (R > 0.8), only the one with the lower
p value in the unconditional associations was retained.
The significant predictors from the univariable analysis were then offered to a multivariable model, and a manual backward elimination procedure was used to the least significant variable (
p > 0.05) if their exclusion from the model did not result in a greater than 30% change (confounding effect) in the effects of the remaining variables in the model [
20].
The generated final model included only variables with a p value ≤ 0.05. In this model, two-way interactions between the remaining significant variables were investigated and were retained if significant. The p value and regression coefficient (b) with a 95% confidence interval (95% CI) were recorded for each variable. In all statistical analyses, the results were considered to be significant with a p ≤ 0.05.
All statistical analyses were conducted using R version 3.3.3 (R Core Team, 2015).
4. Discussion
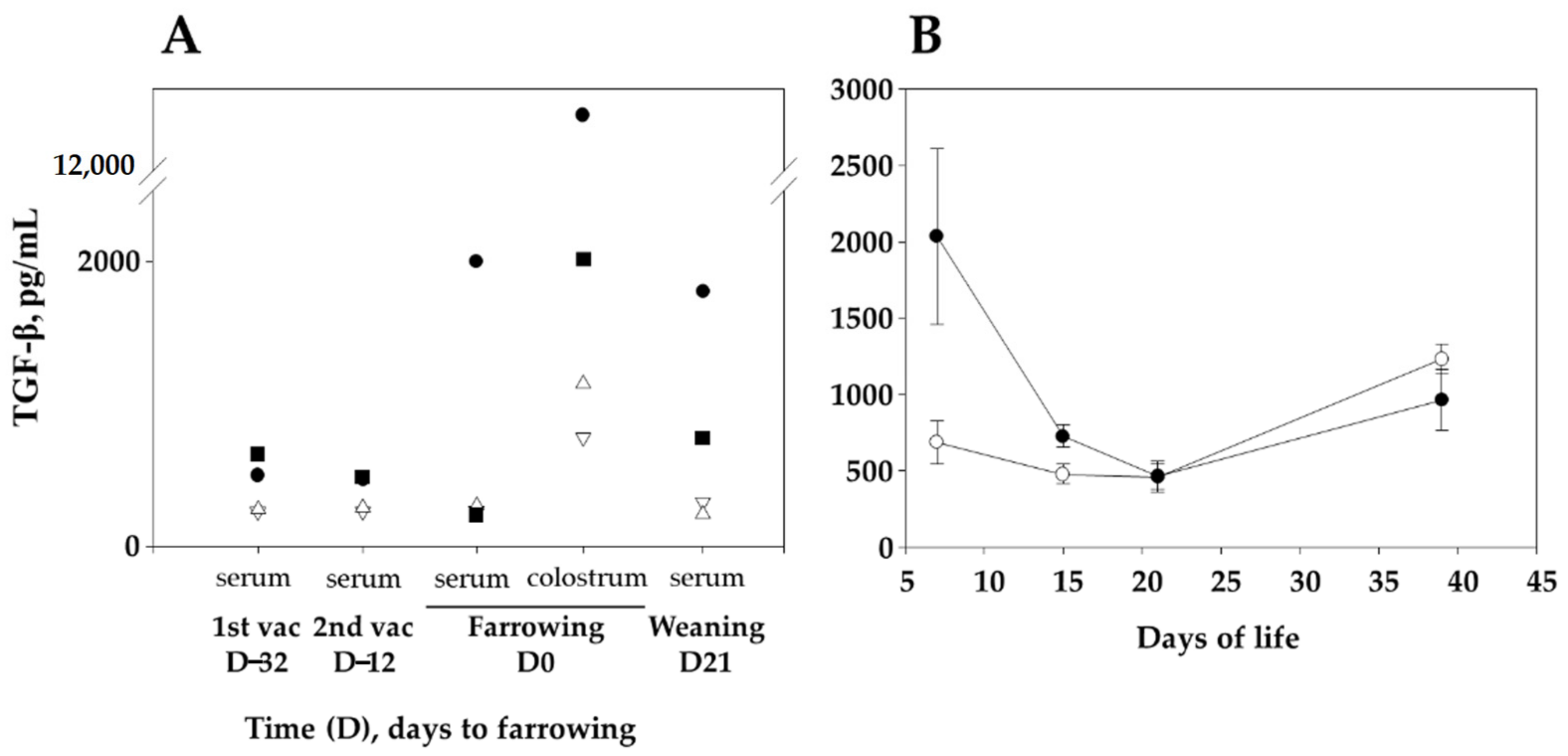
Maternal-derived immunity through the colostrum is essential for the protection of young piglets, and sow vaccination is a way to increase maternal-derived antibody levels in the piglets, which may help preventing infectious diseases in the postweaning period. In our study, sow vaccination with the VtaA fragment F4 resulted in increased specific antibody and high TGF-β levels in the sera of sows and their offspring. After the challenge with virulent G. parasuis, piglets from vaccinated sows presented milder clinical signs and increased SP-D and anti-F4 IgG antibody levels in their lungs. Besides these parameters, piglets from vaccinated sows gained more weight after the infection than animals from unvaccinated sows, reflecting a healthier status. These results confirm the importance of maternal immunity in piglets, where sow vaccination may represent a good alternative in contrast to piglet immunization for the control of early life diseases, since the immature piglet immune system could benefit from their mother’s immunity transfer.
Sow sera and colostrum contained high anti-F4 IgG levels in response to the vaccine, which were subsequently transferred to their offspring. It is reported that the transference of maternal antibodies and other immune components can protect newborn piglets against pathogens during lactation until their own immune system matures [
21]. Together with specific antibodies, an increase of TGF-β in piglets born to vaccinated sows was observed in our study. These outcomes are in line with those reported in human [
22], where an upregulation of TGF-β in the mucosa of the upper airways was detected in neonates from mothers vaccinated with Influenza pdm09 H1N1 adjuvanted vaccine during the pregnancy. Likewise, TGF-β levels were increased in vaccinated sow sera and especially more markedly in colostrum. According to the literature, TGF-β represents one of the most important cytokines transferred by lactation and helps in the maturation of the gastrointestinal tract of the suckling animals helping in IgA class-switching [
23,
24,
25]. A similar trend of maternal transference, although much more diminished than that of TGF-β, was also observed in our experiment with IL-10. These two cytokines have among its pleiotropic functions an immune regulation activity and are principally secreted by T-regulatory cells. This immune suppression in such young stage plays an important role in preventing the exacerbation of the immune system, when the animals begin their contact with the environment and their mucosae begin to be colonized [
26,
27]. Interestingly, an upregulated gene expression of TGF-β was observed in the lungs of animals that were fully-resistant to Glässer’s disease in comparison to susceptible ones [
28]. In that study the authors relate this upregulation with early tissue-repair and antibacterial activity. Moreover, an in vitro experiment with PK-15 cells inoculated with virulent
G. parasuis showed that TGF-β may also play an important protective function by inducing the expression of fibronectin, collagen and integrins necessary to preserve the integrity of the tissue and therefore prevent the invasion of the pathogen [
29]. Glässer’s disease is an inflammatory illness where TGF-β may help in controlling an excessive inflammatory response to the infection, which may facilitate bacterial clearance without the deleterious effects of an excessive activation that causes the typical lesions of polyserositis of the disease. Induction of TGF-β was not observed in sows vaccinated with vaccines against
Mycoplasma hyopneumoniae or porcine circovirus type 2 (unpublished results). However, an increase in TGF-β was detected in piglets vaccinated with F4 protein adjuvanted with Alum in comparison with its corresponding controls and with other adjuvants like carbomer and complete Freund (unpublished results). For these reasons, we consider that the mechanism by which TGF-β increases after vaccination with F4 deserves further studies, as we cannot rule out a role of the adjuvant in the stimulation or a direct induction by the F4 protein, as it has been observed with other bacterial adhesins with anti-inflammatory activity [
30].
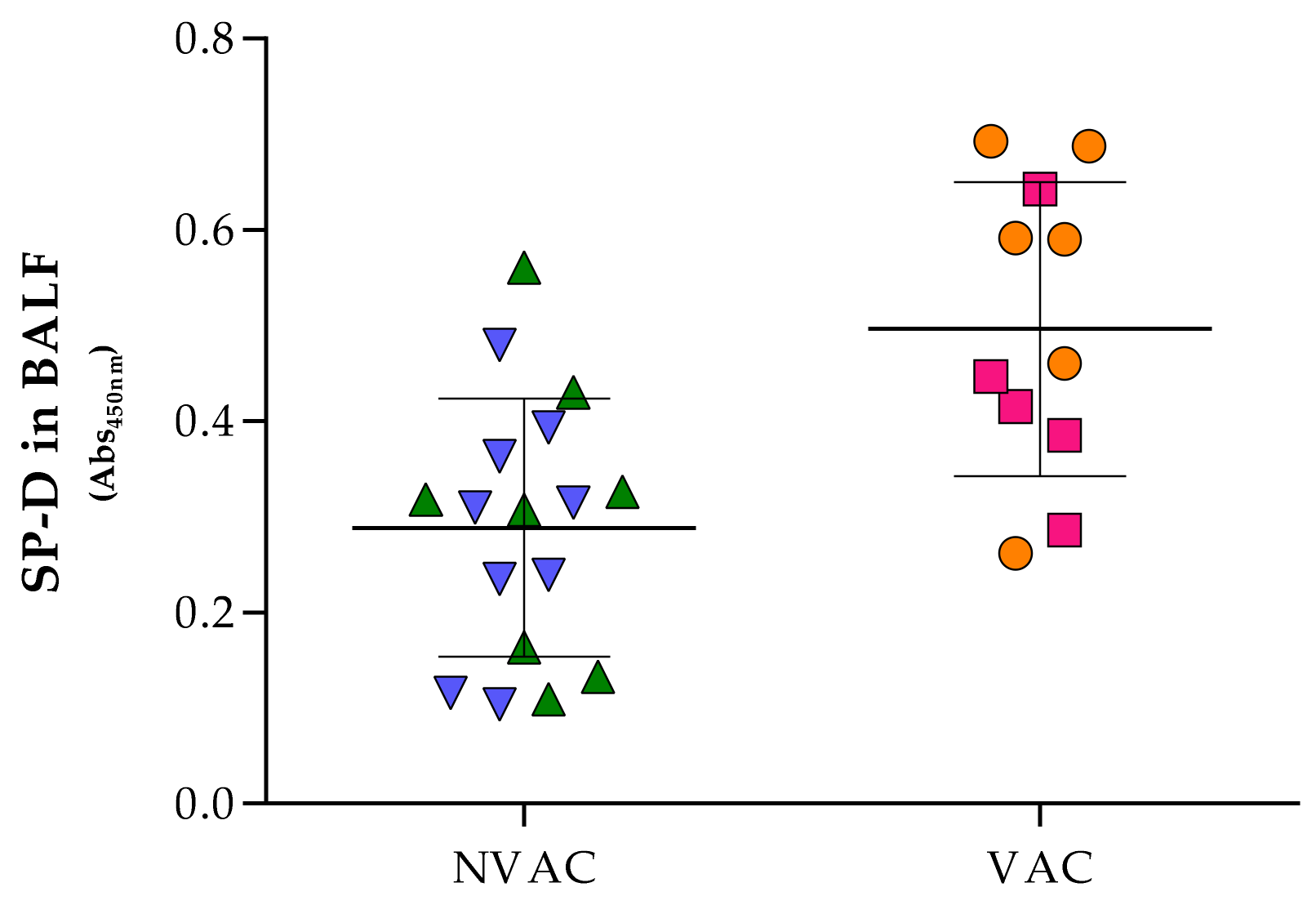
To our knowledge, this is the first report showing that maternal vaccination significantly increased the levels of SP-D in the piglets’ lungs. SP-D is a collectin, one of the first humoral defenses against pathogens. SP-D is secreted by different epithelia throughout the body, specially abundant in digestive and respiratory tracts, with functions comprising from the opsonization and neutralization of pathogens to modulation of the immune response [
31]. However, the link between sow vaccination and SP-D production in piglets’ lungs is not well understood and further research would be needed. In this study we also found a correlation between sow vaccination and F4 antibodies in the BALF of the offspring, which can play a role in bacterial opsonization to allow clearing by alveolar macrophages [
2].
Virulent
G. parasuis strains colonize the upper respiratory tract before progressing into the lung, where innate immunity mechanisms survive [
2,
32]. Our experiment showed that both strains used for challenge were able to colonize the nasal cavity of the piglets with different severity outcomes afterwards. Animals challenged with the Nagasaki strain showed more inflammation in the nasal cavity (via inoculation) and a greater production of SP-D and lung anti-F4 antibodies in comparison to P555/04, which we attribute to the higher virulence of Nagasaki. Although we observed one fatal case of Glässer’s disease in the nonvaccinated animal group challenged with the Nagasaki strain, we could not fully evaluate the protection ability of the vaccine. Clinical signs were generally mild, probably due to the use of sow-reared piglets [
33]. On the other hand, nasal colonization by virulent strains seemed to be reduced by vaccination, although not significantly, while nonvirulent strains were found in most of the piglets included in the experiment. This result may indicate that sow vaccination with F4, a protein fragment only found in virulent
G. parasuis strains, does not affect those commensal nonvirulent strains that colonize the upper respiratory tract and are unable to reach the lung.