Vaccination by Two DerG LEAPS Conjugates Incorporating Distinct Proteoglycan (PG, Aggrecan) Epitopes Provides Therapy by Different Immune Mechanisms in a Mouse Model of Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptides and Antigen
2.2. Mice, Disease Induction and Assessment of GIA
2.3. Preparation and Administration of Vaccines
2.4. Collection of Specimens from Vaccinated Mice
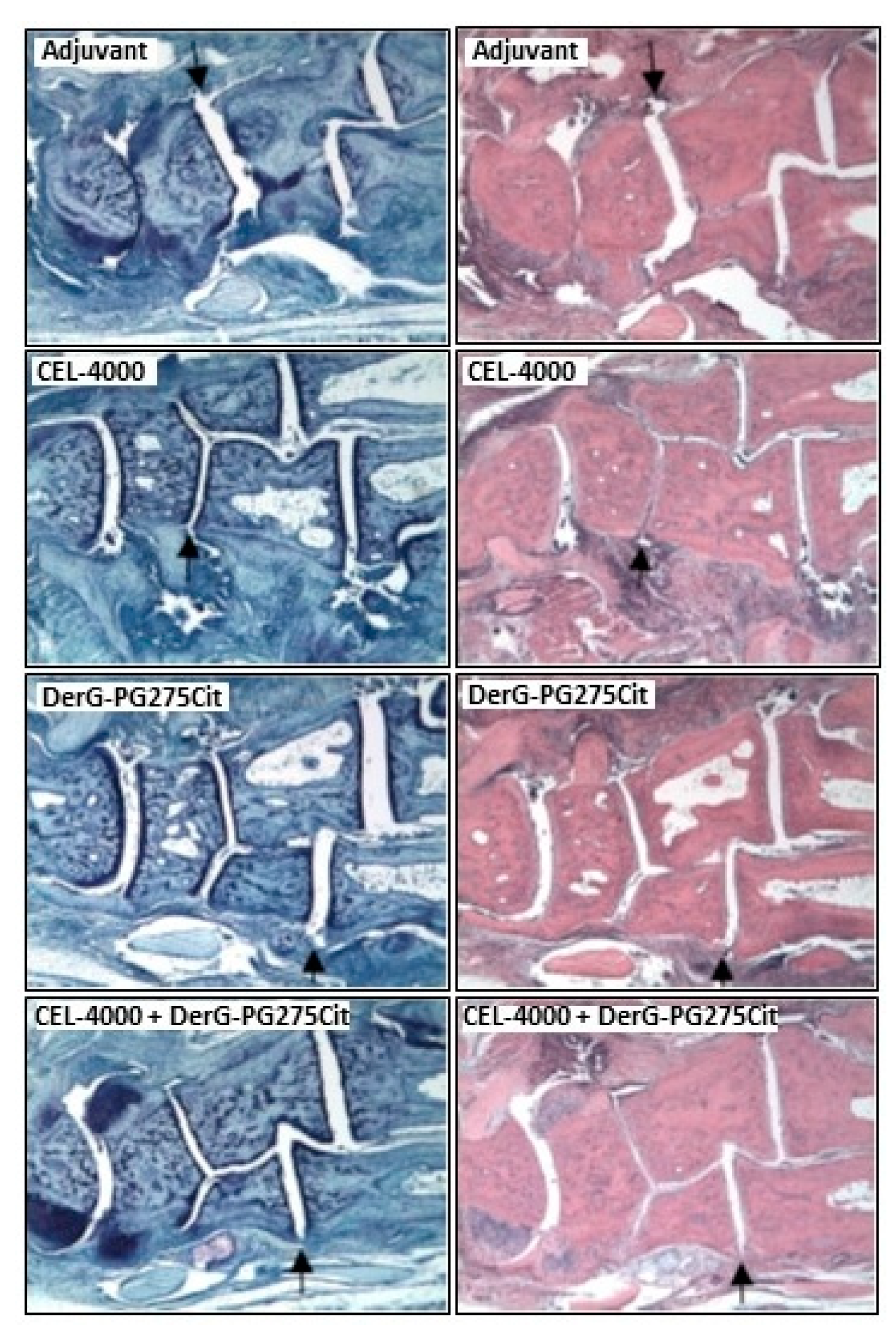
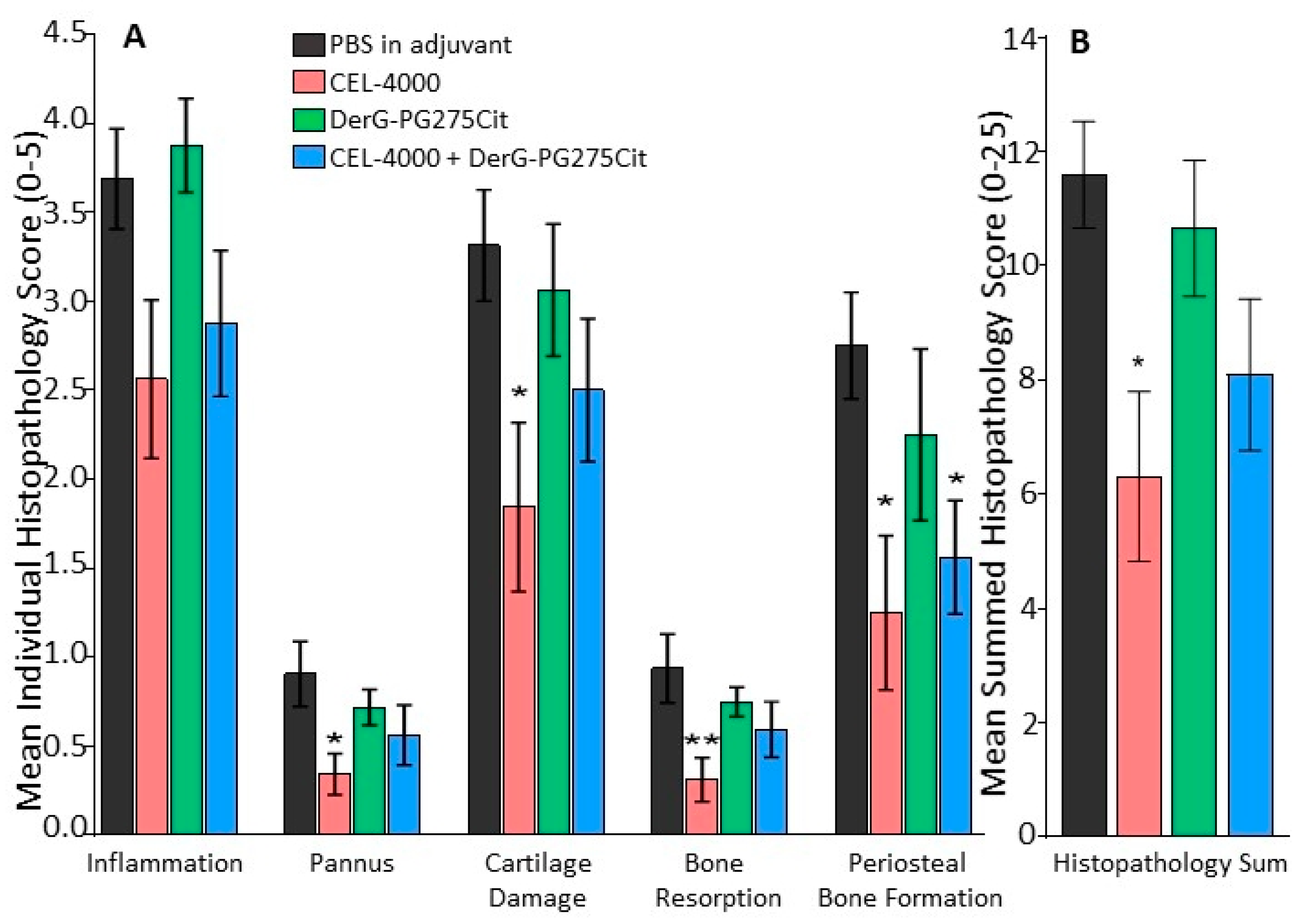
2.5. Histopathology
2.6. Measurement of Serum Anti-Peptide Antibody Levels
2.7. Spleen Cell Cultures
2.8. Flow Cytometry
2.9. Measurement of Cytokines (IL-1β, IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ and TNF-α) Secreted by Spleen Cells
2.10. Experimental Study Design, Animal Use, Powering and Statistical Analysis of Data
3. Results
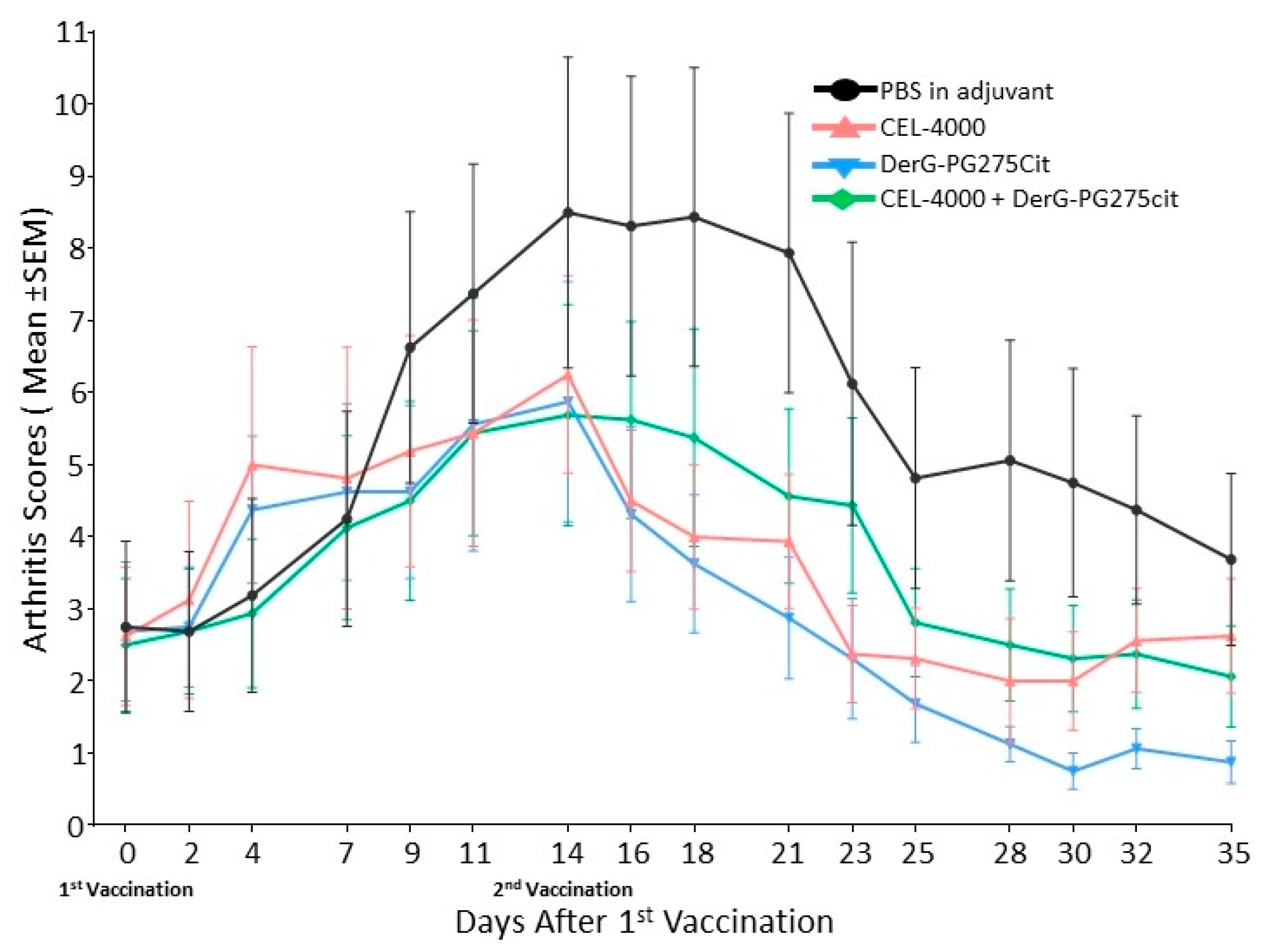
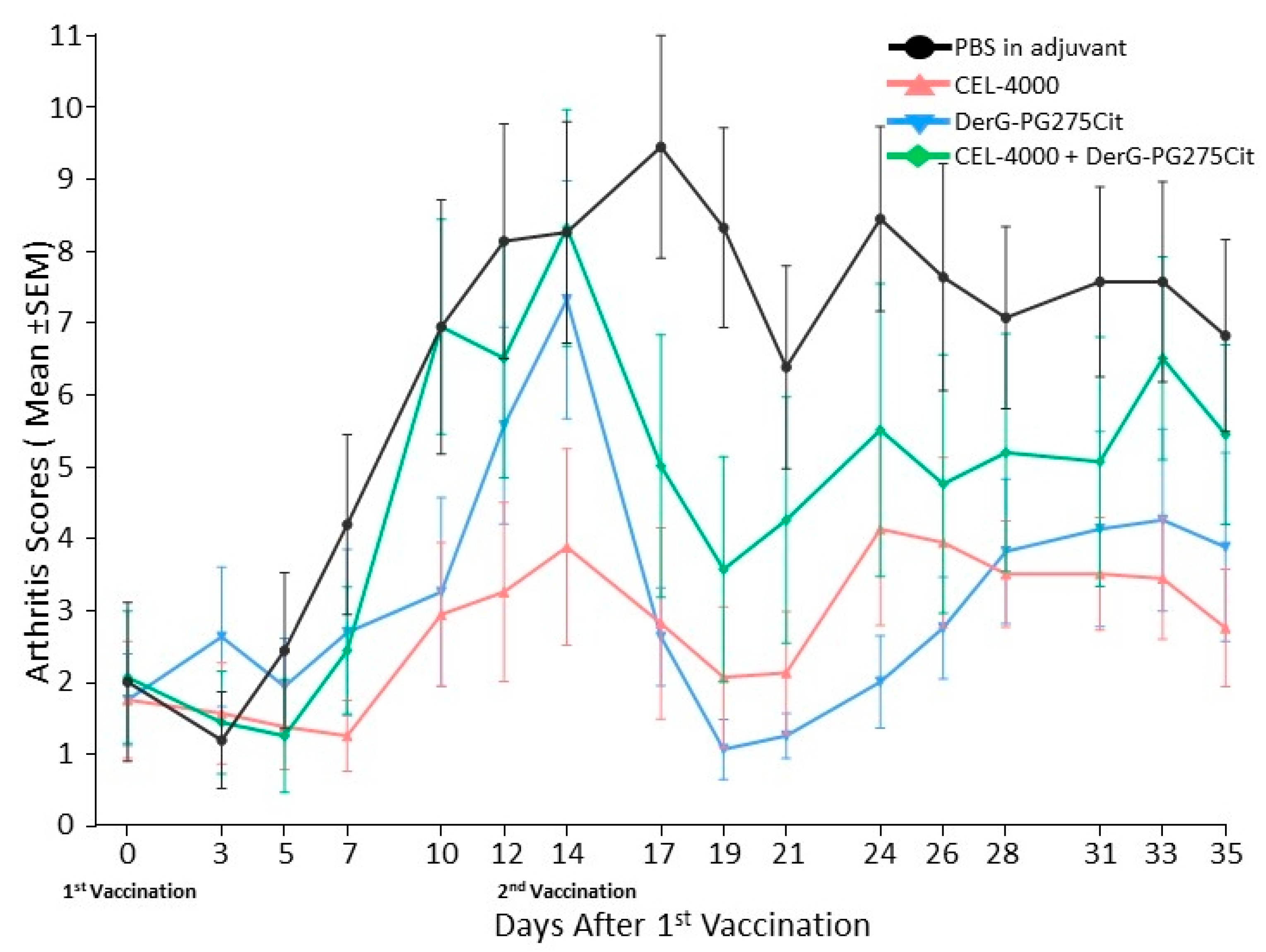
3.1. LEAPS Vaccines Limit Arthritis Progression
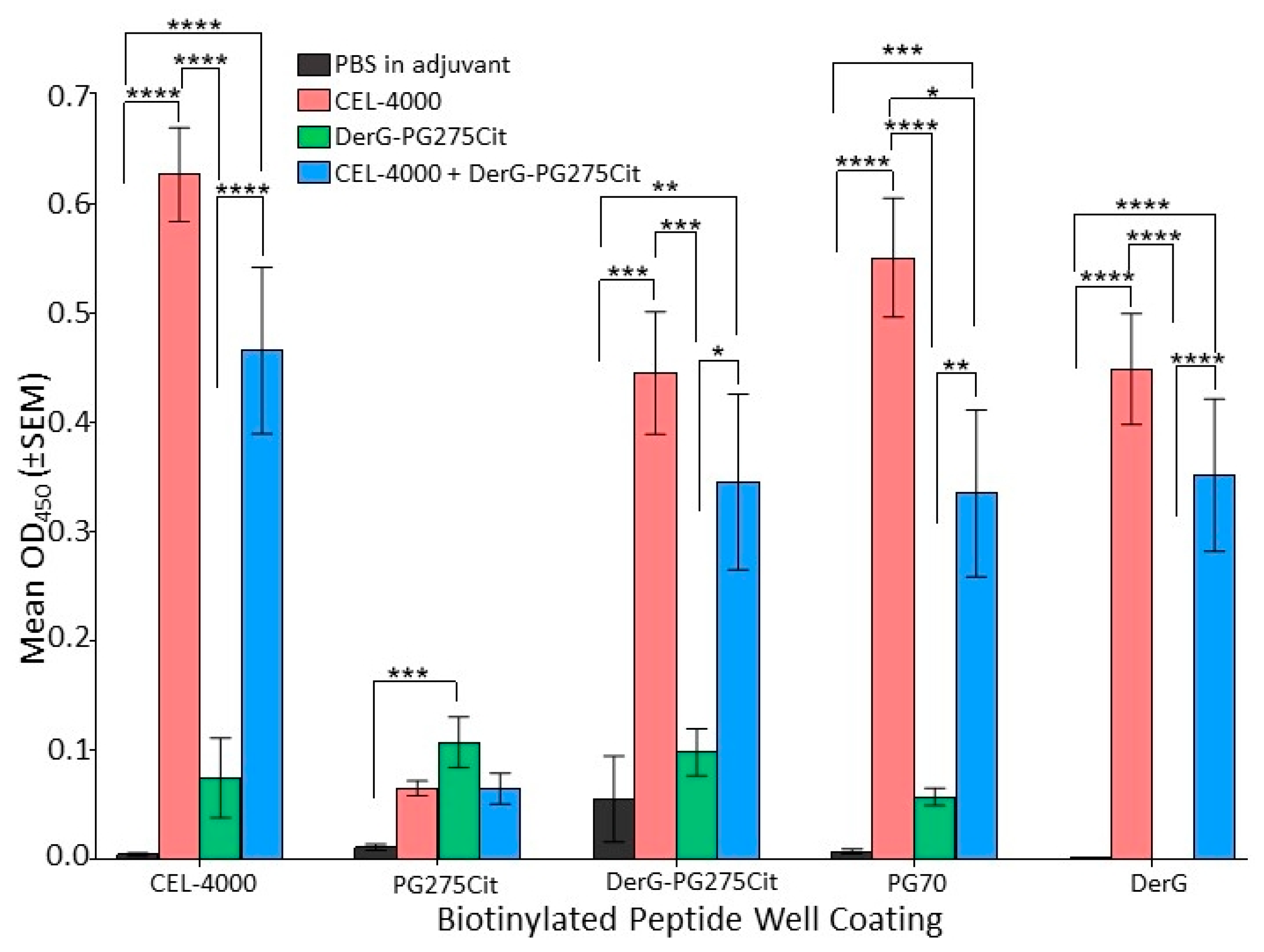
3.2. LEAPS Vaccines Elicit Different Serological Responses
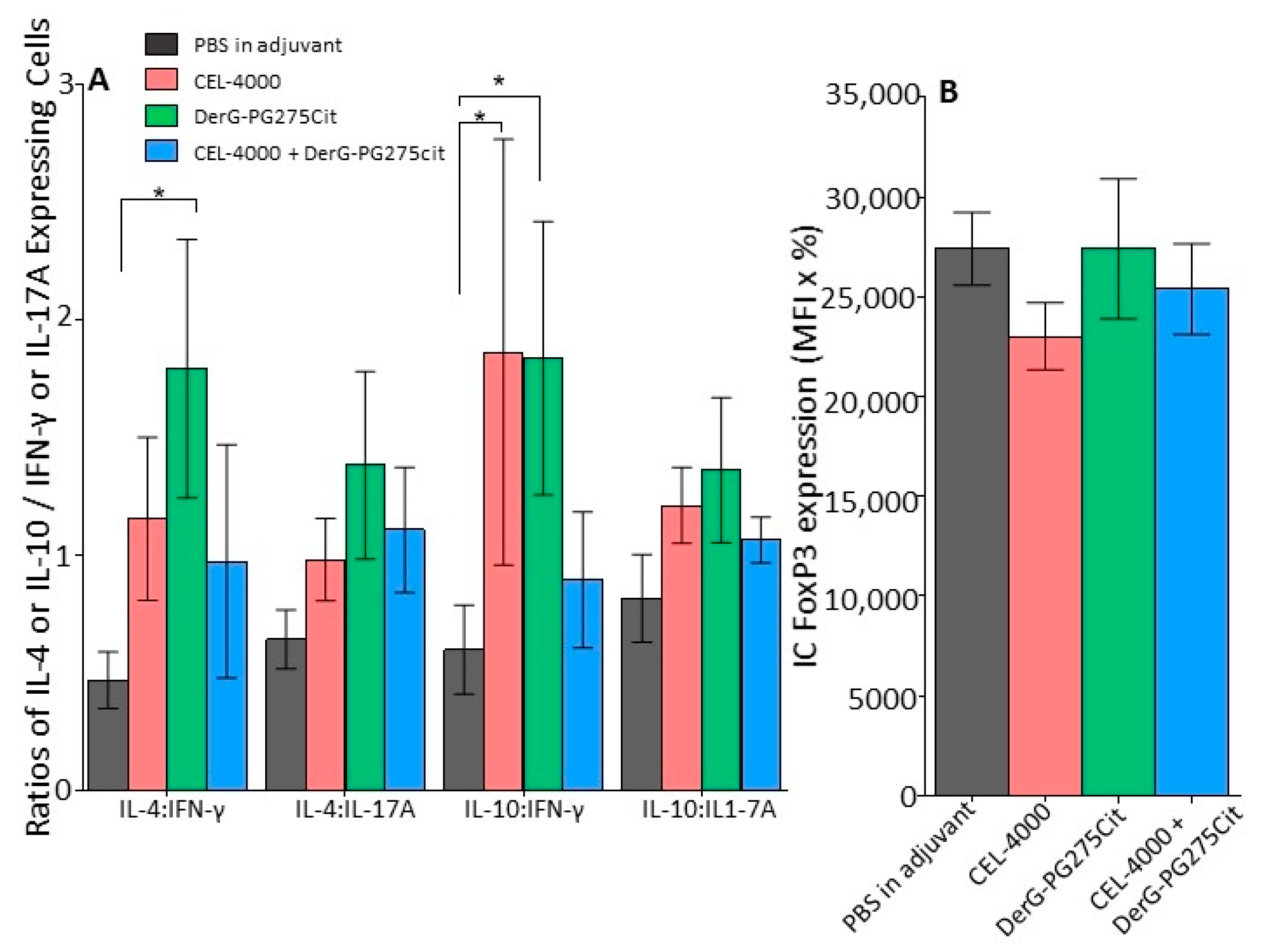
3.3. Intracellular Cytokine Content and FoxP3 in Spleen Cells of Vaccinated GIA Mice
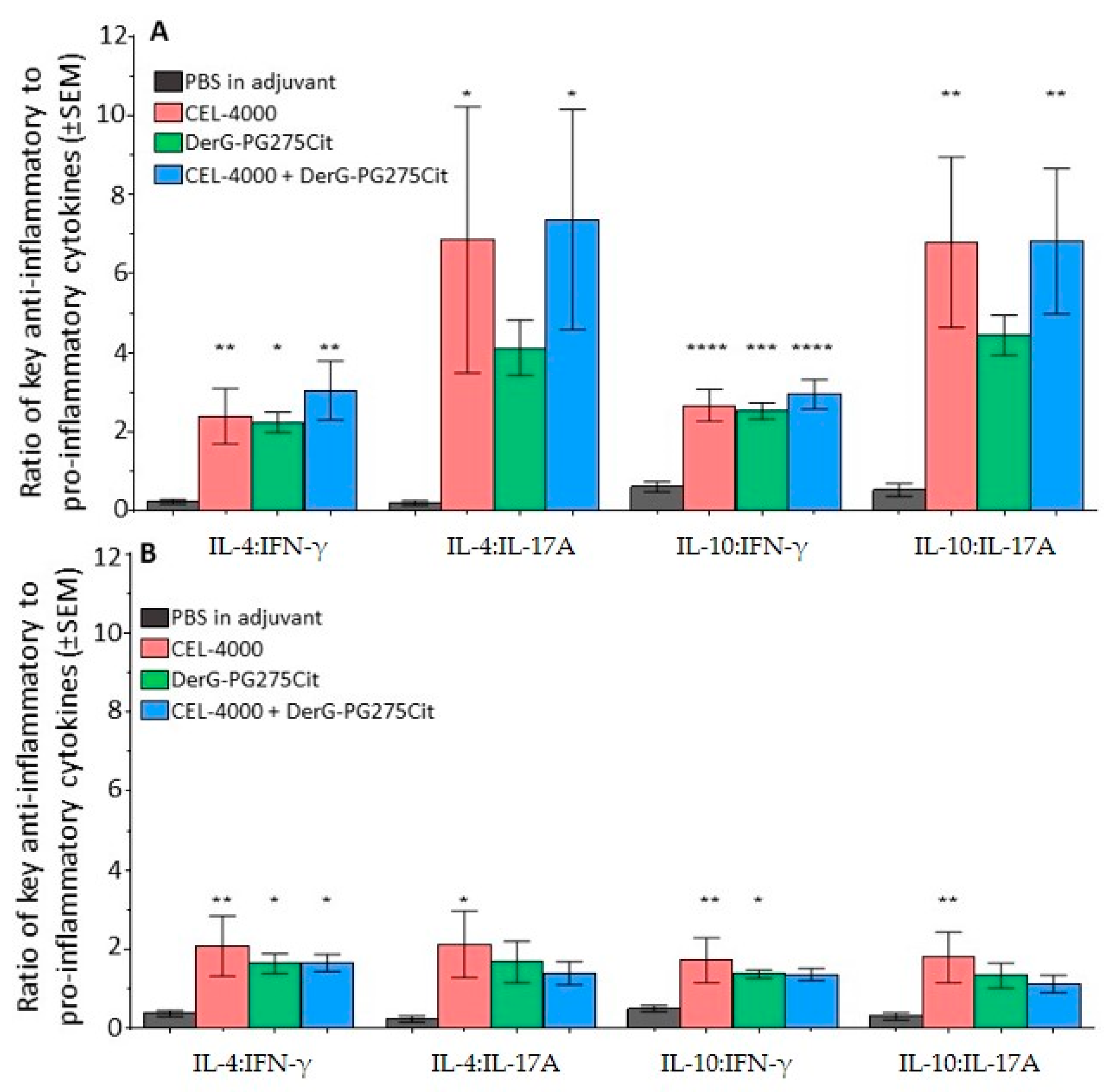
3.4. Soluble Cytokine Production by Spleen Cells from Control and LEAPS Peptide Vaccinated GIA Mice
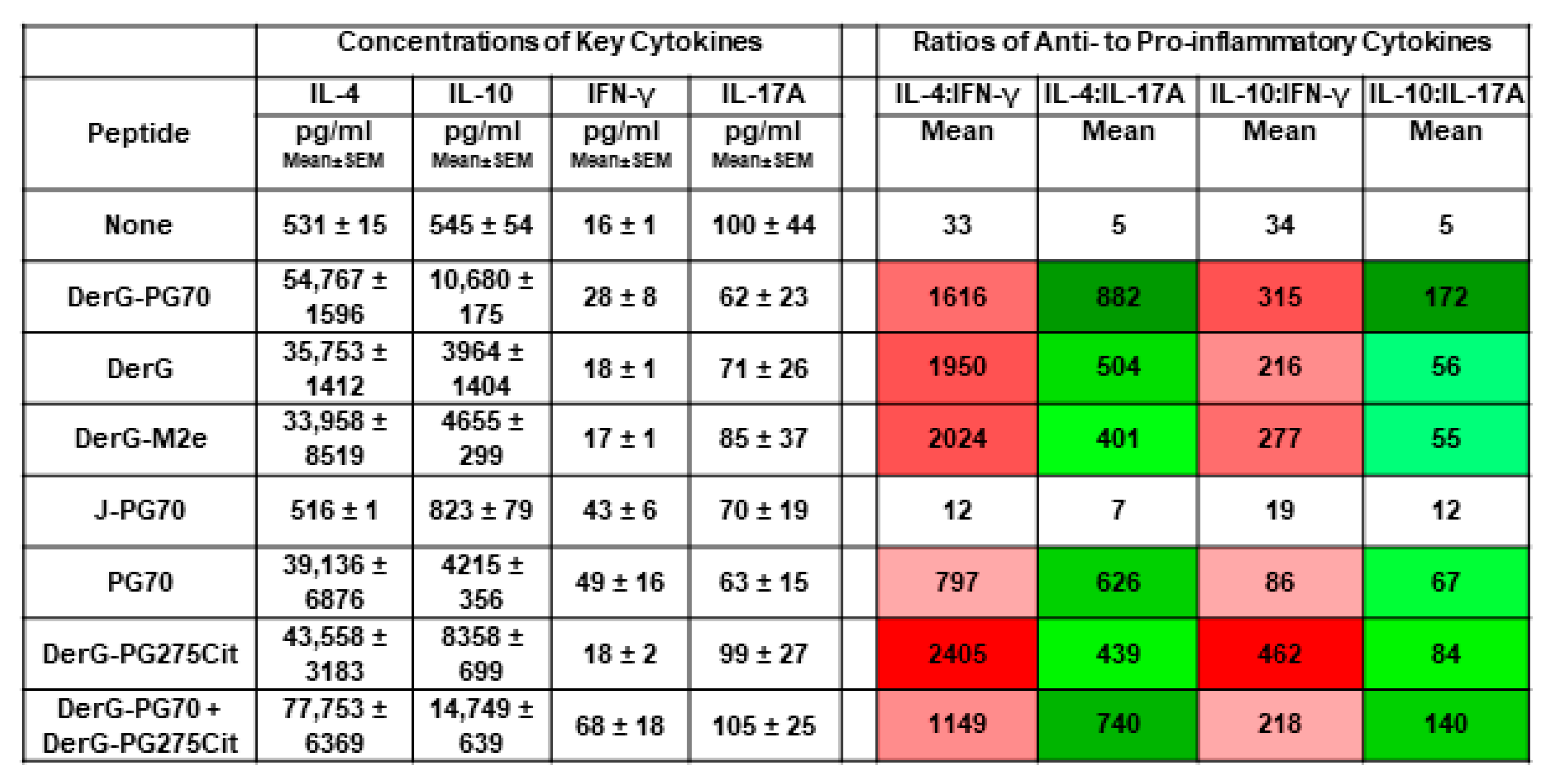
3.5. Cytokine Responses of In Vitro Th Differentiated Spleen CD4+ Cell Subsets from Unvaccinated GIA Mice or from Naïve Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC Arthritis-Related Statistics. Available online: http://www.cdc.gov/arthritis/data_statistics/arthritis_related_stats.htm (accessed on 2 April 2021).
- Research And Markets Global Rheumatoid Arthritis Epidemiology Report 2019–2029. Available online: http://www.globenewswire.com/news-release/2020/12/09/2142440/0/en/Global-Rheumatoid-Arthritis-Epidemiology-Report-2019-2029.html (accessed on 12 September 2020).
- Rosenthal, K.S.; Mikecz, K.; Steiner, H.L.; Glant, T.T.; Finnegan, A.; Carambula, R.E.; Zimmerman, D.H. Rheumatoid arthritis vaccine therapies: Perspectives and lessons from therapeutic ligand epitope antigen presentation system vaccines for models of rheumatoid arthritis. Expert Rev. Vaccines 2015, 14, 891–908. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, S.; Zheng, Q.; Liu, Y.; Shi, G. Peptide-Based Vaccination Therapy for Rheumatic Diseases. J. Immunol. Res. 2020, 2020, 8060375. [Google Scholar] [CrossRef] [PubMed]
- Yap, H.-Y.; Tee, S.; Wong, M.; Chow, S.-K.; Peh, S.-C.; Teow, S.-Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef]
- Yu, C.; Xi, J.; Li, M.; An, M.; Liu, H. Bioconjugate Strategies for the Induction of Antigen-Specific Tolerance in Autoimmune Diseases. Bioconjug. Chem. 2018, 29, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Angelini, J.; Talotta, R.; Roncato, R.; Fornasier, G.; Barbiero, G.; Cin, L.D.; Brancati, S.; Scaglione, F. JAK-inhibitors for the treatment of rheumatoid arthritis: A focus on the present and an outlook on the future. Biomolecules 2020, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef]
- Raimondo, M.G.; Biggioggero, M.; Crotti, C.; Becciolini, A.; Favalli, E.G. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des. Dev. Ther. 2017, 11, 1593–1603. [Google Scholar] [CrossRef]
- Senolt, L. Emerging therapies in rheumatoid arthritis: Focus on monoclonal antibodies [version 1; peer review: 2 approved]. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Rafael-Vidal, C.; Pérez, N.; Altabás, I.; Garcia, S.; Pego-Reigosa, J.M. Blocking il-17: A promising strategy in the treatment of systemic rheumatic diseases. Int. J. Mol. Sci. 2020, 21, 7100. [Google Scholar] [CrossRef]
- Burisch, J.; Eigner, W.; Schreiber, S.; Aletaha, D.; Weninger, W.; Trauner, M.; Reinisch, W.; Narula, N. Risk for development of inflammatory bowel disease under inhibition of interleukin 17: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0233781. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, G.E.; Mcinnes, I.B.; Siebert, S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (U. K.) 2019, 58, i43–i54. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, D.H.; Taylor, P.; Bendele, A.; Carambula, R.; Duzant, Y.; Lowe, V.; O’Neill, S.P.; Talor, E.; Rosenthal, K.S. CEL-2000: A therapeutic vaccine for rheumatoid arthritis arrests disease development and alters serum cytokine/chemokine patterns in the bovine collagen type II induced arthritis in the DBA mouse model. Int. Immunopharmacol. 2010, 10, 412–421. [Google Scholar] [CrossRef]
- Taylor, P.R.; Koski, G.K.; Paustian, C.C.; Bailey, E.; Cohen, P.A.; Moore, F.B.-G.; Zimmerman, D.H.; Rosenthal, K.S. J-LEAPS vaccines initiate murine Th1 responses by activating dendritic cells. Vaccine 2010, 28, 5533–5542. [Google Scholar] [CrossRef]
- Boonnak, K.; Vogel, L.; Orandle, M.; Zimmerman, D.; Talor, E.; Subbarao, K. Antigen-activated dendritic cells ameliorate influenza A infections. J. Clin. Investig. 2013, 123, 2850–2861. [Google Scholar] [CrossRef] [PubMed]
- Parham, P.; Androlewicz, M.J.; Holmes, N.J.; Rothenberg, B.E. Arginine 45 is a major part of the antigenic determinant of human beta 2-microglobulin recognized by mouse monoclonal antibody BBM.1. J. Biol. Chem. 1983, 258, 6179–6186. [Google Scholar] [CrossRef]
- Cihakova, D.; Barin, J.G.; Baldeviano, G.C.; Kimura, M.; Talor, M.V.; Zimmerman, D.H.; Talor, E.; Rose, N.R. LEAPS heteroconjugate is able to prevent and treat experimental autoimmune myocarditis by altering trafficking of autoaggressive cells to the heart. Int. Immunopharmacol. 2008, 8, 624–633. [Google Scholar] [CrossRef][Green Version]
- Mikecz, K.; Glant, T.T.; Markovics, A.; Rosenthal, K.S.; Kurko, J.; Carambula, R.E.; Cress, S.; Steiner, H.L.; Zimmerman, D.H. An epitope-specific DerG-PG70 LEAPS vaccine modulates T cell responses and suppresses arthritis progression in two related murine models of rheumatoid arthritis. Vaccine 2017, 35, 4048–4056. [Google Scholar] [CrossRef]
- Kobezda, T.; Ghassemi-Nejad, S.; Mikecz, K.; Glant, T.T.; Szekanecz, Z. Of mice and men: How animal models advance our understanding of T-cell function in RA. Nat. Rev. Rheumatol. 2014, 10, 160–170. [Google Scholar] [CrossRef]
- Rodeghero, R.; Cao, Y.; Olalekan, S.A.; Iwakua, Y.; Glant, T.T.; Finnegan, A. Location of CD4+ T cell priming regulates the differentiation of Th1 and Th17 cells and their contribution to arthritis. J. Immunol. 2013, 190, 5423–5435. [Google Scholar] [CrossRef] [PubMed]
- Domingues, H.S.; Mues, M.; Lassmann, H.; Wekerle, H.; Krishnamoorthy, G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS ONE 2010, 5, e15531. [Google Scholar] [CrossRef] [PubMed]
- Luger, D.; Silver, P.B.; Tang, J.; Cua, D.; Chen, Z.; Iwakura, Y.; Bowman, E.P.; Sgambellone, N.M.; Chan, C.-C.; Caspi, R.R. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J. Exp. Med. 2008, 205, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Lubberts, E.; Koenders, M.I.; van den Berg, W.B. The role of T-cell interleukin-17 in conducting destructive arthritis: Lessons from animal models. Arthritis Res. Ther. 2005, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Glant, T.T.; Radacs, M.; Nagyeri, G.; Olasz, K.; Laszlo, A.; Boldizsar, F.; Hegyi, A.; Finnegan, A.; Mikecz, K. Proteoglycan-induced arthritis and recombinant human proteoglycan aggrecan G1 domain-induced arthritis in BALB/c mice resembling two subtypes of rheumatoid arthritis. Arthritis Rheum. 2011, 63, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Glant, T.T.; Mikecz, K. Proteoglycan aggrecan-induced arthritis: A murine autoimmune model of rheumatoid arthritis. Methods Mol. Med. 2004, 102, 313–338. [Google Scholar] [PubMed]
- König, R.; Shen, X.; Germain, R.N. Involvement of both major histocompatibility complex class II alpha and beta chains in CD4 function indicates a role for ordered oligomerization in T cell activation. J. Exp. Med. 1995, 182, 779–787. [Google Scholar] [CrossRef]
- König, R.; Huang, L.Y.; Germain, R.N.N. MHC class II interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature 1992, 356, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Scheirle, A.; Takacs, B.; Doran, D.M.; Knorr, R.; Bannwarth, W.; Guardiola, J.; Sinigaglia, F. Identification of a CD4 binding site on the beta 2 domain of HLA-DR molecules. Nature 1992, 356, 799–801. [Google Scholar] [CrossRef]
- Shen, X.; Hu, B.; McPhie, P.; Wu, X.; Fox, A.; Germain, R.N.; König, R. Peptides corresponding to CD4-interacting regions of murine MHC class II molecules modulate immune responses of CD4+ T lymphocytes in vitro and in vivo. J. Immunol. 1996, 157, 87–100. [Google Scholar]
- Zimmerman, D.H.; Steiner, H.; Carmabula, R.; Talor, E.; Rosenthal, K.S. LEAPS therapeutic vaccines as antigen specific suppressors of inflammation in infectious and autoimmune diseases. J. Vaccines Vaccin. 2012, 3, 1000149. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, Y.; Thiel, A.; Rudwaleit, M.; Shi, S.-L.; Radbruch, A.; Poole, R.; Braun, J.; Sieper, J. Predominant cellular immune response to the cartilage autoantigenic G1 aggrecan in ankylosing spondylitis and rheumatoid arthritis. Rheumatology (Oxf.) 2003, 42, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Markovics, A.; Ocskó, T.; Katz, R.S.; Buzás, E.I.; Glant, T.T.; Mikecz, K. Immune Recognition of Citrullinated Proteoglycan Aggrecan Epitopes in Mice with Proteoglycan-Induced Arthritis and in Patients with Rheumatoid Arthritis. PLoS ONE 2016, 11, e0160284. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.-C.; Manning, H.B.; Jain, A.; Troeberg, L.; Dudhia, J.; Essex, D.; Sandison, A.; Seiki, M.; Nanchahal, J.; Nagase, H.; et al. Membrane type 1 matrix metalloproteinase is a crucial promoter of synovial invasion in human rheumatoid arthritis. Arthritis Rheum. 2009, 60, 686–697. [Google Scholar] [CrossRef]
- Maresz, K.J.; Hellvard, A.; Sroka, A.; Adamowicz, K.; Bielecka, E.; Koziel, J.; Gawron, K.; Mizgalska, D.; Marcinska, K.A.; Benedyk, M.; et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 2013, 9, e1003627. [Google Scholar] [CrossRef]
- Misják, P.; Bősze, S.; Horváti, K.; Pásztói, M.; Pálóczi, K.; Holub, M.C.; Szakács, F.; Aradi, B.; György, B.; Szabó, T.G.; et al. The role of citrullination of an immunodominant proteoglycan (PG) aggrecan T cell epitope in BALB/c mice with PG-induced arthritis. Immunol. Lett. 2013, 152, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kidd, B.A.; Ho, P.P.; Sharpe, O.; Zhao, X.; Tomooka, B.H.; Kanter, J.L.; Steinman, L.; Robinson, W.H. Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination. Arthritis Res. Ther. 2008, 10, R119. [Google Scholar] [CrossRef]
- Khmaladze, I.; Saxena, A.; Nandakumar, K.S.; Holmdahl, R. B-cell epitope spreading and inflammation in a mouse model of arthritis is associated with a deficiency in reactive oxygen species production. Eur. J. Immunol. 2015, 45, 2243–2251. [Google Scholar] [CrossRef]
- Didona, D.; Di Zenzo, G. Humoral epitope spreading in autoimmune bullous diseases. Front. Immunol. 2018, 9, 779. [Google Scholar] [CrossRef]
- Kurkó, J.; Vida, A.; Ocskó, T.; Tryniszewska, B.; Rauch, T.A.; Glant, T.T.; Szekanecz, Z.; Mikecz, K. Suppression of proteoglycan-induced autoimmune arthritis by myeloid-derived suppressor cells generated in vitro from murine bone marrow. PLoS ONE 2014, 9, e111815. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies: Washington, DC, USA, 2011; ISBN 9780309154000. [Google Scholar]
- Ko, E.; Park, S.; Lee, J.H.; Cui, C.H.; Hou, J.; Kim, M.H.; Kim, S.C. Ginsenoside Rh2 ameliorates atopic dermatitis in NC/Nga mice by suppressing NF-kappab-mediated thymic stromal lymphopoietin expression and t helper type 2 differentiation. Int. J. Mol. Sci. 2019, 20, 6111. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Malik Tyagi, A.; Li, J.Y.; Adams, J.; Denning, T.L.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. PTH induces bone loss via microbial-dependent expansion of intestinal TNF+ T cells and Th17 cells. Nat. Commun. 2020, 11, 1–17. [Google Scholar]
- Miller, S.A.; Weinmann, A.S. Common themes emerge in the transcriptional control of T helper and developmental cell fate decisions regulated by the T-box, GATA and ROR families. Immunology 2009, 126, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Josefowicz, S.Z.; Rudensky, A. Control of Regulatory T Cell Lineage Commitment and Maintenance. Immunity 2009, 30, 616–625. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the Arrive Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+ T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Roncarolo, M.G.; Gregori, S.; Bacchetta, R.; Battaglia, M.; Gagliani, N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity 2018, 49, 1004–1019. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmerman, D.H.; Mikecz, K.; Markovics, A.; Carambula, R.E.; Ciemielewski, J.C.; Toth, D.M.; Glant, T.T.; Rosenthal, K.S. Vaccination by Two DerG LEAPS Conjugates Incorporating Distinct Proteoglycan (PG, Aggrecan) Epitopes Provides Therapy by Different Immune Mechanisms in a Mouse Model of Rheumatoid Arthritis. Vaccines 2021, 9, 448. https://doi.org/10.3390/vaccines9050448
Zimmerman DH, Mikecz K, Markovics A, Carambula RE, Ciemielewski JC, Toth DM, Glant TT, Rosenthal KS. Vaccination by Two DerG LEAPS Conjugates Incorporating Distinct Proteoglycan (PG, Aggrecan) Epitopes Provides Therapy by Different Immune Mechanisms in a Mouse Model of Rheumatoid Arthritis. Vaccines. 2021; 9(5):448. https://doi.org/10.3390/vaccines9050448
Chicago/Turabian StyleZimmerman, Daniel H., Katalin Mikecz, Adrienn Markovics, Roy E. Carambula, Jason C. Ciemielewski, Daniel M. Toth, Tibor T. Glant, and Ken S. Rosenthal. 2021. "Vaccination by Two DerG LEAPS Conjugates Incorporating Distinct Proteoglycan (PG, Aggrecan) Epitopes Provides Therapy by Different Immune Mechanisms in a Mouse Model of Rheumatoid Arthritis" Vaccines 9, no. 5: 448. https://doi.org/10.3390/vaccines9050448
APA StyleZimmerman, D. H., Mikecz, K., Markovics, A., Carambula, R. E., Ciemielewski, J. C., Toth, D. M., Glant, T. T., & Rosenthal, K. S. (2021). Vaccination by Two DerG LEAPS Conjugates Incorporating Distinct Proteoglycan (PG, Aggrecan) Epitopes Provides Therapy by Different Immune Mechanisms in a Mouse Model of Rheumatoid Arthritis. Vaccines, 9(5), 448. https://doi.org/10.3390/vaccines9050448





