Influenza Vaccination and COVID-19 Mortality in the USA: An Ecological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Processing
2.2. Confounding Adjustment with Generalized Propensity Score
2.3. Modeling the Effect of Vaccine Coverage on COVID-19 Mortality
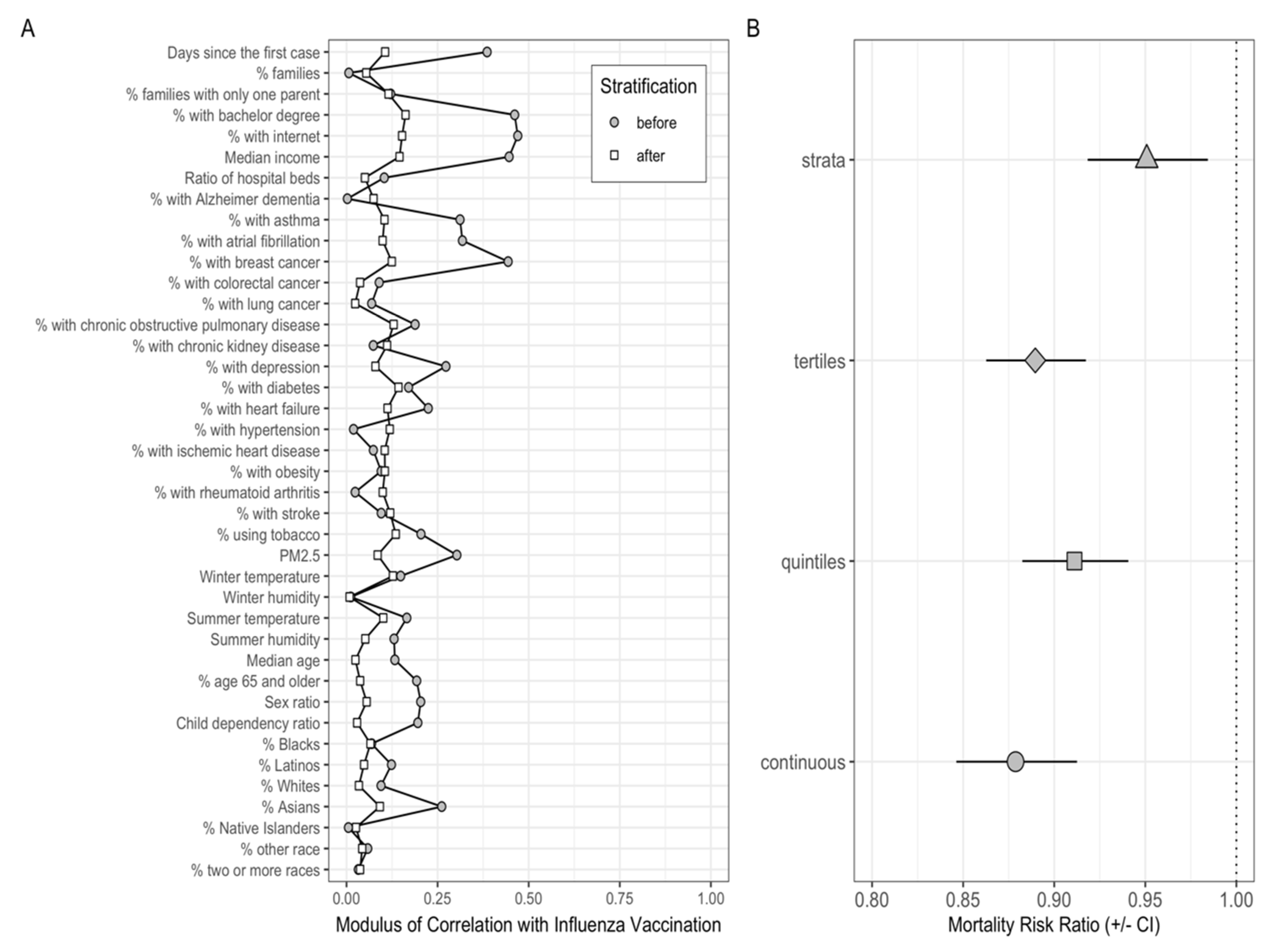
3. Results
3.1. Characteristics of Included Counties
3.2. Evaluating Covariates Balance before and after PS Stratification
3.3. Effect of Influenza Vaccination Coverage on COVID-19 Mortality Rates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roser, M.; Ritchie, H.; Ortiz-Ospina, E.; Hasell, J. Coronavirus Pandemic (COVID-19). Our World Data 2020. Available online: https://ourworldindata.org/coronavirus#citation (accessed on 22 April 2021).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and Gustatory Dysfunctions as a Clinical Presentation of Mild-to-Moderate Forms of the Coronavirus Disease (COVID-19): A Multicenter European Study. Eur. Arch. Oto Rhino Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Konala, V.M.; Adapa, S.; Gayam, V.; Naramala, S.; Daggubati, S.R.; Kammari, C.B.; Chenna, A. Co-Infection with Influenza A and COVID-19. Eur. J. Case Rep. Intern. Med. 2020, 7, 001656. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Doyle, J.D.; Uyeki, T.M. Influenza Virus-Related Critical Illness: Prevention, Diagnosis, Treatment. Crit. Care Lond. Engl. 2019, 23, 214. [Google Scholar] [CrossRef] [Green Version]
- Hak, E.; Nordin, J.; Wei, F.; Mullooly, J.; Poblete, S.; Strikas, R.; Nichol, K.L. Influence of High-Risk Medical Conditions on the Effectiveness of Influenza Vaccination among Elderly Members of 3 Large Managed-Care Organizations. Clin. Infect. Dis. 2002, 35, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Fry, A.M.; Kim, I.K.; Reed, C.; Thompson, M.; Chaves, S.S.; Finelli, L.; Bresee, J. Modeling the Effect of Different Vaccine Effectiveness Estimates on the Number of Vaccine-Prevented Influenza-Associated Hospitalizations in Older Adults. Clin. Infect. Dis. 2014, 59, 406–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taksler, G.B.; Rothberg, M.B.; Cutler, D.M. Association of Influenza Vaccination Coverage in Younger Adults with Influenza-Related Illness in the Elderly. Clin. Infect. Dis. 2015, 61, 1495–1503. [Google Scholar] [CrossRef]
- Wolff, G.G. Influenza Vaccination and Respiratory Virus Interference among Department of Defense Personnel during the 2017-2018 Influenza Season. Vaccine 2020, 38, 350–354. [Google Scholar] [CrossRef]
- Tetro, J.A. Is COVID-19 Receiving ADE from Other Coronaviruses? Microbes Infect. 2020, 22, 72–73. [Google Scholar] [CrossRef]
- Dong, E.; Du, H.; Gardner, L. An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Wu, X.; Nethery, R.C.; Sabath, M.B.; Braun, D.; Dominici, F. Air Pollution and COVID-19 Mortality in the United States: Strengths and Limitations of an Ecological Regression Analysis. Sci. Adv. 2020, 6, eabd4049. [Google Scholar] [CrossRef]
- Brookhart, M.A.; Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stürmer, T. Variable Selection for Propensity Score Models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.L.; Fazio-Eynullayeva, E.; Lane, D.A.; Underhill, P.; Lip, G.Y.H. Comorbidities Associated with Mortality in 31,461 Adults with COVID-19 in the United States: A Federated Electronic Medical Record Analysis. PLoS Med. 2020, 17, e1003321. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors Associated with Hospital Admission and Critical Illness among 5279 People with Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ 2020, 369. [Google Scholar] [CrossRef]
- Prats-Uribe, A.; Paredes, R.; Prieto-Alhambra, D. Ethnicity, Comorbidity, Socioeconomic Status, and Their Associations with COVID-19 Infection in England: A Cohort Analysis of UK Biobank Data. medRxiv 2020. [Google Scholar] [CrossRef]
- Singu, S.; Acharya, A.; Challagundla, K.; Byrareddy, S.N. Impact of Social Determinants of Health on the Emerging COVID-19 Pandemic in the United States. Front. Public Health 2020, 8, 406. [Google Scholar] [CrossRef]
- Gold, J.A.W.; Rossen, L.M.; Ahmad, F.B.; Sutton, P.; Li, Z.; Salvatore, P.P.; Coyle, J.P.; DeCuir, J.; Baack, B.N.; Durant, T.M.; et al. Race, Ethnicity, and Age Trends in Persons Who Died from COVID-19—United States, May–August 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1517–1521. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido, M.M.; Kelley, A.S.; Paris, J.; Roza, K.; Meier, D.E.; Morrison, R.S.; Aldridge, M.D. Methods for Constructing and Assessing Propensity Scores. Health Serv. Res. 2014, 49, 1701–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedderburn, R.W.M. Quasi-Likelihood Functions, Generalized Linear Models, and the Gauss—Newton Method. Biometrika 1974, 61, 439–447. [Google Scholar] [CrossRef]
- Austin, P.C. Assessing Covariate Balance When Using the Generalized Propensity Score with Quantitative or Continuous Exposures. Stat. Methods Med. Res. 2019, 28, 1365–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhou, J.; Cao, W.; Zhang, J. Causal Inference with a Quantitative Exposure. Stat. Methods Med. Res. 2016, 25, 315–335. [Google Scholar] [CrossRef]
- Marín-Hernández, D.; Schwartz, R.E.; Nixon, D.F. Epidemiological Evidence for Association between Higher Influenza Vaccine Uptake in the Elderly and Lower COVID-19 Deaths in Italy. J. Med. Virol. 2021, 93, 64–65. [Google Scholar] [CrossRef]
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between Influenza Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef]
- Fink, G.; Orlova-Fink, N.; Schindler, T.; Grisi, S.; Ferrer, A.P.S.; Daubenberger, C.; Brentani, A. Inactivated Trivalent Influenza Vaccination Is Associated with Lower Mortality among Patients with COVID-19 in Brazil. BMJ Evid. Based Med. 2020. [Google Scholar] [CrossRef]
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Hofmann Bowman, M.A. Impact of the Influenza Vaccine on COVID-19 Infection Rates and Severity. Am. J. Infect. Control 2021. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Islam, N.; Dambha-Miller, H. Association between Influenza Vaccination and Hospitalisation or All-Cause Mortality in People with COVID-19: A Retrospective Cohort Study. BMJ Open Respir. Res. 2021, 8, e000857. [Google Scholar] [CrossRef]
- Ragni, P.; Marino, M.; Formisano, D.; Bisaccia, E.; Scaltriti, S.; Bedeschi, E.; Grilli, R. Association between Exposure to Influenza Vaccination and COVID-19 Diagnosis and Outcomes. Vaccines 2020, 8, 675. [Google Scholar] [CrossRef]
- Green, I.; Ashkenazi, S.; Merzon, E.; Vinker, S.; Golan-Cohen, A. The Association of Previous Influenza Vaccination and Coronavirus Disease-2019. Hum. Vaccines Immunother. 2020, 1–7. [Google Scholar] [CrossRef]
- Noale, M.; Trevisan, C.; Maggi, S.; Antonelli Incalzi, R.; Pedone, C.; Di Bari, M.; Adorni, F.; Jesuthasan, N.; Sojic, A.; Galli, M.; et al. The Association between Influenza and Pneumococcal Vaccinations and SARS-Cov-2 Infection: Data from the EPICOVID19 Web-Based Survey. Vaccines 2020, 8, 471. [Google Scholar] [CrossRef]
- Udell, J.A.; Zawi, R.; Bhatt, D.L.; Keshtkar-Jahromi, M.; Gaughran, F.; Phrommintikul, A.; Ciszewski, A.; Vakili, H.; Hoffman, E.B.; Farkouh, M.E.; et al. Association between Influenza Vaccination and Cardiovascular Outcomes in High-Risk Patients: A Meta-Analysis. JAMA 2013, 310, 1711–1720. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Bhaskaran, K.; Hayward, A.; Leung, G.M.; Lo, S.-V.; Wong, C.-M.; Ellis, J.; Pebody, R.; Smeeth, L.; Cowling, B.J. Circulating Influenza Virus, Climatic Factors, and Acute Myocardial Infarction: A Time Series Study in England and Wales and Hong Kong. J. Infect. Dis. 2011, 203, 1710–1718. [Google Scholar] [CrossRef]
- Madjid, M.; Miller, C.C.; Zarubaev, V.V.; Marinich, I.G.; Kiselev, O.I.; Lobzin, Y.V.; Filippov, A.E.; Casscells, S.W. Influenza Epidemics and Acute Respiratory Disease Activity Are Associated with a Surge in Autopsy-Confirmed Coronary Heart Disease Death: Results from 8 Years of Autopsies in 34,892 Subjects. Eur. Heart J. 2007, 28, 1205–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, N.D.; Ovsyannikova, I.G.; Pankratz, V.S.; Jacobson, R.M.; Poland, G.A. Understanding the Immune Response to Seasonal Influenza Vaccination in Older Adults: A Systems Biology Approach. Expert Rev. Vaccines 2012, 11, 985–994. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElhaney, J.E.; Verschoor, C.P.; Andrew, M.K.; Haynes, L.; Kuchel, G.A.; Pawelec, G. The Immune Response to Influenza in Older Humans: Beyond Immune Senescence. Immun. Ageing 2020, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Tu, W.; Liu, Y.; Qin, G.; Zheng, J.; Chan, P.-L.; Lam, K.-T.; Peiris, J.S.M.; Lau, Y.-L. Inhibition of Human Natural Killer Cell Activity by Influenza Virions and Hemagglutinin. J. Virol. 2010, 84, 4148–4157. [Google Scholar] [CrossRef] [Green Version]
- Pizzolla, A.; Nguyen, T.H.O.; Sant, S.; Jaffar, J.; Loudovaris, T.; Mannering, S.I.; Thomas, P.G.; Westall, G.P.; Kedzierska, K.; Wakim, L.M. Influenza-Specific Lung-Resident Memory T Cells Are Proliferative and Polyfunctional and Maintain Diverse TCR Profiles. J. Clin. Investig. 2018, 128, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory Shock in Children during COVID-19 Pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Vella, L.A.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Diorio, C.; Kuri-Cervantes, L.; Alanio, C.; Pampena, M.B.; Wu, J.E.; Chen, Z.; et al. Deep Immune Profiling of MIS-C Demonstrates Marked but Transient Immune Activation Compared to Adult and Pediatric COVID-19. Sci. Immunol. 2021, 6, eabf7570. [Google Scholar] [CrossRef] [PubMed]
- Connors, T.J.; Ravindranath, T.M.; Bickham, K.L.; Gordon, C.L.; Zhang, F.; Levin, B.; Baird, J.S.; Farber, D.L. Airway CD8+ T Cells Are Associated with Lung Injury during Infant Viral Respiratory Tract Infection. Am. J. Respir. Cell Mol. Biol. 2016, 54, 822–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, J.R.; Lee, R.D. Demographic Perspectives on the Mortality of COVID-19 and Other Epidemics. Proc. Natl. Acad. Sci. USA 2020, 117, 22035–22041. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanettini, C.; Omar, M.; Dinalankara, W.; Imada, E.L.; Colantuoni, E.; Parmigiani, G.; Marchionni, L. Influenza Vaccination and COVID-19 Mortality in the USA: An Ecological Study. Vaccines 2021, 9, 427. https://doi.org/10.3390/vaccines9050427
Zanettini C, Omar M, Dinalankara W, Imada EL, Colantuoni E, Parmigiani G, Marchionni L. Influenza Vaccination and COVID-19 Mortality in the USA: An Ecological Study. Vaccines. 2021; 9(5):427. https://doi.org/10.3390/vaccines9050427
Chicago/Turabian StyleZanettini, Claudio, Mohamed Omar, Wikum Dinalankara, Eddie Luidy Imada, Elizabeth Colantuoni, Giovanni Parmigiani, and Luigi Marchionni. 2021. "Influenza Vaccination and COVID-19 Mortality in the USA: An Ecological Study" Vaccines 9, no. 5: 427. https://doi.org/10.3390/vaccines9050427
APA StyleZanettini, C., Omar, M., Dinalankara, W., Imada, E. L., Colantuoni, E., Parmigiani, G., & Marchionni, L. (2021). Influenza Vaccination and COVID-19 Mortality in the USA: An Ecological Study. Vaccines, 9(5), 427. https://doi.org/10.3390/vaccines9050427





