Dissolvable Microneedle Patches to Enable Increased Access to Vaccines against SARS-CoV-2 and Future Pandemic Outbreaks
Abstract
1. Introduction
2. Discussion
2.1. Overcoming Barriers to Effective Vaccination
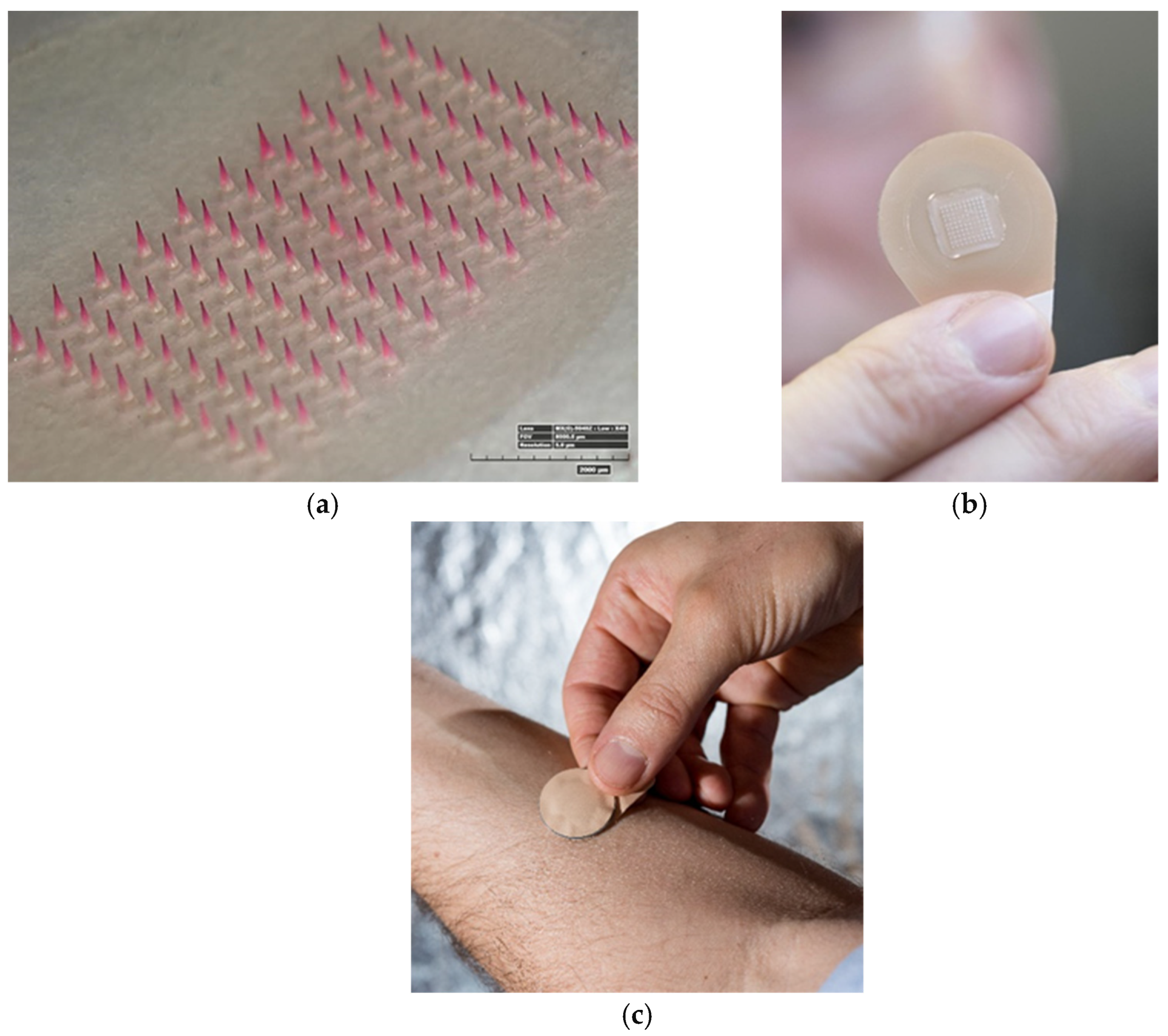
2.2. COVID-19 Microneedle Applications
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Webby, R.J.; Webster, R.G. Are we ready for pandemic influenza? Science 2003, 302, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- World Health Organization. WHO Concept for Fair Access and Equitable Allocation of COVID-19 Health Prod-ucts; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/m/item/fair-allocation-mechanism-for-covid-19-vaccines-through-the-covax-facility (accessed on 29 January 2021).
- Rose, A.; Triano, C.; Alatovic, J.; Maas, S. “Pfizer and Biontech Conclude Phase 3 Study of Covid-19 Vaccine Candidate.” Meeting All Primary Efficacy Endpoints. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine (accessed on 29 January 2021).
- Moderna Therapeutics. Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. Moderna. Available online: https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-longer-shelf-life-its-covid-19-vaccine (accessed on 29 January 2021).
- Voysey, M.; Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Corum, J.; Wee, S.; Zimmer, C. Coronavirus Vaccine Tracker. 2020. Available online: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html (accessed on 29 January 2021).
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 29 January 2021).
- Fisher, K.A.; Bloomstone, S.J.; Walder, J.; Crawford, S.; Fouayzi, H.; Mazor, K.M. Attitudes toward a potential SARS-CoV-2 vac-cine: A survey of US adults. Ann. Intern. Med. 2020, 173, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of po-tential acceptance of a COVID-19 vaccine. Nat. Med. 2020, 27, 225–228. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar]
- Rodgers, A.M.; Cordeiro, A.S.; Donnelly, R.F. Technology update: Dissolvable microneedle patches for vaccine delivery. Med. Devices Évid. Res. 2019, 12, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.P.; Koutsonanos, D.G.; Martin, M.D.P.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef]
- Kim, Y.C.; Song, J.M.; Lipatov, A.S.; Choi, S.O.; Lee, J.W.; Donis, R.O.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Increased immuno-genicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. Eur. J. Phar-Maceutics Biopharm. 2012, 81, 239–247. [Google Scholar] [CrossRef]
- Arya, J.; Prausnitz, M.R. Microneedle patches for vaccination in developing countries. J. Control. Release 2016, 240, 135–141. [Google Scholar] [CrossRef]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef]
- Frew, P.M.; Paine, M.B.; Rouphael, N.; Schamel, J.; Chung, Y.; Mulligan, M.J.; Prausnitz, M.R. Acceptability of an inactivated in-fluenza vaccine delivered by microneedle patch: Results from a phase I clinical trial of safety, reactogenicity, and im-munogenicity. Vaccine 2020, 38, 7175–7181. [Google Scholar] [CrossRef]
- Yang, H.-W.; Ye, L.; Guo, X.D.; Yang, C.; Compans, R.W.; Prausnitz, M.R. Ebola Vaccination Using a DNA Vaccine Coated on PLGA-PLL/γPGA Nanoparticles Administered Using a Microneedle Patch. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Matsuo, K.; Hirobe, S.; Yokota, Y.; Ayabe, Y.; Seto, M.; Quan, Y.S.; Kamiyama, F.; Tougan, T.; Horii, T.; Mukai, Y.; et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J. Control. Release 2012, 160, 495–501. [Google Scholar] [CrossRef]
- Hiraishi, Y.; Nakagawa, T.; Quan, Y.-S.; Kamiyama, F.; Hirobe, S.; Okada, N.; Nakagawa, S. Performance and characteristics evaluation of a sodium hyaluronate-based microneedle patch for a transcutaneous drug delivery system. Int. J. Pharm. 2013, 441, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Romeijn, S.; Du, G.; Dévédec, S.E.; Vrieling, H.; O’Mahony, C.; Bouwstra, J.A.; Kersten, G. Diphtheria toxoid dis-solving microneedle vaccination: Adjuvant screening and effect of repeated-fractional dose administration. Int. J. Pharm. 2020, 580, 119182. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, A.; Allen, E.A.; Ferrara, F.; Temperton, N.J.; Crean, A.M.; Moore, A.C. Induction of broad immunity by thermostabi-lised vaccines incorporated in dissolvable microneedles using novel fabrication methods. J. Control. Release 2016, 225, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Erdos, G.; Donahue, C.; Zhang, J.; Ozdoganlar, B.; Gambotto, A.; Falo, L. Dissolvable microneedle arrays deliver live adeno-virus to the skin for genetic immunization. J. Immunol. 2012, 188, 58. [Google Scholar]
- Erdos, G.; Balmert, S.C.; Carey, C.D.; Falo, G.D.; Patel, N.A.; Zhang, J.; Gambotto, A.; Korkmaz, E.; Falo, L.D. Improved cutaneous genetic immunization by microneedle array delivery of an adjuvanted adenovirus vaccine. J. Investig. Dermatol. 2020, 140, 2528–2531.e2. [Google Scholar] [CrossRef]
- Bachy, V.; Hervouet, C.; Becker, P.D.; Chorro, L.; Carlin, L.M.; Herath, S.; Papagatsias, T.; Barbaroux, J.B.; Oh, S.J.; Benlahrech, A.; et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live ad-enovirus vaccine microneedle arrays. Proc. Natl. Acad. Sci. USA 2013, 110, 3041–3046. [Google Scholar] [CrossRef]
- Becker, P.D.; Hervouet, C.; Mason, G.M.; Kwon, S.-Y.; Klavinskis, L.S. Skin vaccination with live virus vectored microneedle arrays induce long lived CD8+ T cell memory. Vaccine 2015, 33, 4691–4698. [Google Scholar] [CrossRef]
- Zaric, M.; Becker, P.D.; Hervouet, C.; Kalcheva, P.; Yus, B.I.; Cocita, C.; O’Neill, L.A.; Kwon, S.Y.; Klavinskis, L.S. Long-lived tissue resident HIV-1 specific memory CD8+ T cells are generated by skin immunization with live virus vectored micronee-dle arrays. J. Control. Release 2017, 268, 166–175. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, L.; Zhang, S.; Xu, B.; Gao, Y.; Hu, Y.; Hou, J.; Bai, B.; Shen, H.; Mao, P. DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug Deliv. 2016, 23, 2391–2398. [Google Scholar] [CrossRef]
- Zhu, Z.; Ye, X.; Ku, Z.; Liu, Q.; Shen, C.; Luo, H.; Luan, H.; Zhang, C.; Tian, S.; Lim, C.; et al. Transcutaneous immunization via rapidly dissolvable microneedles protects against hand-foot-and-mouth disease caused by enterovirus 71. J. Control. Release 2016, 243, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Nakatsukasa, A.; Kuruma, K.; Okamatsu, M.; Hiono, T.; Suzuki, M.; Matsuno, K.; Kida, H.; Oyamada, T.; Sakoda, Y. Potency of whole virus particle and split virion vaccines using dissolving microneedle against challenges of H1N1 and H5N1 in-fluenza viruses in mice. Vaccine 2017, 35, 2855–2861. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Prausnitz, M.R. Separable arrowhead microneedles. J. Control. Release 2011, 149, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Ye, L.; Dong, K.; Compans, R.W.; Yang, C.; Prausnitz, M.R. Enhanced stability of inactivated influenza vaccine en-capsulated in dissolving microneedle patches. Pharm. Res. 2016, 33, 868–878. [Google Scholar] [CrossRef]
- Raphael, A.P.; Prow, T.W.; Crichton, M.L.; Chen, X.; Fernando, G.J.; Kendall, M.A. Targeted, needle-free vaccinations in skin us-ing multilayered, densely-packed dissolving microprojection arrays. Small 2010, 6, 1785–1793. [Google Scholar] [CrossRef]
- Kuwentrai, C.; Yu, J.; Rong, L.; Zhang, B.Z.; Hu, Y.F.; Gong, H.R.; Dou, Y.; Deng, J.; Huang, J.D.; Xu, C. Intradermal delivery of re-ceptor-binding domain of SARS-CoV-2 spike protein with dissolvable microneedles to induce humoral and cellular responses in mice. Bioeng. Transl. Med. 2020, 6, e10202. [Google Scholar]
- Liao, J.F.; Lee, J.C.; Lin, C.K.; Wei, K.C.; Chen, P.Y.; Yang, H.W. Self-assembly DNA polyplex vaccine inside dissolving mi-croneedles for high-potency intradermal vaccination. Theranostics 2017, 7, 2593. [Google Scholar] [CrossRef]
- Poirier, D.; Renaud, F.; Dewar, V.; Strodiot, L.; Wauters, F.; Janimak, J.; Shimada, T.; Nomura, T.; Kabata, K.; Kuruma, K.; et al. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials 2017, 145, 256–265. [Google Scholar] [CrossRef]
- Cuevas, M.B.; Kodani, M.; Choi, Y.; Joyce, J.; O’connor, S.M.; Kamili, S.; Prausnitz, M.R. Hepatitis B vaccination using a dissolvable microneedle patch is immunogenic in mice and rhesus macaques. Bioeng. Transl. Med. 2018, 3, 186–196. [Google Scholar] [CrossRef]
- Wang, T.; Zhen, Y.; Ma, X.; Wei, B.; Li, S.; Wang, N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids Surfaces B Biointerfaces 2015, 126, 520–530. [Google Scholar] [CrossRef]
- Edens, C.; Collins, M.L.; Goodson, J.L.; Rota, P.A.; Prausnitz, M.R. A microneedle patch containing measles vaccine is immu-nogenic in non-human primates. Vaccine 2015, 33, 4712–4718. [Google Scholar] [CrossRef]
- Joyce, J.C.; Carroll, T.D.; Collins, M.L.; Chen, M.H.; Fritts, L.; Dutra, J.C.; Rourke, T.L.; Goodson, J.L.; McChesney, M.B.; Prausnitz, M.R.; et al. A microneedle patch for measles and rubella vaccination is immunogenic and protective in infant rhesus ma-caques. J. Infect. Dis. 2018, 218, 124–132. [Google Scholar] [CrossRef]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.; Falo, L.D., Jr.; Gambotto, A. Preventative vaccines for Zika virus outbreak: Prelim-inary evaluation. EBioMedicine 2016, 13, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Arya, J.M.; Dewitt, K.; Scott-Garrard, M.; Chiang, Y.W.; Prausnitz, M.R. Rabies vaccination in dogs using a dissolving mi-croneedle patch. J. Control. Release 2016, 239, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Pattani, A.; McKay, P.F.; Garland, M.J.; Curran, R.M.; Migalska, K.; Cassidy, C.M.; Malcolm, R.K.; Shattock, R.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J. Control. Release 2012, 162, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liu, H.; Cheng, Z.; Xue, Y.; Cheng, Z.; Dai, X.; Shan, W.; Chen, F. Immunotherapeutic effect of BCG-polysaccharide nu-cleic acid powder on Mycobacterium tuberculosis-infected mice using microneedle patches. Drug Deliv. 2017, 24, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.; Ali, A.A.; McCrudden, C.M.; McBride, J.W.; McCaffrey, J.; Robson, T.; Kett, V.L.; Dunne, N.J.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer: Strategic optimisation of RALA mediated gene delivery from a biodegradable microneedle system. Eur. J. Pharm. Biopharm. 2018, 127, 288–297. [Google Scholar] [CrossRef]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 921–932. [Google Scholar] [CrossRef]
- Wang, N.; Zhen, Y.; Jin, Y.; Wang, X.; Li, N.; Jiang, S.; Wang, T. Combining different types of multifunctional liposomes loaded with ammonium bicarbonate to fabricate microneedle arrays as a vaginal mucosal vaccine adjuvant-dual delivery sys-tem (VADDS). J. Control. Release 2017, 246, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Gala, R.P.; Zaman, R.U.; D’Souza, M.J.; Zughaier, S.M. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Donadei, A.; Kraan, H.; Ophorst, O.; Flynn, O.; O’Mahony, C.; Soema, P.C.; Moore, A.C. Skin delivery of trivalent Sabin inacti-vated poliovirus vaccine using dissolvable microneedle patches induces neutralizing antibodies. J. Control. Release 2019, 311, 96–103. [Google Scholar] [CrossRef]
- Edens, C.; Dybdahl-Sissoko, N.C.; Weldon, W.C.; Oberste, M.S.; Prausnitz, M.R. Inactivated polio vaccination using a mi-croneedle patch is immunogenic in the rhesus macaque. Vaccine 2015, 33, 4683–4690. [Google Scholar] [CrossRef]
- Kolluru, C.; Gomaa, Y.; Prausnitz, M.R. Development of a thermostable microneedle patch for polio vaccination. Drug Deliv. Transl. Res. 2019, 9, 192–203. [Google Scholar] [CrossRef]
- Vassilieva, E.V.; Kalluri, H.; McAllister, D.; Taherbhai, M.T.; Esser, E.S.; Pewin, W.P.; Pulit-Penaloza, J.A.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. Improved immunogenicity of individual influenza vaccine components delivered with a novel dis-solving microneedle patch stable at room temperature. Drug Deliv. Transl. Res. 2015, 5, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, S.; Baudner, B.C.; Oh, S.; Kwon, S.-Y.; Singh, M.; O’Hagan, D.T. Dissolvable Microneedle Patches for the Delivery of Cell-Culture-Derived Influenza Vaccine Antigens. J. Pharm. Sci. 2012, 101, 1021–1027. [Google Scholar] [CrossRef]
- Allen, E.A.; O’Mahony, C.; Cronin, M.; O’Mahony, T.; Moore, A.C.; Crean, A.M. Dissolvable microneedle fabrication using pi-ezoelectric dispensing technology. Int. J. Pharm. 2016, 500, 1–10. [Google Scholar] [CrossRef]
- Mistilis, M.J.; Bommarius, A.S.; Prausnitz, M.R. Development of a thermostable microneedle patch for influenza vaccina-tion. J. Pharm. Sci. 2015, 104, 740–749. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Wu, M.X. Effective and lesion-free cutaneous influenza vaccination. Proc. Natl. Acad. Sci. USA 2015, 112, 5005–5010. [Google Scholar] [CrossRef] [PubMed]
- Mistilis, M.J.; Joyce, J.C.; Esser, E.S.; Skountzou, I.; Compans, R.W.; Bommarius, A.S.; Prausnitz, M.R. Long-term stability of influ-enza vaccine in a dissolving microneedle patch. Drug Deliv. Transl. Res. 2017, 7, 195–205. [Google Scholar] [CrossRef]
- Vassilieva, E.V.; Wang, S.; Li, S.; Prausnitz, M.R.; Compans, R.W. Skin immunization by microneedle patch overcomes statin-induced suppression of immune responses to influenza vaccine. Sci. Rep. 2017, 7, 17855. [Google Scholar] [CrossRef]
- Littauer, E.Q.; Mills, L.K.; Brock, N.; Esser, E.S.; Romanyuk, A.; Pulit-Penaloza, J.A.; Vassilieva, E.V.; Beaver, J.T.; Antao, O.; Krammer, F.; et al. Stable incorporation of GM-CSF into dissolvable microneedle patch improves skin vaccination against influenza. J. Control. Release 2018, 276, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Esser, E.S.; Pulit-Penaloza, J.A.; Kalluri, H.; McAllister, D.; Vassilieva, E.V.; Littauer, E.Q.; Lelutiu, N.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. Microneedle patch delivery of influenza vaccine during pregnancy enhances maternal immune re-sponses promoting survival and long-lasting passive immunity to offspring. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Hirobe, S.; Azukizawa, H.; Hanafusa, T.; Matsuo, K.; Quan, Y.-S.; Kamiyama, F.; Katayama, I.; Okada, N.; Nakagawa, S. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials 2015, 57, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Pewin, W.; Wang, C.; Luo, Y.; Gonzalez, G.X.; Mohan, T.; Prausnitz, M.R.; Wang, B.-Z. A boosting skin vaccination with dissolving microneedle patch encapsulating M2e vaccine broadens the protective efficacy of conventional influenza vaccines. J. Control. Release 2017, 261, 1–9. [Google Scholar] [CrossRef]
- Deng, L.; Chang, T.Z.; Wang, Y.; Li, S.; Wang, S.; Matsuyama, S.; Yu, G.; Compans, R.W.; Li, J.D.; Prausnitz, M.R.; et al. Het-erosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E7758–E7767. [Google Scholar] [CrossRef]
- Zhu, W.; Li, S.; Wang, C.; Yu, G.; Prausnitz, M.R.; Wang, B.-Z. Enhanced Immune Responses Conferring Cross-Protection by Skin Vaccination With a Tri-Component Influenza Vaccine Using a Microneedle Patch. Front. Immunol 2018, 9, 1705. [Google Scholar] [CrossRef]
- Rodgers, A.M.; McCrudden, M.T.; Vincente-Perez, E.M.; Dubois, A.V.; Ingram, R.J.; Larrañeta, E.; Kissenpfennig, A.; Donnelly, R.F. Design and characterisation of a dissolving microneedle patch for intradermal vaccination with heat-inactivated bacte-ria: A proof of concept study. Int. J. Pharm. 2018, 549, 87–95. [Google Scholar] [CrossRef]
- Lanza, J.S.; Vucen, S.; Flynn, O.; Donadei, A.; Cojean, S.; Loiseau, P.M.; Fernandes, A.P.S.; Frézard, F.; Moore, A.C. A TLR9-adjuvanted vaccine formulated into dissolvable microneedle patches or cationic liposomes protects against leishmaniasis after skin or subcutaneous immunization. Int. J. Pharm 2020, 586, 119390. [Google Scholar] [CrossRef] [PubMed]
- Resch, T.K.; Wang, Y.; Moon, S.S.; Joyce, J.; Li, S.; Prausnitz, M.; Jiang, B. Inactivated rotavirus vaccine by parenteral administra-tion induces mucosal immunity in mice. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine 2020, 55, 102743. [Google Scholar] [CrossRef]
- Hsueh, K.J.; Chen, M.C.; Cheng, L.T.; Lee, J.W.; Chung, W.B.; Chu, C.Y. Transcutaneous immunization of Streptococcus suis bac-terin using dissolving microneedles. Comp. Immunol. Microbiol. Infect. Dis. 2017, 50, 78–87. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Duan, Y.; Niu, Y.; Gu, H.; Zhao, Z.; Zhang, S.; Yang, Y.; Wang, X.; Gao, Y.; et al. Transcutaneous immunization of recombinant Staphylococcal enterotoxin B protein using a dissolving microneedle provides potent protection against lethal enterotoxin challenge. Vaccine 2019, 37, 3810–3819. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yan, Q.; Yu, Y.; Wu, M.X. BCG vaccine powder-laden and dissolvable microneedle arrays for lesion-free vaccina-tion. J. Control. Release 2017, 255, 36–44. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Kim, S.; Lahiji, S.F.; Ha, N.-Y.; Yang, H.; Kang, G.; Nguyen, H.Y.T.; Kim, Y.; Choi, M.-S.; et al. Comparative Study of Two Droplet-Based Dissolving Microneedle Fabrication Methods for Skin Vaccination. Adv. Healthc. Mater. 2018, 7, e1701381. [Google Scholar] [CrossRef]
- Esser, E.S.; Romanyuk, A.; Vassilieva, E.V.; Jacob, J.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy. J. Control. Release 2016, 236, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, S.; Azukizawa, H.; Matsuo, K.; Zhai, Y.; Quan, Y.S.; Kamiyama, F.; Suzuki, H.; Katayama, I.; Okada, N.; Nakagawa, S. De-velopment and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharm. Res. 2013, 30, 2664–2674. [Google Scholar] [CrossRef]
- Yan, Q.; Cheng, Z.; Liu, H.; Shan, W.; Cheng, Z.; Dai, X.; Xue, Y.; Chen, F. Enhancement of Ag85B DNA vaccine immunogenicity against tuberculosis by dissolving microneedles in mice. Vaccine 2018, 36, 4471–4476. [Google Scholar] [CrossRef]
- Arya, J.; Henry, S.; Kalluri, H.; McAllister, D.V.; Pewin, W.P.; Prausnitz, M.R. Tolerability, usability and acceptability of dis-solving microneedle patch administration in human subjects. Biomaterials 2017, 128, 1–7. [Google Scholar] [CrossRef]
- De Perio, M.A. Needlestick injuries among employees at a retail pharmacy chain-nationwide. In Health Hazard Evaluation Report 2011-0063-3154 Needlestick Injuries among Employees at retail Pharmacy Chain–Nationwide; Centers for Dis-ease Control and Prevention: Atlanta, GA, USA, 2012. [Google Scholar]
- PrüssÜstün, A.; Rapiti, E.; Hutin, Y.J. Sharps Injuries: Global Burden of Disease from Sharps Injuries to Health-Care Workers; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Quinn, H.L.; Kearney, M.-C.; Courtenay, A.J.; McCrudden, M.T.C.; Donnelly, R.F. The role of microneedles for drug and vaccine delivery. Expert Opin. Drug Deliv. 2014, 11, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, M.; Shan, H.; Tong, C. Microneedle Patches as Drug and Vaccine Delivery Platform. Curr. Med. Chem. 2017, 24, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Alliance, T.W.B.G. Immunization Financing Toolkit: A Resource for Policy-Makers and Program Managers; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Lee, B.Y.; Bartsch, S.M.; Mvundura, M.; Jarrahian, C.; Zapf, K.M.; Marinan, K.; Wateska, A.R.; Snyder, B.; Swaminathan, S.; Jacoby, E.; et al. An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. Vaccine 2015, 33, 4727–4736. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Arya, J.M.; McClain, M.A.; Frew, P.M.; Meltzer, M.I.; Prausnitz, M.R. Microneedle patches: Usability and acceptabil-ity for self-vaccination against influenza. Vaccine 2014, 32, 1856–1862. [Google Scholar] [CrossRef]
- BARDA’s Rapidly Expanding COVID-19 Medical Countermeasure Portfolio. Available online: https://www.medicalcountermeasures.gov/app/barda/coronavirus/COVID19.aspx (accessed on 29 January 2021).
| Nucleic Acid | Protein-Based (Including Virus-Like Particle (VLP)) | Inactivated/Live Attenuated | Viral Vector |
|---|---|---|---|
| Ebola [18] | Diphtheria [19,20,21] | Adenovirus [22,23,24] | HIV [25,26,27] |
| Hepatitis B virus [28] | EV71 hand-foot-and-mouth disease (HFMD) [29] | Influenza [16,30,31,32,33] | Middle East Respiratory Syndrome (MERS-CoV-S1) [34] |
| Porcine circovirus type 2 [35] | Hepatitis B [36,37,38] | Measles [39,40] | Zika [41] |
| Rabies [42] | Human immunodeficiency virus (HIV) [43] | Modified vaccinia virus Ankara (MVA) [22] | |
| Tuberculosis bacillus Calmette–Guérin (BCG) [44] | Human papillomavirus infection (HPV) [45,46] | Neisseria gonorrhoeae [47] | |
| Herpes simplex virus 2 [48] | Poliovirus [49,50,51] | ||
| Influenza [19,22,52,53,54,55,56,57,58,59,60,61,62,63,64] | Pseudomonas aeruginosa [65] | ||
| Leishmania [66] | Rotavirus [67] | ||
| Malaria [19] | Rubella [39] | ||
| SARS-2-CoV [34,68] | Streptococcus [69] | ||
| Staphylococcal [70] | Tuberculosis bacillus Calmette–Guérin (BCG) [71] | ||
| Scrub typhus [72] | |||
| Tetanus (toxoid) [20,73,74] | |||
| Mycobacterium tuberculosis [75] | |||
| Zika [41] |
| Increased Immunogenicity |
|---|
| Faster virus clearance |
| Dose-sparing effect |
| Reduction in vaccination wastage |
| Avoidance of reconstitution |
| Increased acceptance and less hesitancy |
| Little or no pain |
| Self-administration and reduced need for healthcare workforce |
| Reduced risk of sharps injury and contamination |
| Improved stability |
| Less reliance on cold chain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Shea, J.; Prausnitz, M.R.; Rouphael, N. Dissolvable Microneedle Patches to Enable Increased Access to Vaccines against SARS-CoV-2 and Future Pandemic Outbreaks. Vaccines 2021, 9, 320. https://doi.org/10.3390/vaccines9040320
O’Shea J, Prausnitz MR, Rouphael N. Dissolvable Microneedle Patches to Enable Increased Access to Vaccines against SARS-CoV-2 and Future Pandemic Outbreaks. Vaccines. 2021; 9(4):320. https://doi.org/10.3390/vaccines9040320
Chicago/Turabian StyleO’Shea, Jesse, Mark R. Prausnitz, and Nadine Rouphael. 2021. "Dissolvable Microneedle Patches to Enable Increased Access to Vaccines against SARS-CoV-2 and Future Pandemic Outbreaks" Vaccines 9, no. 4: 320. https://doi.org/10.3390/vaccines9040320
APA StyleO’Shea, J., Prausnitz, M. R., & Rouphael, N. (2021). Dissolvable Microneedle Patches to Enable Increased Access to Vaccines against SARS-CoV-2 and Future Pandemic Outbreaks. Vaccines, 9(4), 320. https://doi.org/10.3390/vaccines9040320






