Systematic Booster after Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
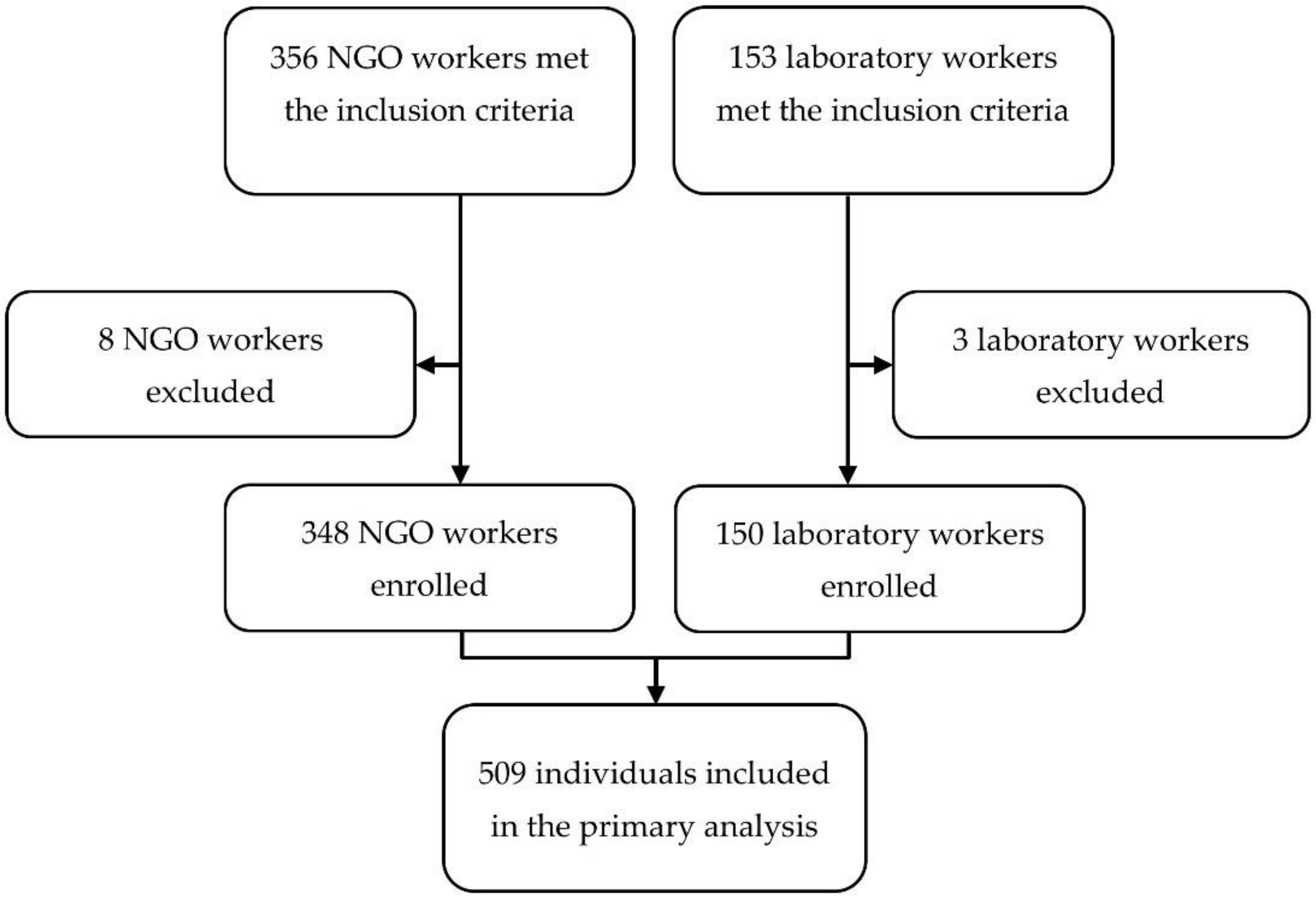
2.2. Population and Data
2.3. Postvaccination Rabies Antibodies Titration
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
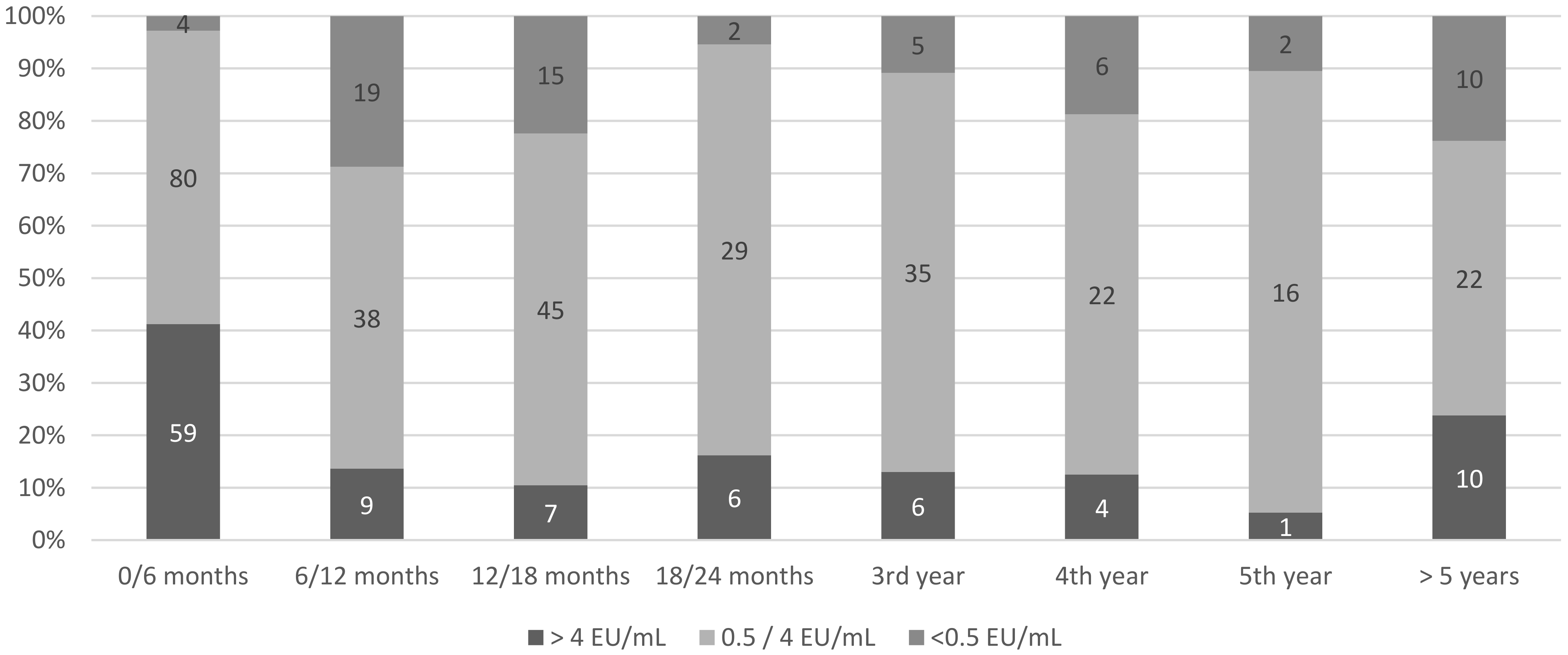
3.2. Postprimary Rabies Vaccination Rresponse
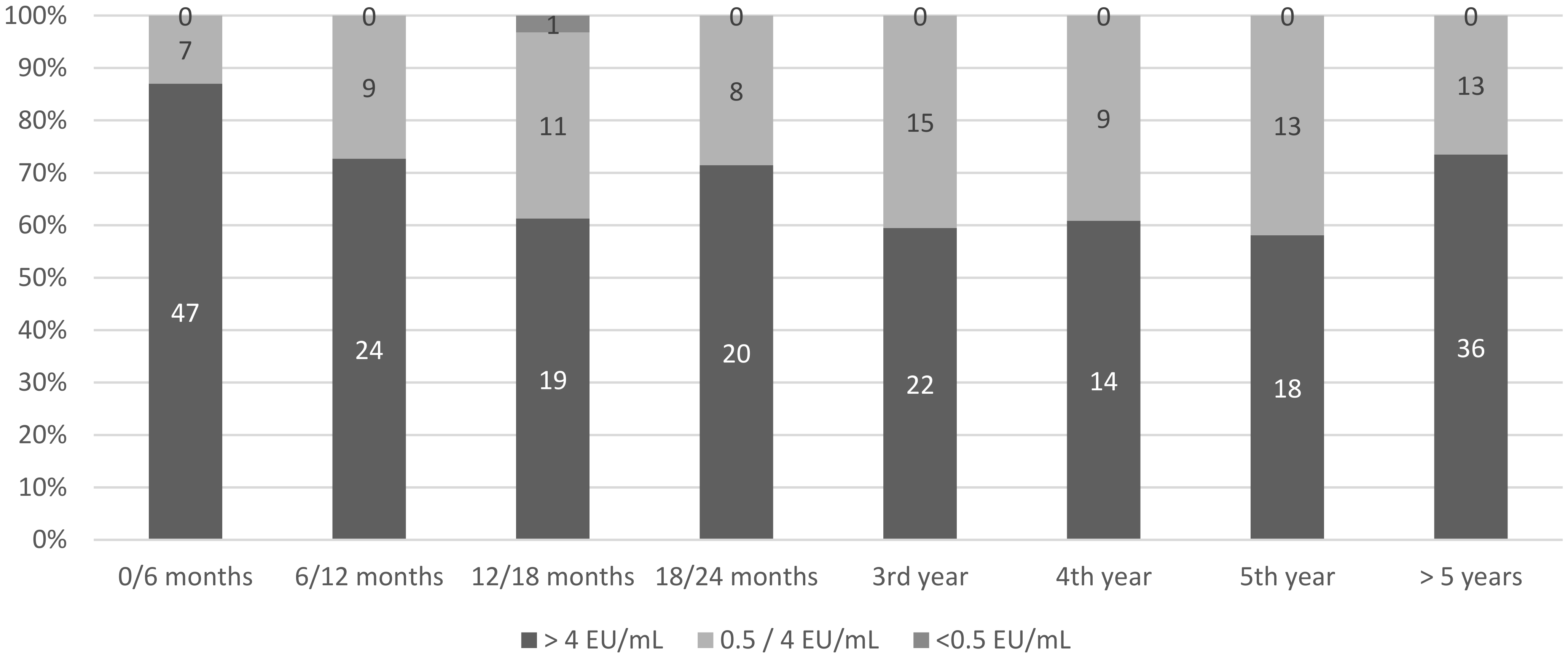
3.3. Postbooster Rabies Vaccine Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primer 2017, 3, 17091. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Rabies Vaccines: WHO Position Paper, April 2018—Recommendations. Vaccine 2018, 36, 5500–5503. [Google Scholar] [CrossRef] [PubMed]
- Hemachudha, T.; Ugolini, G.; Wacharapluesadee, S.; Sungkarat, W.; Shuangshoti, S.; Laothamatas, J. Human rabies: Neuropath-ogenesis, diagnosis, and management. Lancet Neurol. 2013, 12, 498–513. [Google Scholar] [CrossRef]
- Langedijk, A.C.; De Pijper, C.A.; Spijker, R.; Holman, R.; Grobusch, M.P.; Stijnis, C. Rabies Antibody Response after Booster Im-munization: A Systematic Review and Meta-analysis. Clin Infect. Dis. 2018, 67, 1932–1947. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Consultation on Rabies: Third Report; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2018; p. 183. [Google Scholar]
- Kessels, J.A.; Recuenco, S.; Navarro-Vela, A.M.; Deray, R.; Vigilato, M.; Ertl, H.; Durrheim, D.; Rees, H.; Nel, L.H.; Abela-Ridder, B.; et al. Pre-exposure rabies prophylaxis: A systematic review. Bull. World Health Organ. 2016, 95, 210C–219C. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Consultation on Rabies; World Health Organisation Technical Report Series; World Health Organization: Geneva, Switzerland, 2005; pp. 1–88. [Google Scholar]
- World Health Organization. WHO Expert Consultation on Rabies. Second Report; World Health Organisation Technical Report Series; World Health Organization: Geneva, Switzerland, 2013; pp. 1–139. [Google Scholar]
- Lim, P.L.; Barkham, T.M. Serologic response to rabies pre-exposure vaccination in persons with potential occupational exposure in Singapore. Int. J. Infect. Dis. 2010, 14, e511–e513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mansfield, K.L.; Andrews, N.; Goharriz, H.; Goddard, T.; McElhinney, L.M.; Brown, K.E.; Fooks, A.R. Rabies pre-exposure prophylaxis elicits long-lasting immunity in humans. Vaccine 2016, 34, 5959–5967. [Google Scholar] [CrossRef] [PubMed]
- Dougas, G.; Mavrouli, M.; Vrioni, G.; Lytras, T.; Mellou, K.; Metallidis, S.; Istikoglou, I.; Mitrou, K.; Tzani, M.; Georgopoulou, I.; et al. Antibody Response Following Pre-Exposure Immunization Against Rabies in High-Risk Professionals. Vector Borne Zoonotic Dis. 2019, 20, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Feyssaguet, M.; Dacheux, L.; Audry, L.; Compoint, A.; Morize, J.; Blanchard, I.; Bourhy, H. Multicenter comparative study of a new ELISA, PLATELIA™ RABIES II, for the detection and titration of anti-rabies glycoprotein antibodies and comparison with the rapid fluorescent focus inhibition test (RFFIT) on human samples from vaccinated and non-vaccinated people. Vaccine 2007, 25, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Banga, N.; Guss, P.; Banga, A.; Rosenman, K.D. Incidence and variables associated with inadequate antibody titers after pre-exposure rabies vaccination among veterinary medical students. Vaccine 2014, 32, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Jusot, V.; Houillon, G.; Rasuli, A.; Martorelli, L.; Kataoka, A.P.; Ben Mechlia, M.; Le Guern, A.-S.; Rodrigues, L.; Assef, R.; et al. Persistence of Rabies Virus-Neutralizing Antibodies after Vaccination of Rural Population following Vampire Bat Rabies Outbreak in Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0004920. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.F. Sexual dimorphism of humoral immunity with human vaccines. Vaccine 2008, 26, 3551–3555. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Verthelyi, D. Sex hormones as immunomodulators in health and disease. Int. Immunopharmacol. 2001, 1, 983–993. [Google Scholar] [CrossRef]
- Mills, D.J.; Lau, C.L.; Fearnley, E.J.; Weinstein, P. The Immunogenicity of a Modified Intradermal Pre-exposure Rabies Vaccination Schedule—A Case Series of 420 Travelers. J. Travel Med. 2011, 18, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Ramsey, L.; Manson, M.; Gilbert, B.; Lau, C.L. Intradermal rabies pre-exposure vaccination schedules in older travellers: Comparison of immunogenicity post-primary course and post-booster. J. Travel Med. 2020, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- ACIP. Timing and Spacing Guidelines for Immunization. Recommendations. CDC. Available online: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/timing.html (accessed on 19 June 2020).
- Venkataswamy, M.M.; Madhusudana, S.N.; Sanyal, S.S.; Taj, S.; Belludi, A.Y.; Mani, R.S.; Hazra, N. Cellular immune response following pre-exposure and postexposure rabies vaccination by intradermal and intramuscular routes. Clin. Exp. Vaccine Res. 2015, 4, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yu, P.; Shan, Y.; Thirumeni, N.; Li, M.; Lv, Y.; Lid, J.; Rend, W.; Huang, L.; Weif, J.; et al. Rabies virus glycoprotein serology ELISA for measurement of neu-tralizing antibodies in sera of vaccinated human subjects. Vaccine 2019, 37, 6060–6067. [Google Scholar] [CrossRef] [PubMed]
| Variable | n (%) | Median (Interquartile Range) |
|---|---|---|
| Female sex at birth | 259 (52%) | |
| Age (years) | 32 (27–39) | |
| Laboratory workers | 150 (30.1%) | |
| NGO workers | 348 (69.9%) | |
| Specific medical conditions | 6 (1.2%) | |
| Pre-exposure rabies Prophylaxis (PrEP) | 498 (100%) | |
| Interval between first and last dose of vaccine | 23 (21–28) * | |
| Simultaneous administration of a nonrabies vaccine | 234 (47%) | |
| Concurrent treatment by chloroquine | 0 | |
| Individuals with post-PrEP serology | 355 (71.3%) | |
| Number of tests | 452 | |
| Time between PrEP and first titration (days) | 351 (72–865) | |
| Individuals with inadequate first titers | 52 (14.6%) | |
| Individuals with at least one inadequate titer during follow-up | 61 (17.2%) | |
| Time between primary vaccination and first inadequate titer (days) | 438 (326–1215) | |
| Individuals with postbooster serology | 220 (44.2%) | |
| Number of tests | 286 | |
| Time between PrEP and first booster (days) | 490 (398–842) | |
| Time between booster and first postbooster titer (days) | 636 (195–1517) | |
| Individuals with at least one inadequate titer during follow-up | 1 (0.5%) |
| Variables | Inadequate Titer <0.5 IU/mL (n = 52) | Adequate Titer ≥0.5 IU/mL (n = 303) | p-Value | Adjusted Odds Ratio (95% Confidence Interval) | p-Value |
|---|---|---|---|---|---|
| Sex at birth | <0.001 | <0.001 | |||
| Female | 12 (23.1) | 175 (57.8) | 1 (ref) | ||
| Male | 40 (76.9) | 128 (42.2) | 3.85 (1.86–7.93) | ||
| Age at PrEP (years) | 0.10 | 0.29 | |||
| <27 | 7 (13.5) | 86 (28.4) | 1 (ref) | ||
| 27–32 | 14 (26.9) | 84 (27.7) | 1.81 (0.65–5.06) | ||
| 33–38 | 15 (28.8) | 61 (20.1) | 2.63 (0.93–7.40) | ||
| >38 | 16 (30.8) | 72 (23.8) | 2.27 (0.81–6.35) | ||
| Occupation | <0.001 | 0.67 | |||
| Laboratory workers | 6 (11.5) | 132 (43.6) | 1 (ref) | ||
| NGO workers | 46 (88.5) | 171 (56.4) | 1.30 (0.39–4.35) | ||
| Duration between first and last PrEP dose | 0.13 | ||||
| <21 days | 4 (7.7) | 15 (5.0) | |||
| 22–28 days | 39 (75.0) | 209 (69.0) | |||
| >28 days | 9 (17.3) | 56 (18.5) | |||
| Time between first PrEP dose and serology | |||||
| <6 months | 3 (5.8) | 133 (43.8) | <0.001 | 1 (ref) | 0.002 |
| 6–24 months | 28 (53.8) | 85 (28.1) | 9.50 (2.70–33.36) | ||
| >24 months | 21 (40.4) | 85 (28.1) | 8.47 (2.37–30.30) | ||
| Nonrabies vaccine concurrent with rabies PrEP | 0.002 | 0.03 | |||
| Yes | 35 (67.3) | 133 (43.9) | 1 (ref) | ||
| No | 17 (32.7) | 170 (56.1) | 2.36 (1.11–5.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parize, P.; Sommé, J.; Schaeffer, L.; Ribadeau-Dumas, F.; Benabdelkader, S.; Durand, A.; Tarantola, A.; Cailhol, J.; Goesch, J.; Kergoat, L.; et al. Systematic Booster after Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure. Vaccines 2021, 9, 309. https://doi.org/10.3390/vaccines9040309
Parize P, Sommé J, Schaeffer L, Ribadeau-Dumas F, Benabdelkader S, Durand A, Tarantola A, Cailhol J, Goesch J, Kergoat L, et al. Systematic Booster after Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure. Vaccines. 2021; 9(4):309. https://doi.org/10.3390/vaccines9040309
Chicago/Turabian StyleParize, Perrine, Jérémie Sommé, Laura Schaeffer, Florence Ribadeau-Dumas, Sheherazade Benabdelkader, Agnès Durand, Arnaud Tarantola, Johann Cailhol, Julia Goesch, Lauriane Kergoat, and et al. 2021. "Systematic Booster after Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure" Vaccines 9, no. 4: 309. https://doi.org/10.3390/vaccines9040309
APA StyleParize, P., Sommé, J., Schaeffer, L., Ribadeau-Dumas, F., Benabdelkader, S., Durand, A., Tarantola, A., Cailhol, J., Goesch, J., Kergoat, L., Le Guern, A.-S., Mousel, M.-L., Dacheux, L., Consigny, P.-H., Fontanet, A., Francuz, B., & Bourhy, H. (2021). Systematic Booster after Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure. Vaccines, 9(4), 309. https://doi.org/10.3390/vaccines9040309








