Pruritus as a Distinctive Feature of Type 2 Inflammation
Abstract
1. Introduction
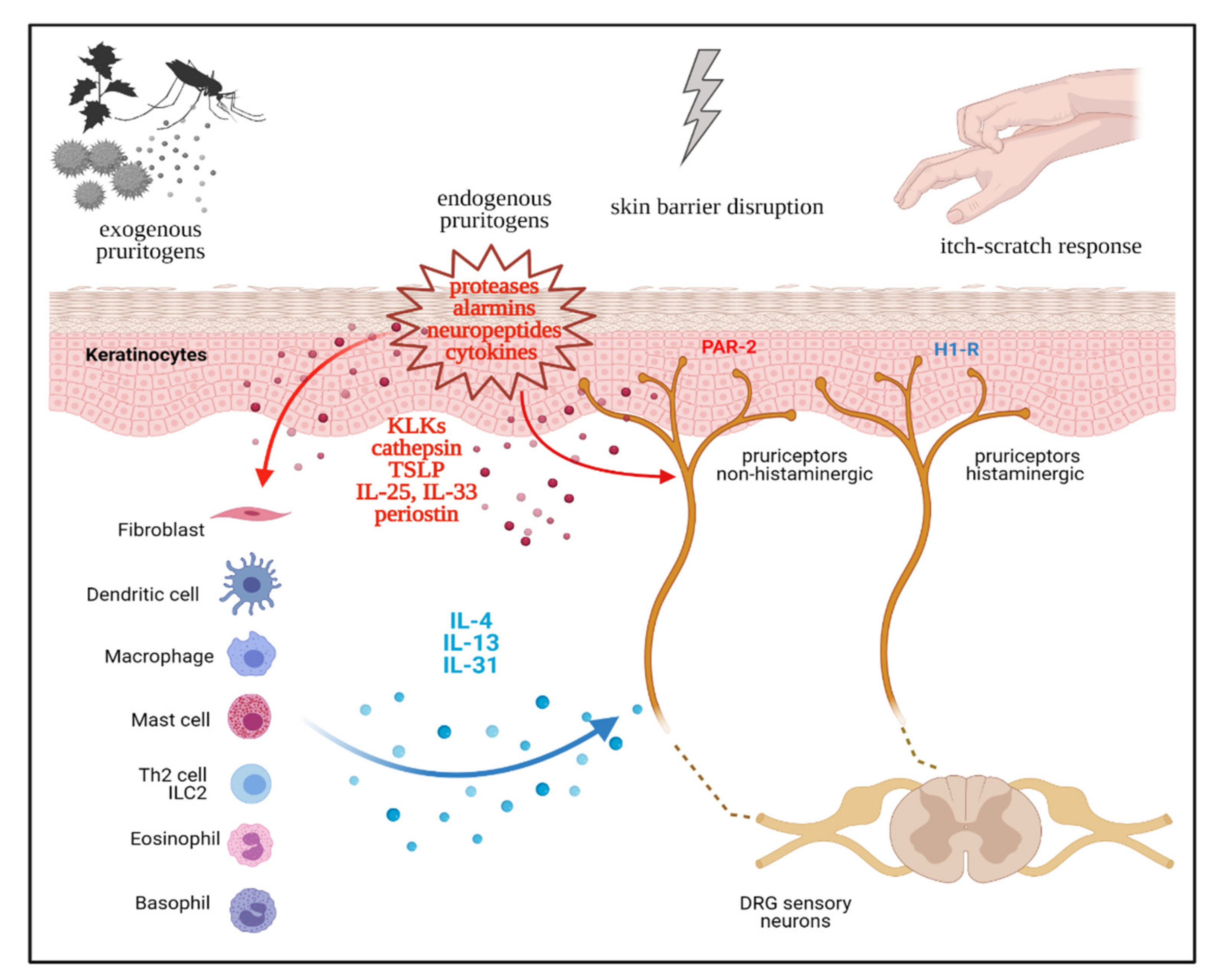
2. Neurophysiology of Pruritus
3. Th2 immunity as a Driver of Chronic Pruritus
4. Th2 Cytokines, Chronic Itch Sensitization, and the Itch-Scratch Cycle
5. The Top Itchy Skin Diseases
5.1. Scabies
5.2. Cutaneous Helminth Infestations
5.3. Insect Bite Reactions
5.4. Atopic Dermatitis
5.5. Prurigo Nodularis
5.6. Chronic Urticaria
5.7. Bullous Pemphigoid
5.8. Mycosis Fungoides and Sézary Syndrome
5.9. Chronic Pruritus of Unknown Origin and of the Elderly
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Weisshaar, E. Epidemiology of Itch. Curr. Probl. Dermatol. 2016, 50, 5–10. [Google Scholar] [PubMed]
- Matterne, U.; Apfelbacher, C.J.; Vogelgsang, L.; Loerbroks, A.; Weisshaar, E. Incidence and Determinants of Chronic Pruritus: A Population-based Cohort Study. Acta Derm. Venereol. 2013, 93, 532–537. [Google Scholar] [CrossRef]
- Ständer, S.; Weisshaar, E.; Mettang, T.; Szepietowski, J.C.; Carstens, E.; Ikoma, A.; Bergasa, N.V.; Gieler, U.; Misery, L.; Wallengren, J.; et al. Clinical Classification of Itch: A Position Paper of the International Forum for the Study of Itch. Acta Derm. Venereol. 2007, 87, 291–294. [Google Scholar] [CrossRef]
- Eyerich, K.; Eyerich, S. Immune Response Patterns in Non-communicable Inflammatory Skin Diseases. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The Immunological Anatomy of the Skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ricardo-Gonzalez, R.R.; Moro, K. Skin-Resident Innate Lymphoid Cells–Cutaneous Innate Guardians and Regulators. Trends Immunol. 2020, 41, 100–112. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From Mechanism to (Novel) Therapeutic Approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Najafi, P.; Dufor, O.; Ben Salem, D.; Misery, L.; Carré, J.-L. Itch Processing in the Brain. J. Eur. Acad. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef]
- Carstens, E.; Akiyama, T. Central Mechanisms of Itch. Curr. Probl. Dermatol. 2016, 50, 11–17. [Google Scholar] [CrossRef]
- Schmelz, M. Itch and Pain Differences and Commonalities. Handb. Exp. Pharmacol. 2015, 227, 285–301. [Google Scholar] [CrossRef]
- Fostini, A.C.; Girolomoni, G. Experimental Elicitation of Itch: Evoking and Evaluation Techniques. J. Dermatol. Sci. 2015, 80, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Hakim, S.; Woolf, C.J. Unraveling the Plastic Peripheral Neuroimmune Interactome. J. Immunol. 2020, 204, 257–263. [Google Scholar] [CrossRef]
- Talagas, M.; Lebonvallet, N.; Leschiera, R.; Sinquin, G.; Elies, P.; Haftek, M.; Pennec, J.-P.; Ressnikoff, D.; La Padula, V.; Le Garrec, R.; et al. Keratinocytes Communicate with Sensory Neurons via Synaptic-like Contacts. Ann. Neurol. 2020, 88, 1205–1219. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Berger, T.; Fassett, M.S. Neuroimmune Interactions in Chronic Itch of Atopic Dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Schwendinger-Schreck, J.; Wilson, S.R.; Bautista, D.M. Interactions between Keratinocytes and Somatosensory Neurons in Itch. Handb. Exp. Pharmacol. 2015, 226, 177–190. [Google Scholar] [CrossRef]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.E. IL-33/ST2 Signaling Excites Sensory Neurons and Mediates Itch Response in a Mouse Model of Poison Ivy Contact Allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The Epithelial Cell-derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef]
- Andoh, T.; Tsujii, K.; Kuraishi, Y. Increase in Pruritogenic Kallikrein 5 in the Skin of NC Mice with Chronic Dermatitis. Exp. Dermatol. 2015, 24, 978–980. [Google Scholar] [CrossRef]
- Guo, C.J.; Mack, M.R.; Oetjen, L.K.; Trier, A.M.; Council, M.L.; Pavel, A.B.; Guttman-Yassky, E.; Kim, B.S.; Liu, Q. Kallikrein 7 Promotes Atopic Dermatitis-Associated Itch Independently of Skin Inflammation. J. Invest. Dermatol. 2020, 140, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.B.; Sun, S.; Azimi, E.; Elmariah, S.B.; Dong, X.; Lerner, E.A. Redefining the Concept of Protease-activated Receptors: Cathepsin S Evokes Itch via Activation of Mrgprs. Nat. Commun. 2015, 6, 7864. [Google Scholar] [CrossRef]
- Chung, K.; Pitcher, T.; Grant, A.D.; Hewitt, E.; Lindstrom, E.; Malcangio, M. Cathepsin S Acts via Protease-activated Receptor 2 to Activate Sensory Neurons and Induce Itch-like Behaviour. Neurobiol. Pain 2019, 6, 100032. [Google Scholar] [CrossRef]
- Mishra, S.K.; Wheeler, J.J.; Pitake, S.; Ding, H.; Jiang, C.; Fukuyama, T.; Paps, J.S.; Ralph, P.; Coyne, J.; Parkington, M.; et al. Periostin Activation of Integrin Receptors on Sensory Neurons Induces Allergic Itch. Cell Rep. 2020, 31, 107472. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, M.; Shiraishi, H.; Ohta, S.; Suzuki, S.; Arima, K.; Aoki, S.; Toda, S.; Inagaki, N.; Kurihara, Y.; Hayashida, S.; et al. Periostin Promotes Chronic Allergic Inflammation in Response to Th2 Cytokines. J. Clin. Invest. 2012, 122, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Campion, M.; Smith, L.; Gatault, S.; Métais, C.; Buddenkotte, J.; Steinhoff, M. Interleukin-4 and Interleukin-13 Evoke Scratching Behaviour in Mice. Exp. Dermatol. 2019, 28, 1501–1504. [Google Scholar] [CrossRef]
- Bao, K.; Reinhardt, R.L. The Differential Expression of IL-4 and IL-13 and Its Impact on Type-2 Immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A New Link between T Cells and Pruritus in Atopic Skin Inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A Sensory Neuron-expressed IL-31 Receptor Mediates T Helper Cell-dependent Itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and Biological Functions of IL-31 and IL-31 Receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356. [Google Scholar] [CrossRef]
- Xu, M.; Dong, C. IL-25 in Allergic Inflammation. Immunol. Rev. 2017, 278, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gupta, K.; Dwivedi, P.D. Pathophysiology of IL-33 and IL-17 in Allergic Disorders. Cytokine Growth Factor Rev. 2017, 38, 22–36. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Hawro, T.; Saluja, R.; Weller, K.; Altrichter, S.; Metz, M.; Maurer, M. Interleukin-31 Does not Induce Immediate Itch in Atopic Dermatitis Patients and Healthy Controls after Skin Challenge. Allergy 2014, 69, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, A.; Fartasch, M.; Heyer, G.; Miyachi, Y.; Handwerker, H.; Schmelz, M. Painful Stimuli Evoke Itch in Patients with Chronic Pruritus: Central Sensitization for Itch. Neurology 2004, 62, 212–217. [Google Scholar] [CrossRef]
- Ständer, S.; Schmelz, M. Chronic Itch and Pain - Similarities and Differences. Eur. J. Pain 2006, 10, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Pogatzki-Zahn, E.M.; Pereira, M.P.; Cremer, A.; Zeidler, C.; Dreyer, T.; Riepe, C.; Wempe, C.; Lotts, T.; Segelcke, D.; Ringkamp, M.; et al. Peripheral Sensitization and Loss of Descending Inhibition Is a Hallmark of Chronic Pruritus. J. Invest. Dermatol. 2020, 140, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Akiyama, T.; Nattkemper, L.A.; van Laarhoven, A.; Elberling, J.; Yosipovitch, G.; Arendt-Nielsen, L. Alloknesis and Hyperknesis-mechanisms, Assessment Methodology, and Clinical Implications of Itch Sensitization. Pain 2018, 159, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Van Laarhoven, A.I.M.; Marker, J.B.; Elberling, J.; Yosipovitch, G.; Arendt-Nielsen, L.; Andersen, H.H. Itch Sensitization? A Systematic Review of Studies Using Quantitative Sensory Testing in Patients with Chronic Itch. Pain 2019, 160, 2661–2678. [Google Scholar] [CrossRef]
- Mack, M.R.; Kim, B.S. The Itch–Scratch Cycle: A Neuroimmune Perspective. Trends Immunol. 2018, 39, 980–991. [Google Scholar] [CrossRef]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The Pruritus- and TH2-associated Cytokine IL-31 Promotes Growth of Sensory Nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508. [Google Scholar] [CrossRef]
- Tominaga, M.; Takamori, K. Itch and Nerve Fibers with Special Reference to Atopic Dermatitis: Therapeutic Implications. J. Dermatol. 2014, 41, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-Dependent Pruritus in IL-13–Induced Chronic Atopic Dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef]
- Litman, T. Personalized Medicine-concepts, Technologies, and Applications in Inflammatory Skin Diseases. APMIS 2019, 127, 386–424. [Google Scholar] [CrossRef]
- Gudjonsson, J.E.; Kabashima, K.; Eyerich, K. Mechanisms of Skin Autoimmunity: Cellular and Soluble Immune Components of the Skin. J. Allergy Clin. Immunol. 2020, 146, 8–16. [Google Scholar] [CrossRef] [PubMed]
- de Bruin-Weller, M.; Gadkari, A.; Auziere, S.; Simpson, E.L.; Puig, L.; Barbarot, S.; Girolomoni, G.; Papp, K.; Pink, A.E.; Saba, G.; et al. The Patient-reported Disease Burden in Adults with Atopic Dermatitis: A Cross-sectional Study in Europe and Canada. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1026–1036. [Google Scholar] [CrossRef]
- Fourzali, K.; Golpanian, R.S.; Yosipovitch, G. Emerging Drugs for the Treatment of Chronic Pruritic Diseases. Expert Opin. Emerg. Drugs 2020, 25, 273–284. [Google Scholar] [CrossRef]
- Walton, S.F. The Immunology of Susceptibility and Resistance to Scabies. Parasite Immunol. 2010, 32, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Lalli, P.N.; Morgan, M.S.; Arlian, L.G. Skewed Th1/Th2 Immune Response to Sarcoptes scabiei. J. Parasitol. 2004, 90, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.F.; Pizzutto, S.; Slender, A.; Viberg, L.; Holt, D.; Hales, B.J.; Kemp, D.J.; Currie, B.J.; Rolland, J.M.; O’Hehir, R. Increased Allergic Immune Response to Sarcoptes scabiei Antigens in Crusted versus Ordinary Scabies. Clin. Vaccine Immunol. 2010, 17, 1428–1438. [Google Scholar] [CrossRef]
- Bhat, S.A.; Mounsey, K.E.; Liu, X.; Walton, S.F. Host Immune Responses to the Itch Mite, Sarcoptes scabiei, in Humans. Parasit. Vectors 2017, 10, 385. [Google Scholar] [CrossRef]
- He, R.; Gu, X.; Lai, W.; Peng, X.; Yang, G. Transcriptome-microRNA Analysis of Sarcoptes scabiei and Host Immune Response. PLoS ONE 2017, 12, e0177733. [Google Scholar] [CrossRef]
- Ständer, S.; Ständer, S. Itch in Scabies—What Do We Know? Front. Med. 2021, 8, 1–6. [Google Scholar] [CrossRef]
- Mounsey, K.E.; Murray, H.C.; Bielefeldt-Ohmann, H.; Pasay, C.; Holt, D.C.; Currie, B.J.; Walton, S.F.; McCarthy, J.S. Prospective Study in a Porcine Model of Sarcoptes scabiei Indicates the Association of Th2 and Th17 Pathways with the Clinical Severity of Scabies. PLoS Negl. Trop. Dis. 2015, 9, e0003498. [Google Scholar] [CrossRef]
- Hashimoto, T.; Satoh, T.; Yokozeki, H. Pruritus in Ordinary Scabies: IL-31 from Macrophages Induced by Overexpression of Thymic Stromal Lymphopoietin and Periostin. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 1727–1737. [Google Scholar] [CrossRef]
- Sanders, K.M.; Nattkemper, L.A.; Rosen, J.D.; Andersen, H.H.; Hsiang, J.; Romanelli, P.; Bernigaud, C.; Guillot, J.; Chosidow, O.; Yosipovitch, G. Non-Histaminergic Itch Mediators Elevated in the Skin of a Porcine Model of Scabies and of Human Scabies Patients. J. Invest. Dermatol. 2019, 139, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Norgan, A.P.; Pritt, B.S. Parasitic Infections of the Skin and Subcutaneous Tissues. Adv. Anat. Pathol. 2018, 25, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Prickett, K.A.; Ferringer, T.C. Helminths: A Clinical Review and Update. Semin. Cutan. Med. Surg. 2014, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Kollipara, R.; Peranteau, A.J.; Nawas, Z.Y.; Tong, Y.; Woc-Colburn, L.; Yan, A.C.; Lupi, O.; Tyring, S.K. Emerging Infectious Diseases with Cutaneous Manifestations: Fungal, Helminthic, Protozoan and Ectoparasitic Infections. J. Am. Acad. Dermatol. 2016, 75, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.M.; Tait Wojno, E.D. The Role of Rare Innate Immune Cells in Type 2 Immune Activation Against Parasitic Helminths. Parasitology 2017, 144, 1288–1301. [Google Scholar] [CrossRef]
- Retzinger, A.C.; Retzinger, G.S. Mites, Ticks, Anaphylaxis and allergy: The Acari Hypothesis. Med. Hypotheses 2020, 144, 110257. [Google Scholar] [CrossRef]
- Méndez-Samperio, P. Molecular Events by Which Dendritic Cells Promote Th2 Immune Protection in Helmith Infection. Infect. Dis. 2016, 48, 715–720. [Google Scholar] [CrossRef]
- Tatsuno, K.; Fujiyama, T.; Matsuoka, H.; Shimauchi, T.; Ito, T.; Tokura, Y. Clinical Categories of Exaggerated Skin Reactions to Mosquito Bites and Their Pathophysiology. J. Dermatol. Sci. 2016, 82, 145–152. [Google Scholar] [CrossRef]
- Wirtz, R.A. Allergic and Toxic Reactions to Non-stinging Arthropods. Annu. Rev. Entomol. 1984, 29, 47–69. [Google Scholar] [CrossRef]
- Ohtsuka, E.; Kawai, S.; Ichikawa, T.; Nojima, H.; Kitagawa, K.; Shirai, Y.; Kamimura, K.; Kuraishi, Y. Roles of Mast Cells and Histamine in Mosquito Bite-induced Allergic Itch-associated Responses in Mice. Jpn. J. Pharmacol. 2001, 86, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, D.A.; Rodewald, H.-R. A Sting in the Tale of TH2 Immunity. Immunity 2013, 39, 803–805. [Google Scholar] [CrossRef][Green Version]
- Elieh Ali Komi, D.; Shafaghat, F.; Zwiener, R.D. Immunology of Bee Venom. Clin. Rev. Allergy Immunol. 2018, 54, 386–396. [Google Scholar] [CrossRef]
- Bellinghausen, I.; Metz, G.; Enk, A.H.; Christmann, S.; Knop, J.; Saloga, J. Insect Venom Immunotherapy Induces Interleukin-10 Production and a Th2-to-Th1 Shift, and Changes Surface Marker Expression in Venom-allergic Subjects. Eur. J. Immunol. 1997, 27, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; de Bruin-Weller, M.; Eckert, L. Epidemiology of Atopic Dermatitis in Adults: Results from an International Survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef]
- Cork, M.J.; Eckert, L.; Simpson, E.L.; Armstrong, A.; Barbarot, S.; Puig, L.; Girolomoni, G.; de Bruin-Weller, M.; Wollenberg, A.; Kataoka, Y.; et al. Dupilumab Improves Patient-reported Symptoms of Atopic Dermatitis, Symptoms of Anxiety and Depression, and Health-related Quality of Life in Moderate-to-severe Atopic Dermatitis: Analysis of Pooled Data from the Randomized Trials SOLO 1 and SOLO 2. J. Dermatolog. Treat. 2020, 31, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.; Kazandjieva, J.; Hristakieva, E.; Fluhr, J.W. Atopic Dermatitis as a Systemic Disease. Clin. Dermatol. 2014, 32, 409–413. [Google Scholar] [CrossRef]
- Möbus, L.; Rodriguez, E.; Harder, I.; Stölzl, D.; Boraczynski, N.; Gerdes, S.; Kleinheinz, A.; Abraham, S.; Heratizadeh, A.; Handrick, C.; et al. Atopic Dermatitis Displays Stable and Dynamic Skin Transcriptome Signatures. J. Allergy Clin. Immunol. 2021, 147, 213–223. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Maurelli, M.; Peris, K.; Girolomoni, G. Targeting IL-4 for the Treatment of Atopic Dermatitis. ImmunoTargets Ther. 2020, 9, 151–156. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Rodriguez, E.; Stölzl, D.; Wehkamp, U.; Sun, J.; Gerdes, S.; Sarkar, M.K.; Hübenthal, M.; Zeng, C.; Uppala, R.; et al. Progression of Acute-to-chronic Atopic Dermatitis is Associated with Quantitative Rather Than Qualitative Changes in Cytokine Responses. J. Allergy Clin. Immunol. 2020, 145, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, L.C.; Rodriguez, E.; Degenhardt, F.; Baurecht, H.; Wehkamp, U.; Volks, N.; Szymczak, S.; Swindell, W.R.; Sarkar, M.K.; Raja, K.; et al. Atopic Dermatitis is an IL-13-Dominant Disease with Greater Molecular Heterogeneity Compared to Psoriasis. J. Invest. Dermatol. 2019, 139, 1480–1489. [Google Scholar] [CrossRef]
- Tubau, C.; Puig, L. Therapeutic Targeting of the IL-13 Pathway in Skin Inflammation. Expert Rev. Clin. Immunol. 2020, 1–11. [Google Scholar] [CrossRef]
- Xuemin, X.; Lihang, L.; Changhua, Z.; Xiaofang, Y.; Yongshan, N.; Zhipeng, L.; Chong, J.; Yue, H. Efficacy and Safety of Nemolizumab for Adult Atopic Dermatitis Treatment: A Meta-analysis of Randomized Clinical Trials. J. Investig. Allergol. Clin. Immunol. 2021, 31. [Google Scholar] [CrossRef] [PubMed]
- Cartron, A.M.; Nguyen, T.H.; Roh, Y.S.; Kwatra, M.M.; Kwatra, S.G. JAK Inhibitors for Atopic Dermatitis: A Promising Treatment Modality. Clin. Exp. Dermatol. 2021. [Google Scholar] [CrossRef]
- Pereira, M.P.; Steinke, S.; Zeidler, C.; Forner, C.; Riepe, C.; Augustin, M.; Bobko, S.; Dalgard, F.; Elberling, J.; Garcovich, S.; et al. European Academy of Dermatology and Venereology European Prurigo Project: Expert Consensus on the Definition, Classification and Terminology of Chronic Prurigo. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Boozalis, E.; Tang, O.; Patel, S.; Semenov, Y.R.; Pereira, M.P.; Stander, S.; Kang, S.; Kwatra, S.G. Ethnic Differences and Comorbidities of 909 Prurigo Nodularis Patients. J. Am. Acad. Dermatol. 2018, 79, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Hoffmann, V.; Weisshaar, E.; Wallengren, J.; Halvorsen, J.A.; Garcovich, S.; Misery, L.; Brenaut, E.; Savk, E.; Potekaev, N.; et al. Chronic Nodular Prurigo: Clinical Profile and Burden. A European Cross-sectional Study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2373–2383. [Google Scholar] [CrossRef]
- Park, K.; Mori, T.; Nakamura, M.; Tokura, Y. Increased Expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in Skin Lesions of Subacute and Chronic Forms of Prurigo. Eur. J. Dermatol. 2011, 21, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wu, X.; Zhang, W.; Zhang, J.; Chen, X.; Chen, S.; Huang, H.; Yang, Y.; Yu, B.; Dou, X. Aberrant Expression of Histamine-independent Pruritogenic Mediators in Keratinocytes may be Involved in the Pathogenesis of Prurigo Nodularis. Acta Derm. Venereol. 2019, 99, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Nattkemper, L.A.; Kim, H.S.; Kursewicz, C.D.; Fowler, E.; Shah, S.M.; Nanda, S.; Fayne, R.A.; Romanelli, P.; Yosipovitch, G. Dermal Periostin: A New Player in Itch of Prurigo Nodularis. Acta Derm. Venereol. 2021, 101, adv00375. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jacobi, H.H.; Reimert, C.M.; Haak-Frendscho, M.; Marcusson, J.A.; Johansson, O. CGRP-immunoreactive Nerves in Prurigo Nodularis–an Exploration of Neurogenic Inflammation. J. Cutan. Pathol. 2000, 27, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Johansson, O.; Liang, Y.; Emtestam, L. Increased Nerve Growth Factor- and Tyrosine Kinase A-like Immunoreactivities in Prurigo Nodularis Skin–an Exploration of the Cause of Neurohyperplasia. Arch. Dermatol. Res. 2002, 293, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Nattkemper, L.A.; Kim, H.S.; Kursewicz, C.D.; Fowler, E.; Shah, S.M.; Nanda, S.; Fayne, R.A.; Paolini, J.F.; Romanelli, P.; et al. Itch Intensity in Prurigo Nodularis is Closely Related to Dermal Interleukin-31, Oncostatin M, IL-31 Receptor Alpha and Oncostatin M Receptor Beta. Exp. Dermatol. 2021. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Maurelli, M.; Gori, N.; Argenziano, G.; De Simone, C.; Calabrese, G.; Girolomoni, G.; Peris, K. Dupilumab Improves Clinical Manifestations, Symptoms, and Quality of Life in Adult Patients with Chronic Nodular Prurigo. J. Am. Acad. Dermatol. 2020, 83, 39–45. [Google Scholar] [CrossRef]
- Ständer, S.; Yosipovitch, G.; Legat, F.J.; Lacour, J.-P.; Paul, C.; Narbutt, J.; Bieber, T.; Misery, L.; Wollenberg, A.; Reich, A.; et al. Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis. N. Engl. J. Med. 2020, 382, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Moy, A.P.; Murali, M.; Nazarian, R.M. Identification of a Th2- and Th17-skewed Immune Phenotype in Chronic Urticaria with Th22 Reduction Dependent on Autoimmunity and Thyroid Disease Markers. J. Cutan. Pathol. 2016, 43, 372–378. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef]
- Raap, U.; Gehring, M.; Kleiner, S.; Rüdrich, U.; Eiz-Vesper, B.; Haas, H.; Kapp, A.; Gibbs, B.F. Human Basophils are a Source of - and are Differentially Activated by - IL-31. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2017, 47, 499–508. [Google Scholar] [CrossRef]
- Kolkhir, P.; Altrichter, S.; Munoz, M.; Hawro, T.; Maurer, M. New Treatments for Chronic Urticaria. Ann. Allergy Asthma Immunol. 2020, 124, 2–12. [Google Scholar] [CrossRef]
- Hendricks, A.J.; Yosipovitch, G.; Shi, V.Y. Dupilumab Use in Dermatologic Conditions Beyond Atopic Dermatitis - a Systematic Review. J. Dermatolog. Treat. 2021, 32, 19–28. [Google Scholar] [CrossRef]
- Bellinato, F.; Maurelli, M.; Schena, D.; Gisondi, P.; Girolomoni, G. Clinical and Immunological Profile of Patients with Dipeptidyl Peptidase-4 Inhibitor-associated Bullous Pemphigoid. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2020. [Google Scholar] [CrossRef]
- Messingham, K.N.; Crowe, T.P.; Fairley, J.A. The Intersection of IgE Autoantibodies and Eosinophilia in the Pathogenesis of Bullous Pemphigoid. Front. Immunol. 2019, 10, 2331. [Google Scholar] [CrossRef]
- Cozzani, E.; Gasparini, G.; Di Zenzo, G.; Parodi, A. Immunoglobulin E and Bullous Pemphigoid. Eur. J. Dermatol. 2018, 28, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Panah, P.-S.; Moravvej, H.; Alirajab, M.; Etaaty, A.; Geranmayeh, M.; Hosseine, F.; Khansari, A.; Mahdian, M.; Mirhashemi, M.; Parvizi, S.; et al. Association between TH2 Cytokine Gene Polymorphisms and Risk of Bullous Pemphigoid. Immunol. Investig. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rüdrich, U.; Gehring, M.; Papakonstantinou, E.; Illerhaus, A.; Engmann, J.; Kapp, A.; Hartmann, K.; Meyer, N.H.; Gibbs, B.F.; Raap, U. Eosinophils are a Major Source of Interleukin-31 in Bullous Pemphigoid. Acta Derm. Venereol. 2018, 98, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Kursewicz, C.D.; Fayne, R.A.; Nanda, S.; Shah, S.M.; Nattkemper, L.; Yokozeki, H.; Yosipovitch, G. Pathophysiologic Mechanisms of Itch in Bullous Pemphigoid. J. Am. Acad. Dermatol. 2020, 83, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Abdat, R.; Waldman, R.A.; de Bedout, V.; Czernik, A.; Mcleod, M.; King, B.; Gordon, S.; Ahmed, R.; Nichols, A.; Rothe, M.; et al. Dupilumab as a Novel Therapy for Bullous Pemphigoid: A Multicenter Case Series. J. Am. Acad. Dermatol. 2020, 83, 46–52. [Google Scholar] [CrossRef] [PubMed]
- A Study to Investigate the Use of Benralizumab in Patients with Bullous Pemphigoid. Available online: https://clinicaltrials.gov/ct2/show/NCT04612790 (accessed on 16 February 2021).
- A Study to Evaluate the Efficacy and Safety of Dupilumab in Adult Patients with Bullous Pemphigoid. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04206553 (accessed on 16 February 2021).
- Maurelli, M.; Tessari, G.; Colato, C.; Schena, D.; Girolomoni, G. Incidence and Ten-year Follow-up of Primary Cutaneous Lymphomas: A Single-centre Cohort Study. Eur. J. Dermatol. 2018, 28, 44–49. [Google Scholar] [CrossRef]
- Furue, M.; Kadono, T. New Aspects of the Clinicopathological Features and Treatment of Mycosis Fungoides and Sézary Syndrome. J. Dermatol. 2015, 42, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Huang, S.; Duvic, M. Inflammatory Cytokines and Peripheral Mediators in the Pathophysiology of Pruritus in Cutaneous T-cell Lymphoma. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1652–1656. [Google Scholar] [CrossRef]
- Takahashi, N.; Sugaya, M.; Suga, H.; Oka, T.; Kawaguchi, M.; Miyagaki, T.; Fujita, H.; Sato, S. Thymic Stromal Chemokine TSLP Acts through Th2 Cytokine Production to Induce Cutaneous T-cell Lymphoma. Cancer Res. 2016, 76, 6241–6252. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Miyagaki, T.; Hirakawa, M.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Fujita, H.; Asano, Y.; Sugaya, M.; et al. Interleukin-25 is Involved in Cutaneous T-cell Lymphoma Progression by Establishing a T helper 2-dominant Microenvironment. Br. J. Dermatol. 2018, 178, 1373–1382. [Google Scholar] [CrossRef]
- Shimizu, K.; Andoh, T.; Makino, T.; Yoshihisa, Y.; Mizawa, M.; Shimizu, T. Mechanisms of Itching in Mycosis Fungoides: Grade of Itching Correlates with Eosinophil Infiltration and Kallikrein 5 Expression. Eur. J. Dermatol. 2019, 29, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Nattkemper, L.A.; Martinez-Escala, M.-E.; Gelman, A.B.; Singer, E.M.; Rook, A.H.; Guitart, J.; Yosipovitch, G. Cutaneous T-cell Lymphoma and Pruritus: The Expression of IL-31 and its Receptors in the Skin. Acta Derm. Venereol. 2016, 96, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Cedeno-Laurent, F.; Singer, E.M.; Wysocka, M.; Benoit, B.M.; Vittorio, C.C.; Kim, E.J.; Yosipovitch, G.; Rook, A.H. Improved Pruritus Correlates with Lower Levels of IL-31 in CTCL Patients under Different Therapeutic Modalities. Clin. Immunol. 2015, 158, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Steck, O.; Bertschi, N.L.; Luther, F.; van den Berg, J.; Winkel, D.J.; Holbro, A.; Schlapbach, C. Rapid and Sustained Control of Itch and Reduction in Th2 Bias by Dupilumab in a Patient with Sézary Syndrome. J. Eur. Acad. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef]
- Kim, B.S.; Berger, T.G.; Yosipovitch, G. Chronic Pruritus of Unknown Origin (CPUO): Uniform Nomenclature and Diagnosis as a Pathway to Standardized Understanding and Treatment. J. Am. Acad. Dermatol. 2019, 81, 1223–1224. [Google Scholar] [CrossRef]
- Andrade, A.; Kuah, C.Y.; Martin-Lopez, J.E.; Chua, S.; Shpadaruk, V.; Sanclemente, G.; Franco, J.V.A. Interventions for Chronic Pruritus of Unknown Origin. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.S.; Khanna, R.; Patel, S.P.; Gopinath, S.; Williams, K.A.; Khanna, R.; Pritchard, T.; Sutaria, N.; Choi, J.; Alphonse, M.P.; et al. Circulating Blood Eosinophils as a Biomarker for Variable Clinical Presentation and Therapeutic Response in Patients with Chronic Pruritus of Unknown Origin. J. Allergy Clin. Immunol. Pract. 2021. [Google Scholar] [CrossRef]
- Zhai, L.L.; Savage, K.T.; Qiu, C.C.; Jin, A.; Valdes-Rodriguez, R.; Mollanazar, N.K. Chronic Pruritus Responding to Dupilumab—A Case Series. Medicines 2019, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Salao, K.; Sawanyawisuth, K.; Winaikosol, K.; Choonhakarn, C.; Chaowattanapanit, S. Interleukin-31 and Chronic Pruritus of Unknown Origin. Biomark. Insights 2020, 15, 1177271920940712. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Morris, C.; Bodet, N.D.; Kim, B.S. Treatment of Refractory Chronic Pruritus of Unknown Origin With Tofacitinib in Patients With Rheumatoid Arthritis. JAMA Dermatol. 2019. [Google Scholar] [CrossRef]
| Mediator | Source | References |
|---|---|---|
| IL-4 | Type 2 lymphocytes, ILC2, mast cells, eosinophils, basophils | [5,7] |
| IL-13 | Type 2 lymphocytes, mast cells, basophils, eosinophils | [5,7,24,25] |
| IL-31 | Type 2 lymphocytes, mast cells, macrophages, dendritic cells | [26,27,28] |
| TSLP | Keratinocytes | [14,15,17] |
| IL-25 | Type 2 lymphocytes, dendritic cells, macrophages, mast cells, basophils, eosinophils, keratinocytes | [14,15,29] |
| IL-33 | Fibroblasts, mast cells, macrophages, endothelial cells, keratinocytes | [16,30] |
| Periostin | Fibroblasts, keratinocytes, endothelial cells | [22,23] |
| Proteases: kallikreins, cathepsins | Keratinocytes, mast cells, S. aureus | [18,19,20,21] |
| Histamine | Mast cells | [31] |
| Immune Response Pattern | Th1/ILC1 | Th2/ILC2 | Th17/ILC3 | iTreg |
|---|---|---|---|---|
| Pruritus burden | ↑↑ | ↑↑↑↑ | ↑ | ↑ |
| Cytokines | IFN-γ; TNF-α | IL-4, IL-13, IL-31 | IL-17, IL-22, TNF-α | IL-10, TGF-β |
| Disease | Lichen planus | Scabies | Psoriasis | Scleroderma |
| Graft vs. host disease, lichenoid | Cutaneous helminth infestations | Psoriasis, pustular | Systemic sclerosis | |
| Contact dermatitis | Insect bite reactions | Acne | Lichen sclerosus | |
| Drug eruption (lichenoid) | Atopic dermatitis | Hidradenitis suppurativa | Granuloma annulare | |
| Toxic epidermal necrolysis | Prurigo nodularis | Neutrophilic dermatoses | Keloid | |
| Erythema multiforme | Chronic urticaria | Rosacea | Granuloma annulare | |
| Cutaneous lupus erythematosus | Bullous pemphigoid | Folliculitis decalvans | Drug reaction, granulomatous | |
| Dermatomyositis | Cutaneous T cell lymphoma | Drug eruption, psoriasiform | Necrobiosis lipoidica | |
| Chronic pruritus of unknown origin | Sarcoidosis |
| Agent | Drug Target | Phase | Indications |
|---|---|---|---|
| Dupilumab | Anti-IL-4/13R | II-IV | AD, PN, CP |
| Tralokinumab | Anti-IL-13 | III | AD |
| Lebrikizumab | Anti-IL-13 | III | AD |
| Vixarelimab | Anti-OSMRβ | II | AD, PN, CP |
| Tezepelumab | Anti-TSLP | II | AD |
| Nemolizumab | Anti-IL-31R | III | AD, PN |
| BMS-981164 | Anti-IL-31 | I | AD |
| MSTT1041A | Anti-IL-33R | II | AD |
| Etokimab | Anti-IL-33R | II | AD |
| Benralizumab | Anti-IL-5R | II | CU |
| Mepolizumab | Anti-IL-5 | I-II | CU, AD |
| Omalizumab | Anti-IgE | IV | CU, AD |
| Ligelizumab | Anti-IgE mAb | III | CU |
| Baricitinib | JAK1/2 inhibitor | III | AD |
| Upadacitinib | JAK1 inhibitor | III | AD |
| Abrocitinib | JAK1 inhibitor | III | AD |
| Tradipitant | NK1R antagonist | III | AD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcovich, S.; Maurelli, M.; Gisondi, P.; Peris, K.; Yosipovitch, G.; Girolomoni, G. Pruritus as a Distinctive Feature of Type 2 Inflammation. Vaccines 2021, 9, 303. https://doi.org/10.3390/vaccines9030303
Garcovich S, Maurelli M, Gisondi P, Peris K, Yosipovitch G, Girolomoni G. Pruritus as a Distinctive Feature of Type 2 Inflammation. Vaccines. 2021; 9(3):303. https://doi.org/10.3390/vaccines9030303
Chicago/Turabian StyleGarcovich, Simone, Martina Maurelli, Paolo Gisondi, Ketty Peris, Gil Yosipovitch, and Giampiero Girolomoni. 2021. "Pruritus as a Distinctive Feature of Type 2 Inflammation" Vaccines 9, no. 3: 303. https://doi.org/10.3390/vaccines9030303
APA StyleGarcovich, S., Maurelli, M., Gisondi, P., Peris, K., Yosipovitch, G., & Girolomoni, G. (2021). Pruritus as a Distinctive Feature of Type 2 Inflammation. Vaccines, 9(3), 303. https://doi.org/10.3390/vaccines9030303








