Immunization with Epstein–Barr Virus Core Fusion Machinery Envelope Proteins Elicit High Titers of Neutralizing Activities and Protect Humanized Mice from Lethal Dose EBV Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, EBV Strain, and Reagents
2.2. Rabbit Immunizations
2.3. Measurement of Serum Titers of EBV gH/gL- and gB-Specific IgG
2.4. Determination of Serum EBV Neutralizing Titers Using the B Lymphocyte Cell Line RAJI
2.5. Determination of Serum EBV Neutralizing Titers Using the Epithelial Cell Line HeLa
2.6. Passive Immune Protection of Humanized Mice from Lethal Dose EBV Challenge
2.7. Statistics
3. Results
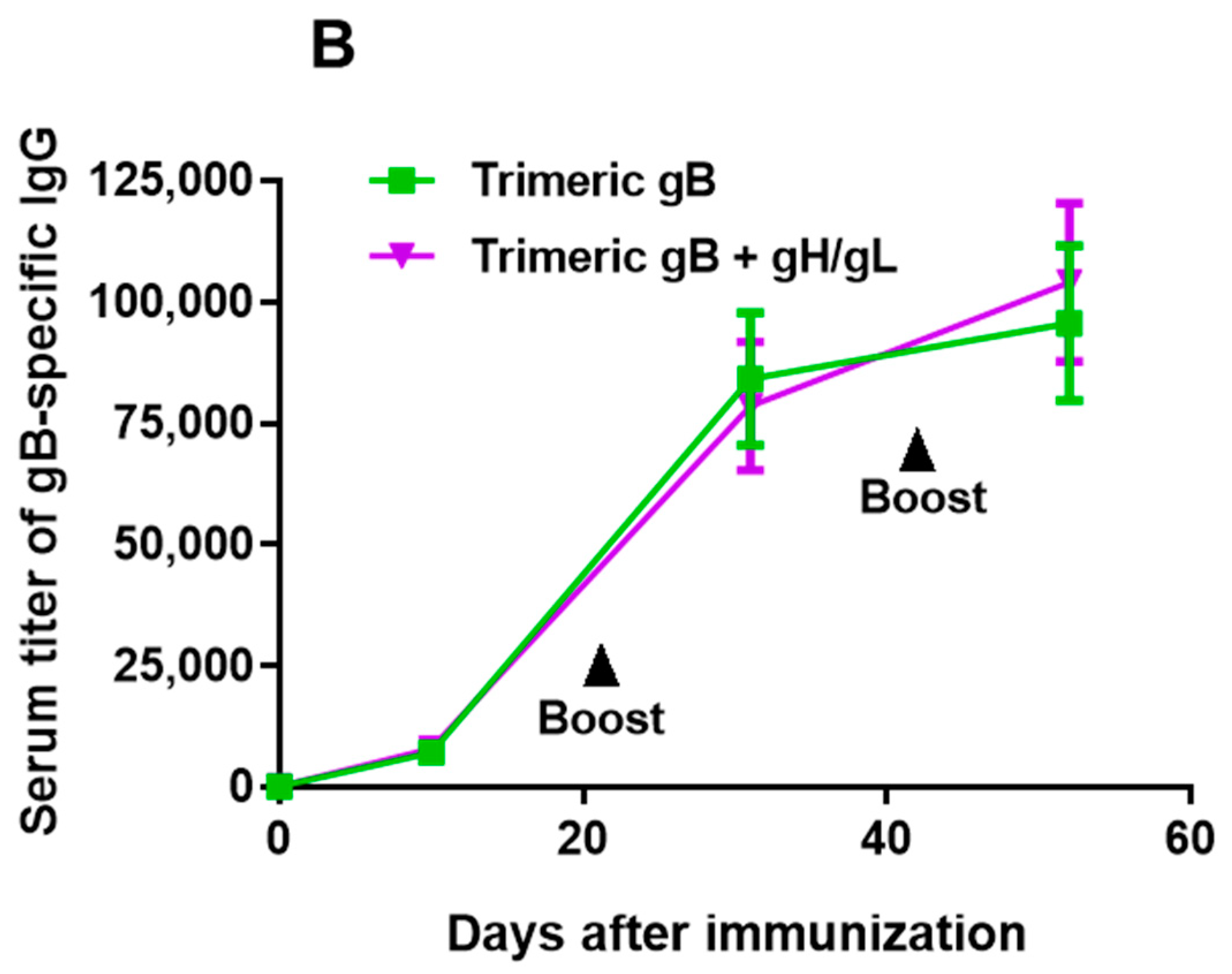
3.1. Immunization of Rabbits with EBV gH/gL in Combination with Trimeric gB Led to Induction of High Serum Titers of gH/gL- and gB-Specific IgG with no Cross-Antigen Interference
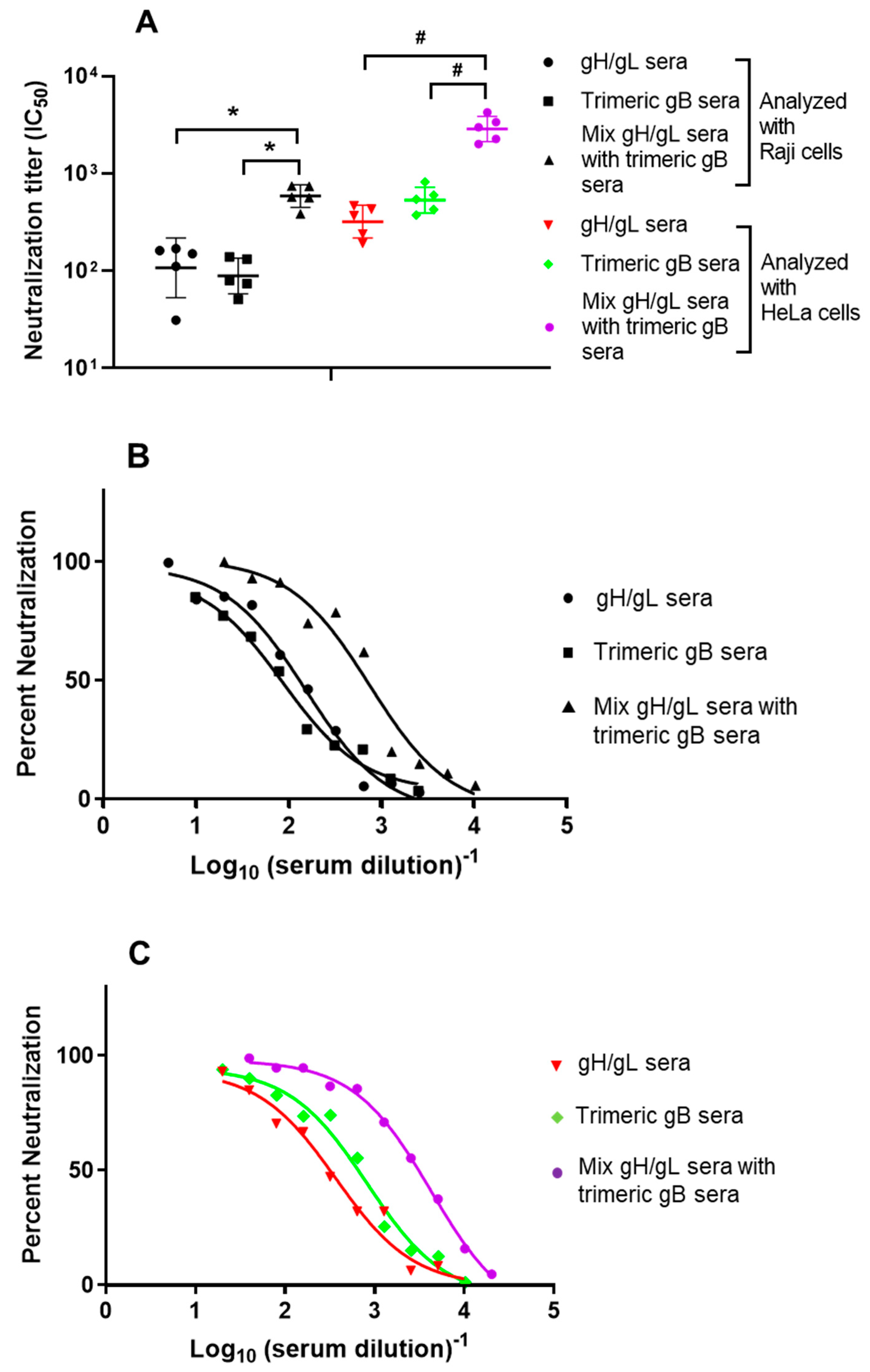
3.2. In Vitro Mixture of the EBV gH/gL Rabbit Immune Sera with EBV gB Rabbit Immune Sera Showed Synergistic EBV Neutralizing Activity for the B Lymphoma Cell Line Raji and the Epithelial Cell Line HeLa
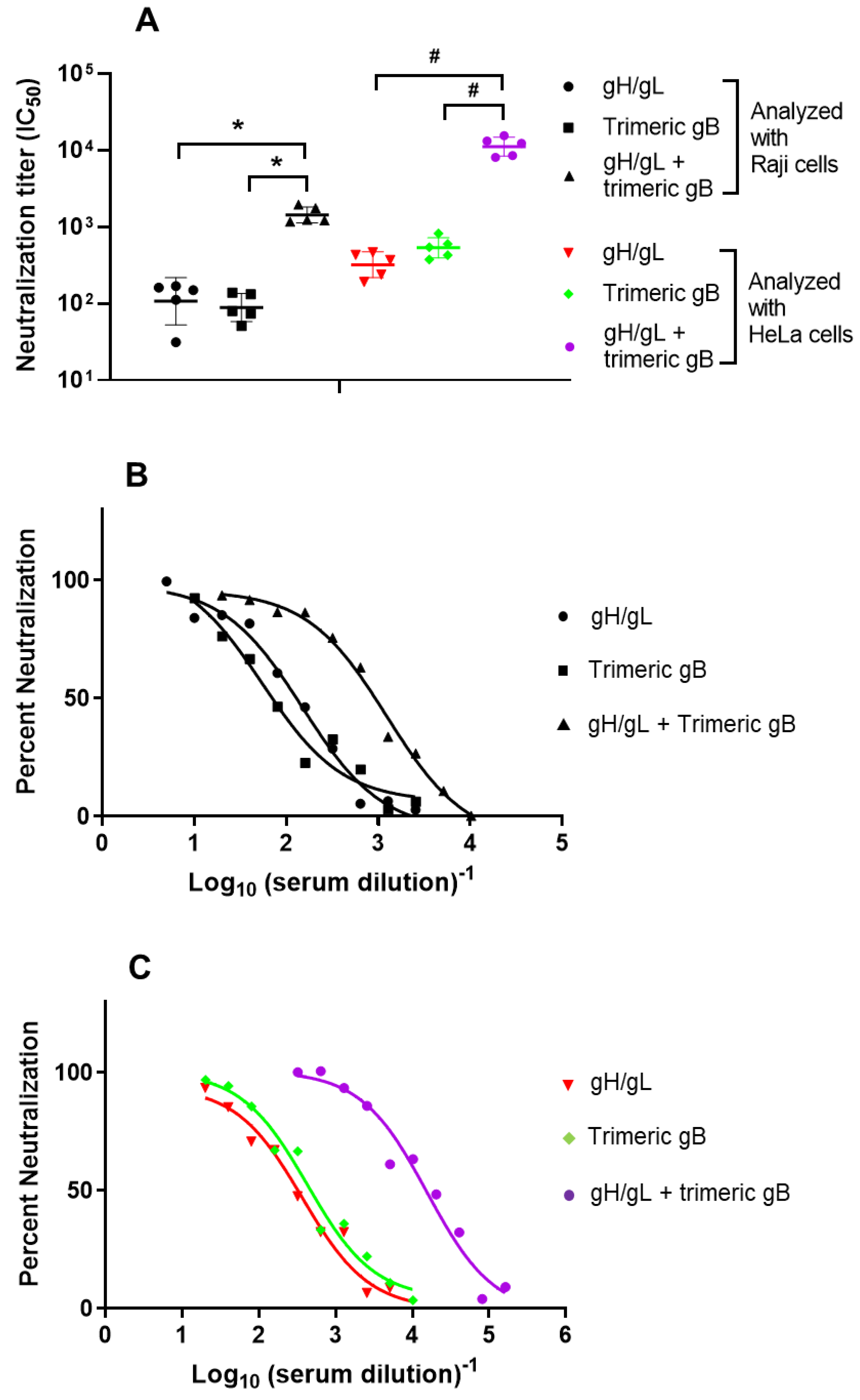
3.3. Immunization with the Combination of EBV gH/gL and Trimeric gB Elicited Strong Synergistic EBV Neutralizing Activity for the B Lymphoma Cell Line Raji and the Epithelial Cell Line HeLa
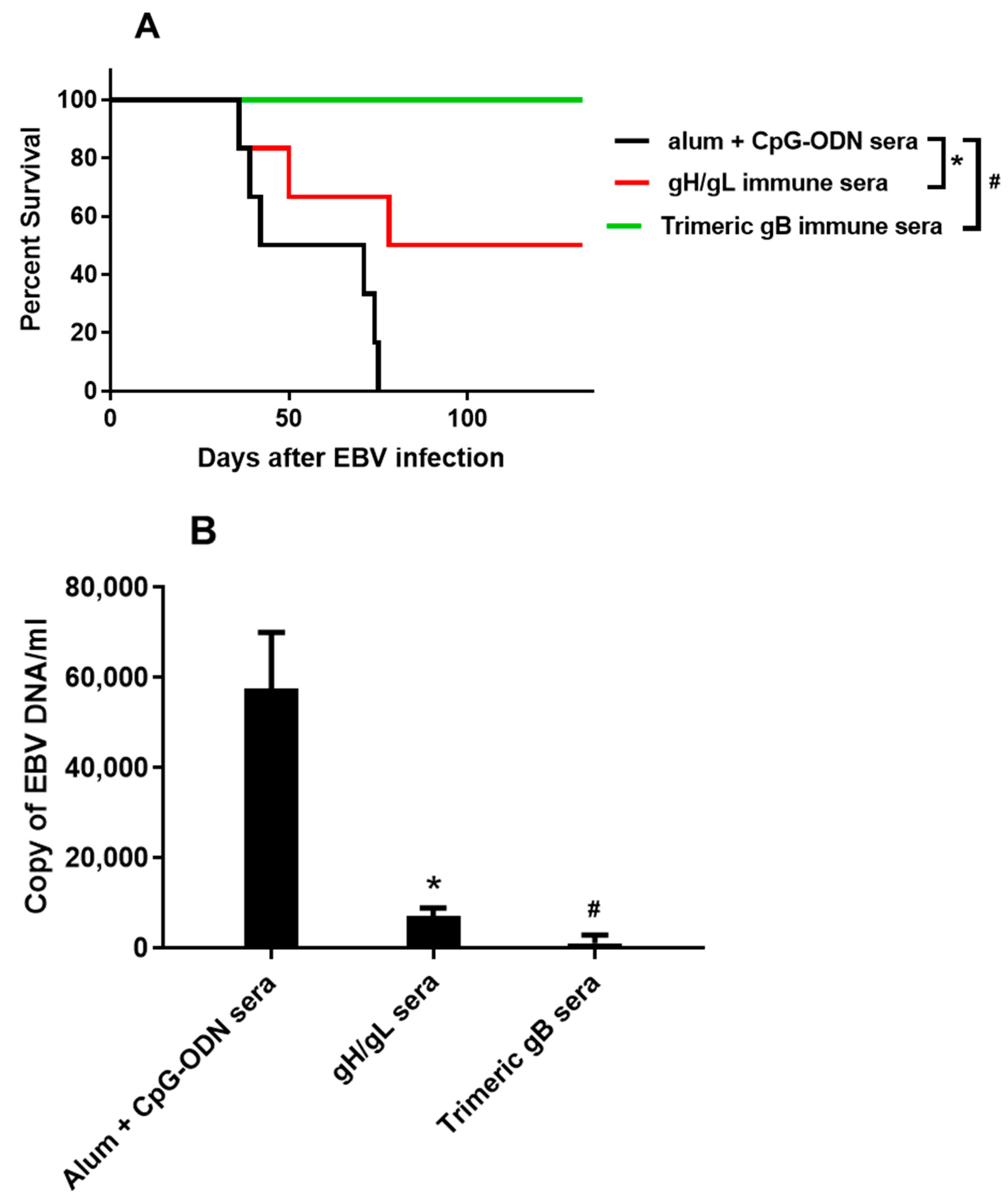
3.4. Immune Sera from Rabbits Immunized with EBV gH/gL or EBV Trimeric gB Protected Humanized Mice from Death Caused by High Dose EBV Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Cohen, J.I.; Fauci, A.S.; Varmus, H.; Nabel, G.J. Epstein-Barr virus: An important vaccine target for cancer prevention. Sci. Transl. Med. 2011, 3, 107fs7. [Google Scholar] [CrossRef]
- Vetsika, E.K.; Callan, M. Infectious mononucleosis and Epstein-Barr virus. Expert Rev. Mol. Med. 2004, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I.; Mocarski, E.S.; Raab-Traub, N.; Corey, L.; Nabel, G.J. The need and challenges for development of an Epstein-Barr virus vaccine. Vaccine 2013, 31 (Suppl. 2), B194–B196. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, K.; Sullivan, J.L. Infectious mononucleosis. N. Engl. J. Med. 2010, 362, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Hallee, T.J.; Evans, A.S.; Niederman, J.C.; Brooks, C.M.; Voegtly, J.H. Infectious mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J. Biol. Med. 1974, 47, 182–195. [Google Scholar]
- Rea, T.D.; Russo, J.E.; Katon, W.; Ashley, R.L.; Buchwald, D.S. Prospective study of the natural history of infectious mononucleosis caused by Epstein-Barr virus. J. Am. Board Fam. Pract. 2001, 14, 234–242. [Google Scholar]
- Cohen, J.I. Vaccine Development for Epstein-Barr Virus. Adv. Exp. Med. Biol. 2018, 1045, 477–493. [Google Scholar]
- Neparidze, N.; Lacy, J. Malignancies associated with epstein-barr virus: Pathobiology, clinical features, and evolving treatments. Clin. Adv. Hematol. Oncol. 2014, 12, 358–371. [Google Scholar] [PubMed]
- Fukayama, M. Epstein-Barr virus and gastric carcinoma. Pathol. Int. 2010, 60, 337–350. [Google Scholar] [CrossRef]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef]
- Hjalgrim, H.; Askling, J.; Sorensen, P.; Madsen, M.; Rosdahl, N.; Storm, H.H.; Hamilton-Dutoit, S.; Eriksen, L.S.; Frisch, M.; Ekbom, A.; et al. Risk of Hodgkin’s disease and other cancers after infectious mononucleosis. J. Natl. Cancer Inst. 2000, 92, 1522–1528. [Google Scholar] [CrossRef]
- Dharnidharka, V.R. Comprehensive review of post-organ transplant hematologic cancers. Am. J. Transplant. 2018, 18, 537–549. [Google Scholar] [CrossRef]
- Al-Mansour, Z.; Nelson, B.P.; Evens, A.M. Post-transplant lymphoproliferative disease (PTLD): Risk factors, diagnosis, and current treatment strategies. Curr. Hematol. Malig. Rep. 2013, 8, 173–183. [Google Scholar] [CrossRef]
- AlDabbagh, M.A.; Gitman, M.R.; Kumar, D.; Humar, A.; Rotstein, C.; Husain, S. The Role of Antiviral Prophylaxis for the Prevention of Epstein-Barr Virus-Associated Posttransplant Lymphoproliferative Disease in Solid Organ Transplant Recipients: A Systematic Review. Am. J. Transplant. 2017, 17, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ito, Y.; Kawabe, S.; Gotoh, K.; Takahashi, Y.; Kojima, S.; Naoe, T.; Esaki, S.; Kikuta, A.; Sawada, A.; et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: Prospective analysis of 108 cases. Blood 2012, 119, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Kim, W.S.; Ko, Y.H.; Kim, K.; Lee, M.H.; Park, K. Aggressive natural killer cell leukemia: Clinical features and treatment outcome. Haematologica 2002, 87, 1343–1345. [Google Scholar]
- Iezzoni, J.C.; Gaffey, M.J.; Weiss, L.M. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am. J. Clin. Pathol. 1995, 103, 308–315. [Google Scholar] [CrossRef]
- Villegas, E.; Santiago, O.; Sorlozano, A.; Gutierrez, J. New strategies and patent therapeutics in EBV-associated diseases. Mini Rev. Med. Chem. 2010, 10, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Dambaugh, T.; Wang, F.; Hennessy, K.; Woodland, E.; Rickinson, A.; Kieff, E. Expression of the Epstein-Barr virus nuclear protein 2 in rodent cells. J. Virol. 1986, 59, 453–462. [Google Scholar] [CrossRef]
- Sample, J.; Young, L.; Martin, B.; Chatman, T.; Kieff, E.; Rickinson, A.; Kieff, E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J. Virol. 1990, 64, 4084–4092. [Google Scholar] [CrossRef]
- Rowe, M.; Young, L.S.; Cadwallader, K.; Petti, L.; Kieff, E.; Rickinson, A.B. Distinction between Epstein-Barr virus type A (EBNA 2A) and type B (EBNA 2B) isolates extends to the EBNA 3 family of nuclear proteins. J. Virol. 1989, 63, 1031–1039. [Google Scholar] [CrossRef]
- Niederman, J.C.; McCollum, R.W.; Henle, G.; Henle, W. Infectious mononucleosis. Clinical manifestations in relation to EB virus antibodies. JAMA 1968, 203, 205–209. [Google Scholar] [CrossRef]
- Connolly, S.A.; Jackson, J.O.; Jardetzky, T.S.; Longnecker, R. Fusing structure and function: A structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 2011, 9, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Hutt-Fletcher, L.M. Epstein-Barr virus entry. J. Virol. 2007, 81, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Shannon-Lowe, C.; Rowe, M. Epstein Barr virus entry; kissing and conjugation. Curr. Opin. Virol. 2014, 4, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Neuhierl, B.; Feederle, R.; Hammerschmidt, W.; Delecluse, H.J. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proc. Natl. Acad. Sci. USA 2002, 99, 15036–15041. [Google Scholar] [CrossRef] [PubMed]
- Heldwein, E.E.; Krummenacher, C. Entry of herpesviruses into mammalian cells. Cell Mol. Life Sci. 2008, 65, 1653–1668. [Google Scholar] [CrossRef]
- Jiang, R.; Gu, X.; Nathan, C.O.; Hutt-Fletcher, L. Laser-capture microdissection of oropharyngeal epithelium indicates restriction of Epstein-Barr virus receptor/CD21 mRNA to tonsil epithelial cells. J. Oral Pathol. Med. 2008, 37, 626–633. [Google Scholar] [CrossRef]
- Birkenbach, M.; Tong, X.; Bradbury, L.E.; Tedder, T.F.; Kieff, E. Characterization of an Epstein-Barr virus receptor on human epithelial cells. J. Exp. Med. 1992, 176, 1405–1414. [Google Scholar] [CrossRef]
- Maruo, S.; Yang, L.; Takada, K. Roles of Epstein-Barr virus glycoproteins gp350 and gp25 in the infection of human epithelial cells. J. Gen. Virol. 2001, 82 Pt 10, 2373–2383. [Google Scholar] [CrossRef]
- Fingeroth, J.D.; Diamond, M.E.; Sage, D.R.; Hayman, J.; Yates, J.L. CD21-Dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J. Virol. 1999, 73, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Hutt-Fletcher, L.M. Epstein-Barr virus replicating in epithelial cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16242–16243. [Google Scholar] [CrossRef]
- Tsao, S.W.; Tsang, C.M.; Pang, P.S.; Zhang, G.; Chen, H.; Lo, K.W. The biology of EBV infection in human epithelial cells. Semin. Cancer Biol. 2012, 22, 137–143. [Google Scholar] [CrossRef]
- Li, Q.X.; Young, L.S.; Niedobitek, G.; Dawson, C.W.; Birkenbach, M.; Wang, F.; Rickinson, A.B. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature 1992, 356, 347–350. [Google Scholar] [CrossRef]
- Backovic, M.; Longnecker, R.; Jardetzky, T.S. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc. Natl. Acad. Sci. USA 2009, 106, 2880–2885. [Google Scholar] [CrossRef]
- Sashihara, J.; Hoshino, Y.; Bowman, J.J.; Krogmann, T.; Burbelo, P.D.; Coffield, V.M.; Kamrud, K.; Cohen, J.I. Soluble rhesus lymphocryptovirus gp350 protects against infection and reduces viral loads in animals that become infected with virus after challenge. PLoS Pathog. 2011, 7, e1002308. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.J.; Epstein, M.A.; North, J.R. Comparative immunogenicity studies on Epstein-Barr virus membrane antigen (MA) gp340 with novel adjuvants in mice, rabbits, and cotton-top tamarins. J. Med. Virol. 1984, 13, 281–292. [Google Scholar] [CrossRef]
- Morgan, A.J.; Allison, A.C.; Finerty, S.; Scullion, F.T.; Byars, N.E.; Epstein, M.A. Validation of a first-generation Epstein-Barr virus vaccine preparation suitable for human use. J. Med. Virol. 1989, 29, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Finerty, S.; Tarlton, J.; Mackett, M.; Conway, M.; Arrand, J.R.; Watkins, P.E.; Morgan, A.J. Protective immunization against Epstein-Barr virus-induced disease in cottontop tamarins using the virus envelope glycoprotein gp340 produced from a bovine papillomavirus expression vector. J. Gen. Virol. 1992, 73 Pt 2, 449–453. [Google Scholar] [CrossRef]
- Cox, C.; Naylor, B.A.; Mackett, M.; Arrand, J.R.; Griffin, B.E.; Wedderburn, N. Immunization of common marmosets with Epstein-Barr virus (EBV) envelope glycoprotein gp340: Effect on viral shedding following EBV challenge. J. Med. Virol. 1998, 55, 255–261. [Google Scholar] [CrossRef]
- Mackett, M.; Cox, C.; Pepper, S.D.; Lees, J.F.; Naylor, B.A.; Wedderburn, N.; Arrand, J.R. Immunisation of common marmosets with vaccinia virus expressing Epstein-Barr virus (EBV) gp340 and challenge with EBV. J. Med. Virol. 1996, 50, 263–271. [Google Scholar] [CrossRef]
- Ragot, T.; Finerty, S.; Watkins, P.E.; Perricaudet, M.; Morgan, A.J. Replication-defective recombinant adenovirus expressing the Epstein-Barr virus (EBV) envelope glycoprotein gp340/220 induces protective immunity against EBV-induced lymphomas in the cottontop tamarin. J. Gen. Virol. 1993, 74 Pt 3, 501–507. [Google Scholar] [CrossRef]
- Morgan, A.J.; Mackett, M.; Finerty, S.; Arrand, J.R.; Scullion, F.T.; Epstein, M.A. Recombinant vaccinia virus expressing Epstein-Barr virus glycoprotein gp340 protects cottontop tamarins against EB virus-induced malignant lymphomas. J. Med. Virol. 1988, 25, 189–195. [Google Scholar] [CrossRef]
- Sokal, E.M.; Hoppenbrouwers, K.; Vandermeulen, C.; Moutschen, M.; Leonard, P.; Moreels, A.; Haumont, M.; Bollen, A.; Smets, F.; Denis, M. Recombinant gp350 vaccine for infectious mononucleosis: A phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J. Infect. Dis. 2007, 196, 1749–1753. [Google Scholar] [CrossRef]
- Moutschen, M.; Leonard, P.; Sokal, E.M.; Smets, F.; Haumont, M.; Mazzu, P.; Bollen, A.; Denamur, F.; Peeters, P.; Dubin, G.; et al. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein-Barr virus vaccine in healthy adults. Vaccine 2007, 25, 4697–4705. [Google Scholar] [CrossRef]
- Cui, X.; Cao, Z.; Chen, Q.; Arjunaraja, S.; Snow, A.L.; Snapper, C.M. Rabbits immunized with Epstein-Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine 2016, 34, 4050–4055. [Google Scholar] [CrossRef]
- Lal, H.; Cunningham, A.L.; Heineman, T.C. Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N. Engl. J. Med. 2015, 373, 1576–1577. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Heineman, T.C.; Lal, H.; Godeaux, O.; Chlibek, R.; Hwang, S.J.; McElhaney, J.E.; Vesikari, T.; Andrews, C.; Choi, W.S.; et al. Immune Responses to a Recombinant Glycoprotein E Herpes Zoster Vaccine in Adults Aged 50 Years or Older. J. Infect. Dis. 2018, 217, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.M.; Foley, J.; Tison, T.; Silva, R.; Ogembo, J.G. Novel Epstein-Barr virus-like particles incorporating gH/gL-EBNA1 or gB-LMP2 induce high neutralizing antibody titers and EBV-specific T-cell responses in immunized mice. Oncotarget 2017, 8, 19255–19273. [Google Scholar] [CrossRef]
- Bu, W.; Joyce, M.G.; Nguyen, H.; Banh, D.V.; Aguilar, F.; Tariq, Z.; Yap, M.L.; Tsujimura, Y.; Gillespie, R.A.; Tsybovsky, Y.; et al. Immunization with Components of the Viral Fusion Apparatus Elicits Antibodies That Neutralize Epstein-Barr Virus in B Cells and Epithelial Cells. Immunity 2019, 50, 1305–1316.e6. [Google Scholar] [CrossRef]
- Berges, B.K.; Tanner, A. Modelling of human herpesvirus infections in humanized mice. J. Gen. Virol. 2014, 95 Pt 10, 2106–2117. [Google Scholar] [CrossRef]
- Cocco, M.; Bellan, C.; Tussiwand, R.; Corti, D.; Traggiai, E.; Lazzi, S.; Mannucci, S.; Bronz, L.; Palummo, N.; Ginanneschi, C.; et al. CD34+ cord blood cell-transplanted Rag2-/- gamma(c)-/- mice as a model for Epstein-Barr virus infection. Am. J. Pathol. 2008, 173, 1369–1378. [Google Scholar] [CrossRef]
- Kuwana, Y.; Takei, M.; Yajima, M.; Imadome, K.; Inomata, H.; Shiozaki, M.; Ikumi, N.; Nozaki, T.; Shiraiwa, H.; Kitamura, N.; et al. Epstein-Barr virus induces erosive arthritis in humanized mice. PLoS ONE 2011, 6, e26630. [Google Scholar] [CrossRef] [PubMed]
- Yajima, M.; Imadome, K.; Nakagawa, A.; Watanabe, S.; Terashima, K.; Nakamura, H.; Ito, M.; Shimizu, N.; Honda, M.; Yamamoto, N.; et al. A new humanized mouse model of Epstein-Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J. Infect. Dis. 2008, 198, 673–682. [Google Scholar] [CrossRef]
- Traggiai, E.; Chicha, L.; Mazzucchelli, L.; Bronz, L.; Piffaretti, J.C.; Lanzavecchia, A.; Manz, M.G. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 2004, 304, 104–107. [Google Scholar] [CrossRef]
- Seung, E.; Tager, A.M. Humoral immunity in humanized mice: A work in progress. J. Infect. Dis. 2013, 208 (Suppl. 2), S155–S159. [Google Scholar] [CrossRef]
- Heuts, F.; Rottenberg, M.E.; Salamon, D.; Rasul, E.; Adori, M.; Klein, G.; Klein, E.; Nagy, N. T cells modulate Epstein-Barr virus latency phenotypes during infection of humanized mice. J. Virol. 2014, 88, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Misawa, N.; Nie, C.; Satou, Y.; Iwakiri, D.; Matsuoka, M.; Takahashi, R.; Kuzushima, K.; Ito, M.; Takada, K.; et al. A novel animal model of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in humanized mice. Blood 2011, 117, 5663–5673. [Google Scholar] [CrossRef]
- Sashihara, J.; Burbelo, P.D.; Savoldo, B.; Pierson, T.C.; Cohen, J.I. Human antibody titers to Epstein-Barr Virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay. Virology 2009, 391, 249–256. [Google Scholar] [CrossRef]
- Delecluse, H.J.; Hilsendegen, T.; Pich, D.; Zeidler, R.; Hammerschmidt, W. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. USA 1998, 95, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Cao, Z.; Wang, S.; Adler, S.P.; McVoy, M.A.; Snapper, C.M. Immunization with Human Cytomegalovirus Core Fusion Machinery and Accessory Envelope Proteins Elicit Strong Synergistic Neutralizing Activities. Vaccines 2020, 8, 179. [Google Scholar] [CrossRef]
- Cui, X.; Cao, Z.; Sen, G.; Chattopadhyay, G.; Fuller, D.H.; Fuller, J.T.; Snapper, D.M.; Snow, A.L.; Mond, J.J.; Snapper, C.M. A novel tetrameric gp350 1-470 as a potential Epstein-Barr virus vaccine. Vaccine 2013, 31, 3039–3045. [Google Scholar] [CrossRef]
- Cui, X.; Cao, Z.; Wang, S.; Flora, M.; Adler, S.P.; McVoy, M.A.; Snapper, C.M. Immunization of Rabbits with Recombinant Human Cytomegalovirus Trimeric versus Monomeric gH/gL Protein Elicits Markedly Higher Titers of Antibody and Neutralization Activity. Int. J. Mol. Sci. 2019, 20, 3158. [Google Scholar] [CrossRef]
- Kimura, H.; Morita, M.; Yabuta, Y.; Kuzushima, K.; Kato, K.; Kojima, S.; Matsuyama, T.; Morishima, T. Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J. Clin. Microbiol. 1999, 37, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Elgui de Oliveira, D.; Muller-Coan, B.G.; Pagano, J.S. Viral Carcinogenesis Beyond Malignant Transformation: EBV in the Progression of Human Cancers. Trends Microbiol. 2016, 24, 649–664. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; Chen, H.; Castro, F.A.; Hu, J.K.; Brenner, H. Epstein-Barr virus infection and gastric cancer: A systematic review. Medicine 2015, 94, e792. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.H.; Han, S.H.; An, J.S.; Lee, E.S.; Kim, Y.S. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: A meta-analysis. J. Gastroenterol. Hepatol. 2009, 24, 354–365. [Google Scholar] [CrossRef]
- Mawson, A.R.; Majumdar, S. Malaria, Epstein-Barr virus infection and the pathogenesis of Burkitt’s lymphoma. Int. J. Cancer 2017, 141, 1849–1855. [Google Scholar] [CrossRef]
- Rochford, R.; Moormann, A.M. Burkitt’s Lymphoma. Curr. Top. Microbiol. Immunol. 2015, 390 Pt 1, 267–285. [Google Scholar]
- Bornkamm, G.W. Epstein-Barr virus and the pathogenesis of Burkitt’s lymphoma: More questions than answers. Int. J. Cancer 2009, 124, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef]
- Ambinder, R.F. Epstein-barr virus and hodgkin lymphoma. Hematol. Am. Soc. Hematol. Educ. Program 2007, 204–209. [Google Scholar] [CrossRef]
- Weiss, L.M.; Movahed, L.A.; Warnke, R.A.; Sklar, J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N. Engl. J. Med. 1989, 320, 502–506. [Google Scholar] [CrossRef]
- Anagnostopoulos, I.; Herbst, H.; Niedobitek, G.; Stein, H. Demonstration of monoclonal EBV genomes in Hodgkin’s disease and Ki-1-positive anaplastic large cell lymphoma by combined Southern blot and in situ hybridization. Blood 1989, 74, 810–816. [Google Scholar] [CrossRef]
- Rooney, C.M.; Smith, C.A.; Ng, C.Y.; Loftin, S.; Li, C.; Krance, R.A.; Brenner, M.K.; Heslop, H.E. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 1995, 345, 9–13. [Google Scholar] [CrossRef]
- Rooney, C.M.; Smith, C.A.; Ng, C.Y.; Loftin, S.K.; Sixbey, J.W.; Gan, Y.; Srivastava, D.K.; Bowman, L.C.; Krance, R.A.; Brenner, M.K.; et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998, 92, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Heslop, H.E.; Ng, C.Y.; Li, C.; Smith, C.A.; Loftin, S.K.; Krance, R.A.; Brenner, M.K.; Rooney, C.M. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 1996, 2, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Heslop, H.E.; Slobod, K.S.; Pule, M.A.; Hale, G.A.; Rousseau, A.; Smith, C.A.; Bollard, C.M.; Liu, H.; Wu, M.F.; Rochester, R.J.; et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010, 115, 925–935. [Google Scholar] [CrossRef]
- Gottschalk, S.; Rooney, C.M. Adoptive T-Cell Immunotherapy. Curr. Top. Microbiol. Immunol. 2015, 391, 427–454. [Google Scholar]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Janz, A.; Oezel, M.; Kurzeder, C.; Mautner, J.; Pich, D.; Kost, M.; Hammerschmidt, W.; Delecluse, H.J. Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J. Virol. 2000, 74, 10142–10152. [Google Scholar] [CrossRef]
- Draper, S.J.; Angov, E.; Horii, T.; Miller, L.H.; Srinivasan, P.; Theisen, M.; Biswas, S. Recent advances in recombinant protein-based malaria vaccines. Vaccine 2015, 33, 7433–7443. [Google Scholar] [CrossRef] [PubMed]
- Hjerrild, K.A.; Jin, J.; Wright, K.E.; Brown, R.E.; Marshall, J.M.; Labbe, G.M.; Silk, S.E.; Cherry, C.J.; Clemmensen, S.B.; Jørgensen, T.; et al. Production of full-length soluble Plasmodium falciparum RH5 protein vaccine using a Drosophila melanogaster Schneider 2 stable cell line system. Sci. Rep. 2016, 6, 30357. [Google Scholar] [CrossRef]
- Diamond, D.J.; La Rosa, C.; Chiuppesi, F.; Contreras, H.; Dadwal, S.; Wussow, F.; Bautista, S.; Nakamura, R.; Zaia, J.A. A fifty-year odyssey: Prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert Rev. Vaccines 2018, 17, 889–911. [Google Scholar] [CrossRef]
- Cui, X.; Snapper, C.M. Development of novel vaccines against human cytomegalovirus. Hum. Vaccines Immunother. 2019, 15, 2673–2683. [Google Scholar] [CrossRef]
- Cui, X.; Cao, Z.; Wang, S.; Lee, R.B.; Wang, X.; Murata, H.; Adler, S.P.; McVoy, M.A.; Snapper, C.M. Novel trimeric human cytomegalovirus glycoprotein B elicits a high-titer neutralizing antibody response. Vaccine 2018, 36, 5580–5590. [Google Scholar] [CrossRef] [PubMed]
- Chiuppesi, F.; Nguyen, J.; Park, S.; Contreras, H.; Kha, M.; Meng, Z.; Kaltcheva, T.; Iniguez, A.; Martinez, J.; La Rosa, C.; et al. Multiantigenic Modified Vaccinia Virus Ankara Vaccine Vectors To Elicit Potent Humoral and Cellular Immune Reponses against Human Cytomegalovirus in Mice. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- McVoy, M.A.; Lee, R.; Saccoccio, F.M.; Hartikka, J.; Smith, L.R.; Mahajan, R.; Wang, J.B.; Cui, X.; Adler, S.P. A cytomegalovirus DNA vaccine induces antibodies that block viral entry into fibroblasts and epithelial cells. Vaccine 2015, 33, 7328–7336. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R.; Permar, S.R.; Plotkin, S.A. Progress toward Development of a Vaccine against Congenital Cytomegalovirus Infection. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Majji, S.; Wijayalath, W.; Shashikumar, S.; Pow-Sang, L.; Villasante, E.; Brumeanu, T.D.; Casares, S. Differential effect of HLA class-I versus class-II transgenes on human T and B cell reconstitution and function in NRG mice. Sci. Rep. 2016, 6, 28093. [Google Scholar] [CrossRef] [PubMed]
- Majji, S.; Wijayalath, W.; Shashikumar, S.; Brumeanu, T.D.; Casares, S. Humanized DRAGA mice immunized with Plasmodium falciparum sporozoites and chloroquine elicit protective pre-erythrocytic immunity. Malar. J. 2018, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.; Ballesteros, A.; Qiu, Q.; Pow Sang, L.; Shashikumar, S.; Casares, S.; Brumeanu, T.D. Generation and testing anti-influenza human monoclonal antibodies in a new humanized mouse model (DRAGA: HLA-A2. HLA-DR4. Rag1 KO. IL-2Rgammac KO. NOD). Hum. Vaccines Immunother. 2018, 14, 345–360. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Cao, Z.; Ishikawa, Y.; Cui, S.; Imadome, K.-I.; Snapper, C.M. Immunization with Epstein–Barr Virus Core Fusion Machinery Envelope Proteins Elicit High Titers of Neutralizing Activities and Protect Humanized Mice from Lethal Dose EBV Challenge. Vaccines 2021, 9, 285. https://doi.org/10.3390/vaccines9030285
Cui X, Cao Z, Ishikawa Y, Cui S, Imadome K-I, Snapper CM. Immunization with Epstein–Barr Virus Core Fusion Machinery Envelope Proteins Elicit High Titers of Neutralizing Activities and Protect Humanized Mice from Lethal Dose EBV Challenge. Vaccines. 2021; 9(3):285. https://doi.org/10.3390/vaccines9030285
Chicago/Turabian StyleCui, Xinle, Zhouhong Cao, Yuriko Ishikawa, Sara Cui, Ken-Ichi Imadome, and Clifford M. Snapper. 2021. "Immunization with Epstein–Barr Virus Core Fusion Machinery Envelope Proteins Elicit High Titers of Neutralizing Activities and Protect Humanized Mice from Lethal Dose EBV Challenge" Vaccines 9, no. 3: 285. https://doi.org/10.3390/vaccines9030285
APA StyleCui, X., Cao, Z., Ishikawa, Y., Cui, S., Imadome, K.-I., & Snapper, C. M. (2021). Immunization with Epstein–Barr Virus Core Fusion Machinery Envelope Proteins Elicit High Titers of Neutralizing Activities and Protect Humanized Mice from Lethal Dose EBV Challenge. Vaccines, 9(3), 285. https://doi.org/10.3390/vaccines9030285




