Antibody Response to a Live-Modified Virus Vaccine against Bovine Viral Diarrhoea in Dairy Cattle in a Field Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vaccination
2.3. Samples Collection
- n—required sample size
- N—herd size (290)
- d—minimum expected number of animals that did not seroconvert after vaccination (25%)
- P—the probability of finding at least one seronegative animal in the sample (i.e., a level of confidence = 95%)
2.4. ELISA
2.5. Virus Neutralization Test (VNT)
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Committee on Taxonomy of Viruses (ICTV). Flaviviridae. In Virus Taxonomy: The Classification and Nomenclature of Viruses; Report of ICTV; Berlin, Germany, 2019; Available online: www.ictv.global/report/flaviviridae (accessed on July 2019).
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Report Consortium. ICTV Virus Taxonomy Profile, Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Houe, H. Economic impact of BVDV infection in dairies. Biologicals 2003, 31, 137–143. [Google Scholar] [CrossRef]
- Rodning, P.S.; Givens, D.M.; Marley, D.S.M.; Zhang, Y.; Riddell, P.K.; Galik, K.P.; Hathcock, T.L.; Gard, J.A.; Prevatt, J.W.; Owsley, F.W. Reproductive and economic impact following controlled introduction of cattle persistently infected with bovine viral diarrhea virus into a naïve group of heifers. Therio 2012, 78, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Stott, A.W.; Humphry, R.W.; Gunn, G.J. Modelling the effects of previous infection and re-infection on the costs of bovine viral diarrhoea outbreaks in beef herds. Vet. J. 2010, 185, 138–143. [Google Scholar] [CrossRef]
- Kuta, A.; Polak, M.P.; Larska, M.; Żmudziński, J.F. Predominance of bovine viral diarrhea virus 1b and 1d subtypes during eight years of survey in Poland. Vet. Microbiol. 2013, 166, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Mirosław, P.; Polak, M.P. Increased genetic variation of bovine viral diarrhea virus in dairy cattle in Poland. BMC Vet. Res. 2019, 15, 278. [Google Scholar] [CrossRef]
- Kuta, A.; Polak, M.P.; Larska, M.; Żmudziński, J.F. Monitoring of Bovine Viral Diarrhoea Virus (BVDV) infection in Polish dairy herds using bulk tank milk samples. Bull. Vet. Inst. Pulawy 2013, 57, 149–156. [Google Scholar] [CrossRef][Green Version]
- Rypuła, K.; Płoneczka-Janeczko, K.; Czopowicz, M.; Klimowicz-Bodys, M.D.; Shabunin, S.; Siegwalt, G. Occurrence of BVDV infection and the presence of potential risk factors in dairy cattle herds in Poland. Animals 2020, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Rypuła, K.; Kumala, A.; Kaba, J.; Płoneczko-Janeczko, K.; Wojewoda-Kotwica, B.; Mazurkiewicz, M. Epidemiological aspects of BVDV infections in dairy cattle herds in Poland. Med. Wet. 2010, 66, 684–687. (In Polish) [Google Scholar]
- Moennig, V.; Houe, H.; Lindberg, A. BVD control in Europe: Current status and perspectives. Anim. Health Res. Rev. 2005, 6, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W. Impact of species and subgenotypes of bovine viral diarrhea virus on control by vaccination. Anim. Health Res. Rev. 2015, 16, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F. Immunology of BVDV vaccines. Biologicals 2013, 41, 14–19. [Google Scholar] [CrossRef]
- Newcomer, B.W.; Chamorro, M.F.; Walz, P.H. Vaccination of cattle against bovine viral diarrhea virus. Vet. Microbiol. 2017, 206, 78–83. [Google Scholar] [CrossRef]
- Surveys. Veterinary Epidemiology, 4th ed.; Thrusfield, M., Christley, R., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2018; pp. 270–296. [Google Scholar]
- Lanyon, S.R.; Anderson, M.L.; Bergman, E.; Reichel, M.P. Validation and evaluation of a commercially available ELISA for the detection of antibodies specific to bovine viral diarrhoea virus (bovine pestivirus). Aust. Vet. J. 2013, 91, 52–56. [Google Scholar] [CrossRef]
- Newcomer, B.W.; Walz, P.H.; Givens, M.D. Efficacy of bovine viral diarrhea virus vaccination to prevent reproductive disease: A meta-analysis. Therio 2015, 83, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Deplanche, M.; Roux, D.; Moulignie, M.; Picard-Hagen, N.; Lyazrhi, F.; Raboisson, D.; Mathevet, P.; Schelcher, F. Fetal protection against bovine viral diarrhoea type 1 virus infection after one administration of a live-attenuated vaccine. Vet. J. 2012, 192, 242–245. [Google Scholar] [CrossRef]
- Sozzi, E.; Righi, C.; Boldini, M.; Bazzucchi, M.; Pezzoni, G.; Gradassi, M.; Petrini, S.; Lelli, D.; Ventura, G.; Pierini, I.; et al. Cross-Reactivity Antibody Response after Vaccination with Modified Live and Killed Bovine Viral Diarrhoea Virus (BVD). Vaccines 2020, 8, 374. [Google Scholar] [CrossRef]
- Rodning, S.P.; Marley, M.S.; Zhang, Y.; Eason, A.B.; Nunley, C.L.; Walz, P.H.; Riddell, K.P.; Galik, P.K.; Brodersen, B.W.; Givens, M.D. Comparison of three commercial vaccines for preventing persistent infection with bovine viral diarrhea virus. Therio 2010, 73, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Griebel, P.J. BVDV vaccination in North America: Risk versus benefits. Anim. Health Res. Rev. 2015, 16, 27–32. [Google Scholar] [CrossRef]
- Hawkes, P.W.; Fetal Bovine Serum (FBS) Irradiation Working Group. The Harmonization and Standardization of Irradiation Procedures for Fetal Bovine Serum (FBS). Available online: https://www.biowest.net (accessed on 20 May 2016).
- Antos, A.; Rola, J.; Bednarski, M.; Krzysiak, M.K.; Kęsik-Maliszewska, J.; Larska, M. Is contamination of bovine-sourced material with bovine viral diarrhea virus still a problem in countries with ongoing eradication campaigns? Ann. Anim. Sci. 2020. [Google Scholar] [CrossRef]
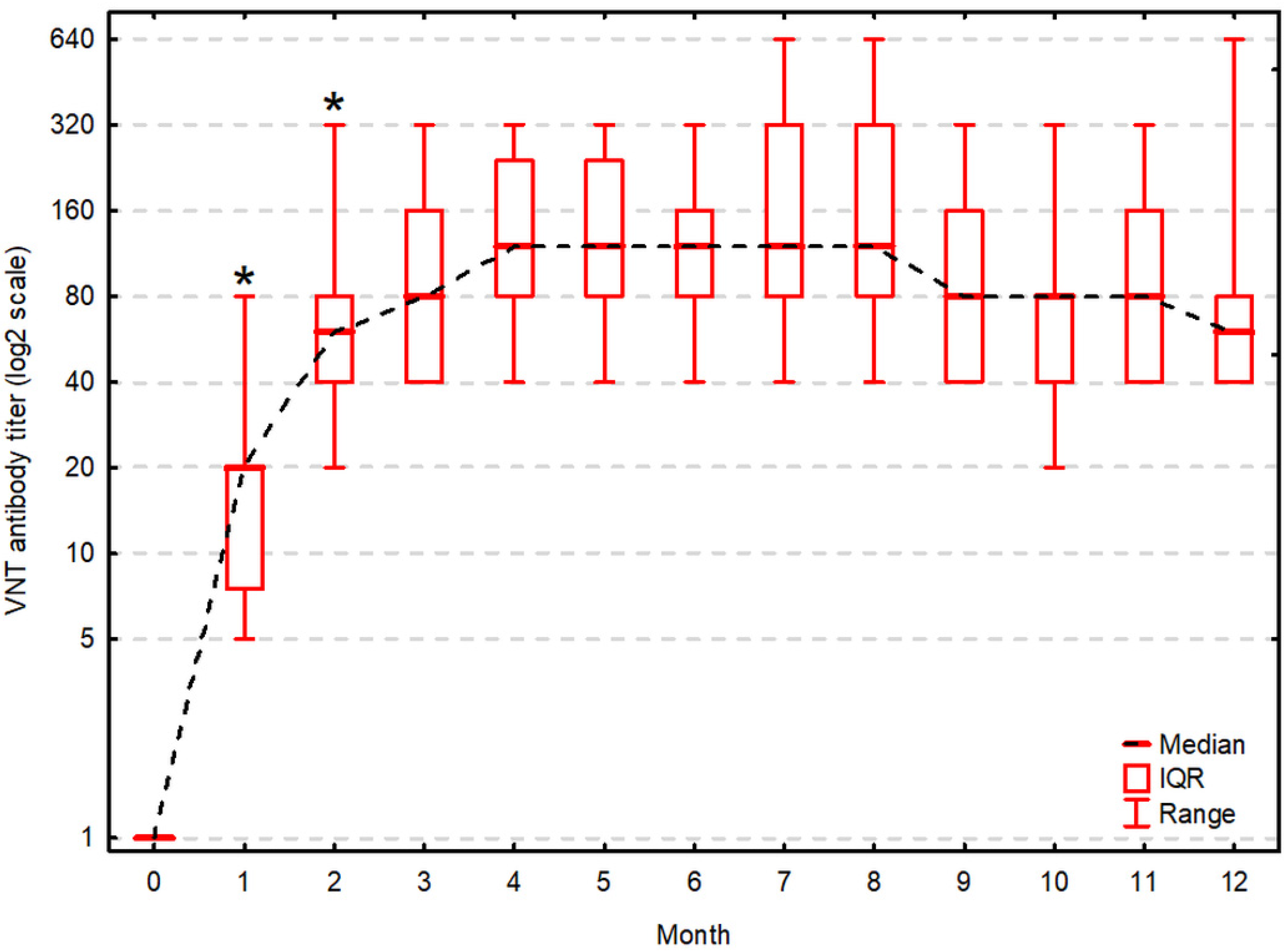
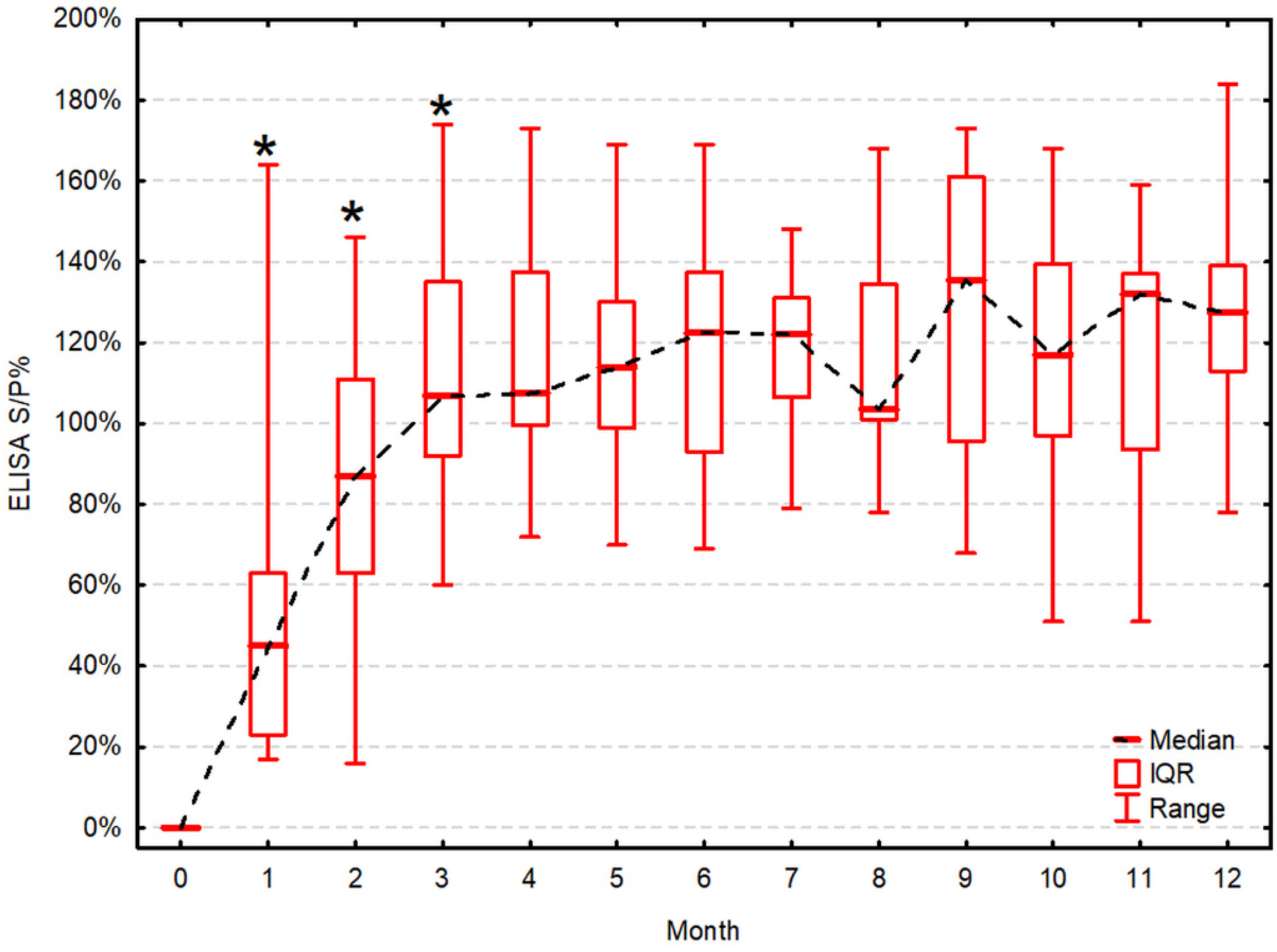
| Test (Month) | VNT [log2 Transformed Titer] | Significance of an Increment Compared to the Previous Testing (p) | ELISA [S/P%] | Significance of an Increment Compared to the Previous Testing (p) | Correlation between VNT and ELISA (Rs, p-Value) |
|---|---|---|---|---|---|
| 0 | 0 | - | 0 | - | - |
| 1 | 4.3, 2.8–4.3 (2.3–6.3) | 0.009 1 | 45, 23–63 (17–164) | 0.009 1 | 0.91, p < 0.001 1 |
| 2 | 5.8, 5.3–6.3 (4.3–8.3) | 0.014 1 | 87, 63–111 (16–146) | 0.030 1 | 0.88, p < 0.001 1 |
| 3 | 6.3, 5.3–7.3 (5.3–8.3) | ns | 107, 92–135 (60–174) | 0.030 1 | 0.90, p < 0.001 1 |
| 4 | 6.8, 6.3–7.8 (5.3–8.3) | ns | 108, 100–138 (72–173) | ns | 0.79, p = 0.003 1 |
| 5 | 6.8, 6.3–7.8 (5.3–8.3) | ns | 114, 99–130 (70–169) | ns | 0.74, p = 0.006 1 |
| 6 | 6.8, 6.3–7.3 (5.3–8.3) | ns | 123, 93–138 (69–169) | ns | 0.84, p = 0.001 1 |
| 7 | 6.8, 6.3–8.3 (5.3–9.3) | ns | 122, 107–131 (79–148) | ns | 0.82, p = 0.001 1 |
| 8 | 6.8, 6.3–8.3 (5.3–9.3) | ns | 104, 101–135 (78–168) | ns | 0.91, p < 0.001 1 |
| 9 | 6.3, 5.3–7.3 (5.3–8.3) | ns | 136, 96–161 (68–173) | ns | 0.30, p = 0.353 |
| 10 | 6.3, 5.3–6.3 (4.3–8.3) | ns | 117, 97–140 (51–168) | ns | 0.90, p < 0.001 1 |
| 11 | 6.3, 5.3–7.3 (5.3–8.3) | ns | 132, 94–137 (51–159) | ns | 0.70, p = 0.011 1 |
| 12 | 5.8, 5.3–6.3 (5.3–9.3) | ns | 128, 113–139 (78–184) | ns | 0.92, p < 0.001 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimowicz-Bodys, M.D.; Płoneczka-Janeczko, K.; Czopowicz, M.; Polak, M.P.; Lachowicz-Wolak, A.; Rypuła, K. Antibody Response to a Live-Modified Virus Vaccine against Bovine Viral Diarrhoea in Dairy Cattle in a Field Trial. Vaccines 2021, 9, 259. https://doi.org/10.3390/vaccines9030259
Klimowicz-Bodys MD, Płoneczka-Janeczko K, Czopowicz M, Polak MP, Lachowicz-Wolak A, Rypuła K. Antibody Response to a Live-Modified Virus Vaccine against Bovine Viral Diarrhoea in Dairy Cattle in a Field Trial. Vaccines. 2021; 9(3):259. https://doi.org/10.3390/vaccines9030259
Chicago/Turabian StyleKlimowicz-Bodys, Małgorzata D., Katarzyna Płoneczka-Janeczko, Michał Czopowicz, Mirosław Paweł Polak, Agnieszka Lachowicz-Wolak, and Krzysztof Rypuła. 2021. "Antibody Response to a Live-Modified Virus Vaccine against Bovine Viral Diarrhoea in Dairy Cattle in a Field Trial" Vaccines 9, no. 3: 259. https://doi.org/10.3390/vaccines9030259
APA StyleKlimowicz-Bodys, M. D., Płoneczka-Janeczko, K., Czopowicz, M., Polak, M. P., Lachowicz-Wolak, A., & Rypuła, K. (2021). Antibody Response to a Live-Modified Virus Vaccine against Bovine Viral Diarrhoea in Dairy Cattle in a Field Trial. Vaccines, 9(3), 259. https://doi.org/10.3390/vaccines9030259







