Safety and Immunogenicity of a Novel Intranasal Influenza Vaccine (NasoVAX): A Phase 2 Randomized, Controlled Trial
Abstract
1. Introduction
2. Material and Methods
2.1. Study Procedures
2.2. Investigational Product
2.3. Safety Assessments
2.4. Immunogenicity Assessments
2.5. Hemagglutination Inhibition Assay (HAI)
2.6. Microneutralization (MN) Assay
2.7. Hemagglutinin-Specific T Cells by IFN-γ ELISpot
2.8. HA-Specific Mucosal IgA
2.9. Ad5 Neutralizing Antibody
2.10. Adenoviral Vector Shedding
2.11. Statistical Analysis
3. Results
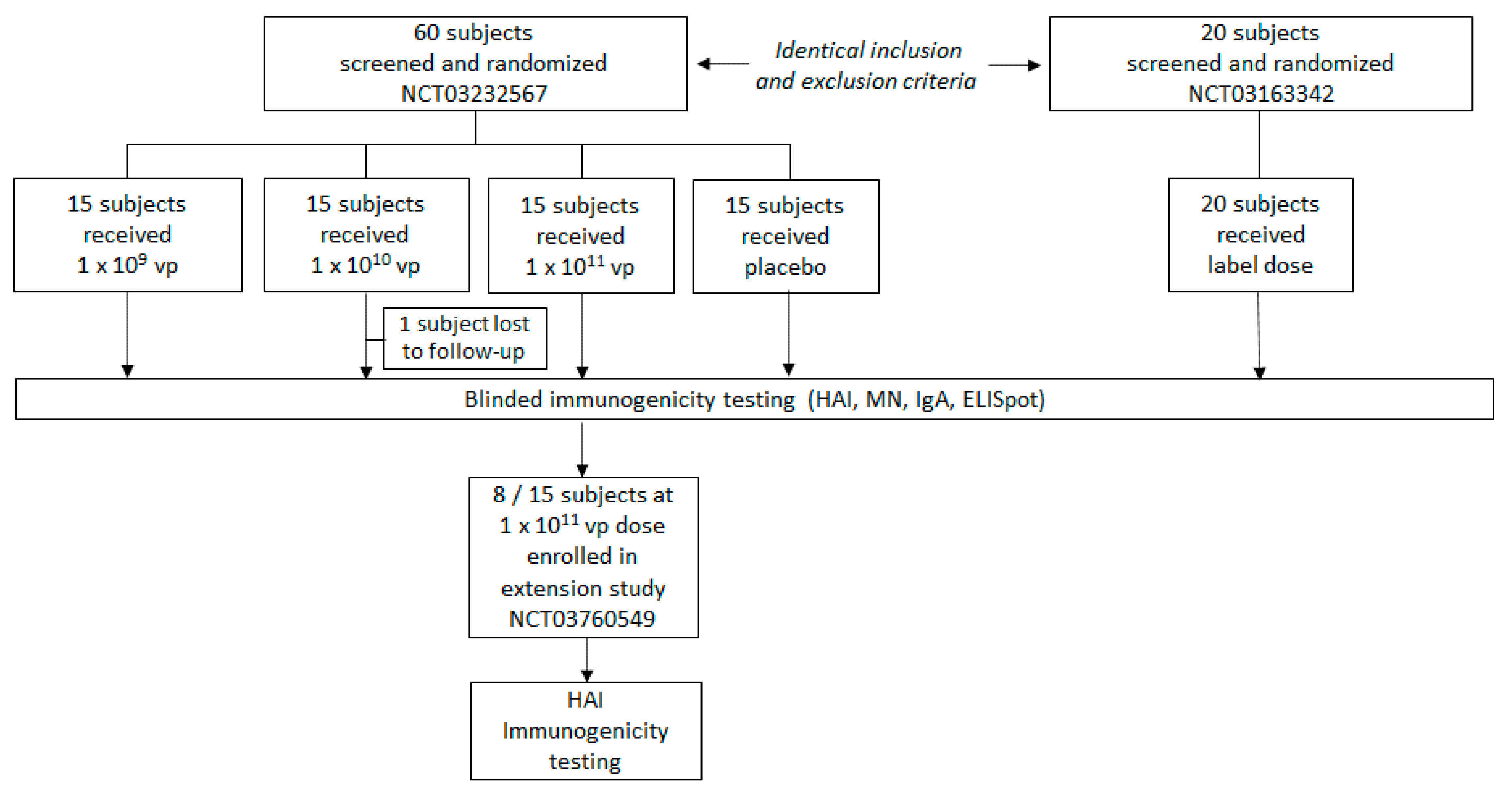
3.1. Study Population
3.2. Safety
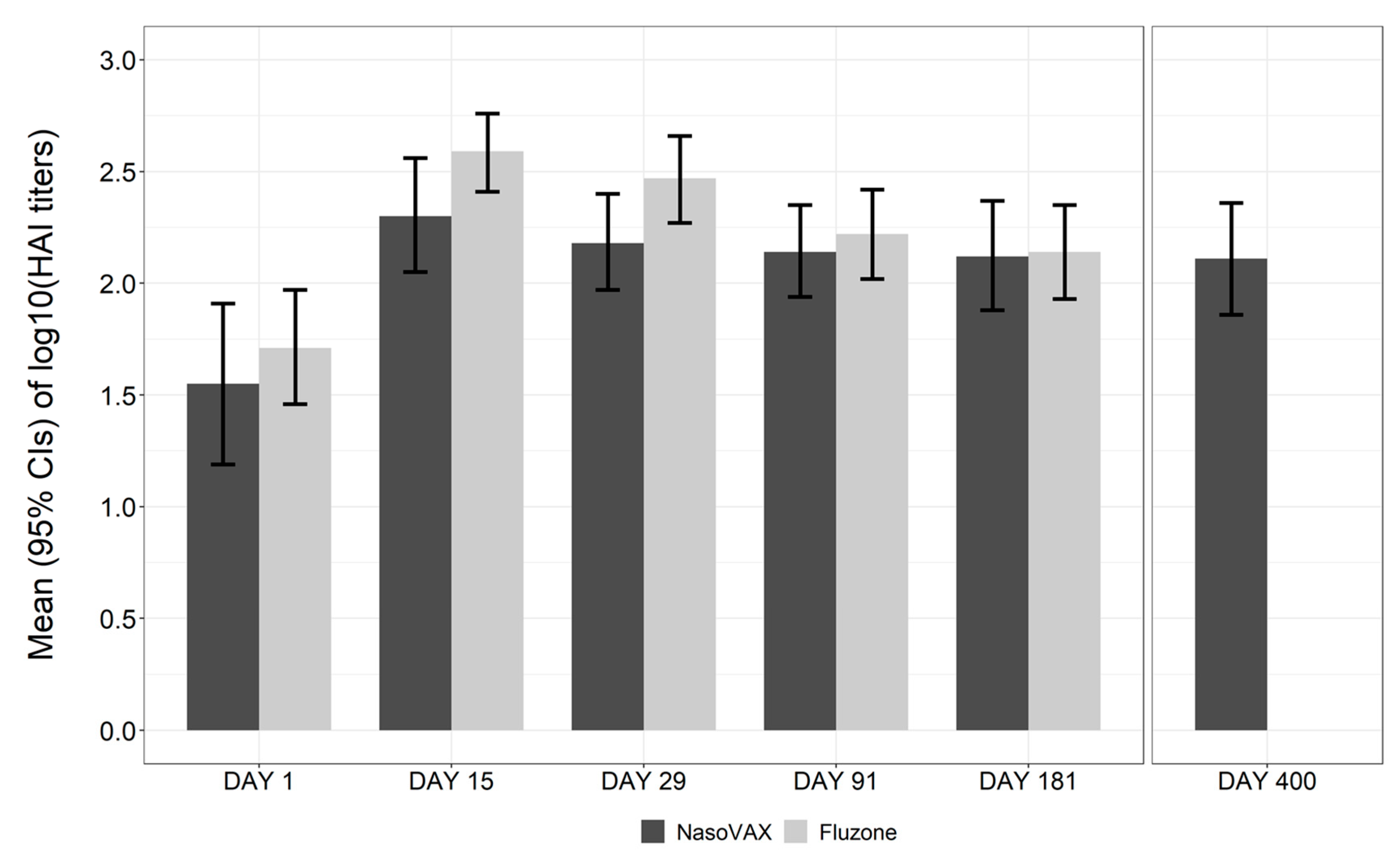
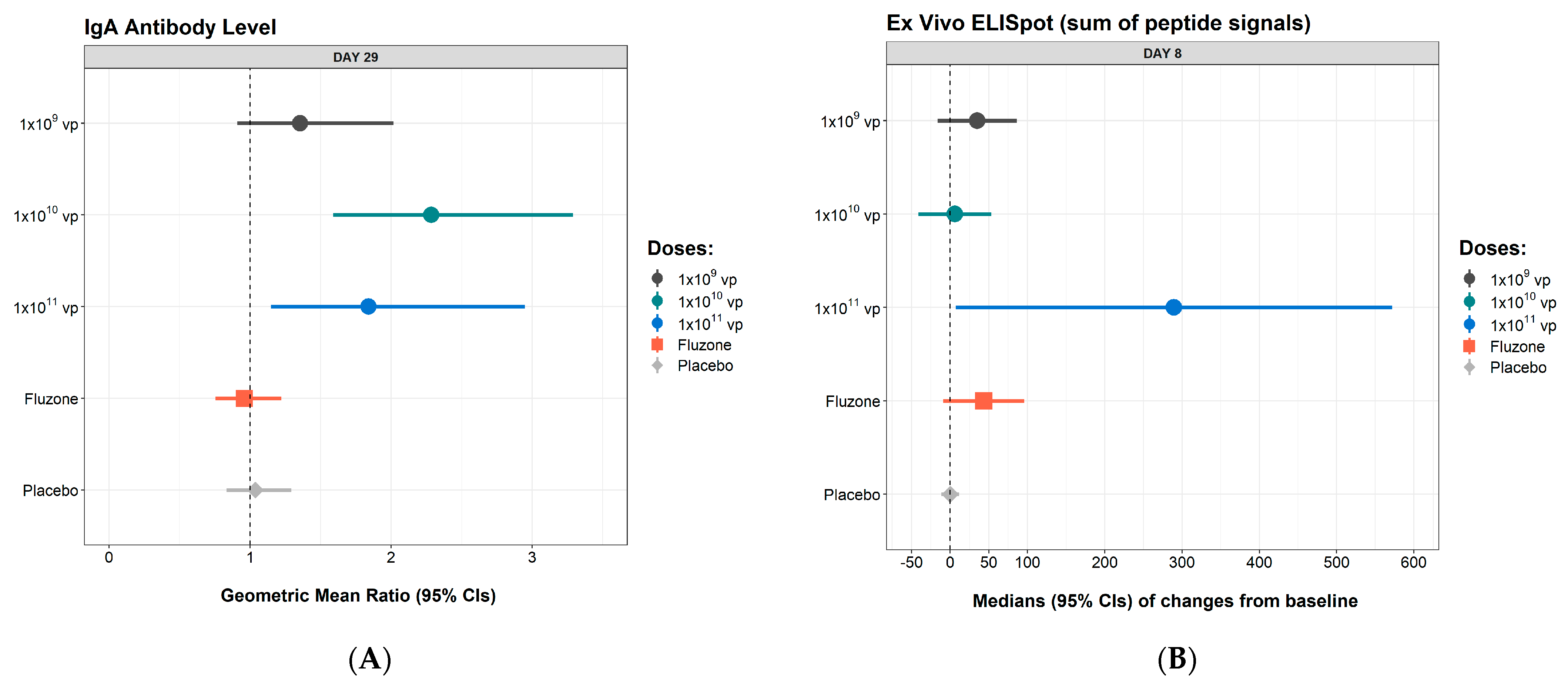
3.3. Immunogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Past Seasons Vaccine Effectiveness Estimates. Available online: https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html (accessed on 29 January 2020).
- Belshe, R.B.; Gruber, W.C.; Mendelman, P.M.; Mehta, H.B.; Mahmood, K.; Reisinger, K.; Treanor, J.; Zangwill, K.; Hayden, F.G.; Bernstein, D.I.; et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J. Infect. Dis. 2000, 181, 1133–1137. [Google Scholar] [CrossRef]
- Clements, M.L.; Betts, R.F.; Tierney, E.L.; Murphy, B.R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J. Clin. Microbiol. 1986, 24, 157–160. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Wu, X.; Jones, T.; Mallory, R.M. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine 2012, 30, 6794–6801. [Google Scholar] [CrossRef] [PubMed]
- Gould, V.M.W.; Francis, J.N.; Anderson, K.J.; Georges, B.; Cope, A.V.; Tregoning, J.S. Nasal IgA provides protection against human influenza challenge in volunteers with low serum influenza antibody titre. Front. Microbiol. 2017, 8, 900. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.; Li, C.K.; Chui, C.S.; Huang, A.K.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Fry, A.M.; Reynolds, S.; Petrie, J.; Flannery, B.; Jackson, M.L.; Belongia, E.A. Intraseason waning of influenza vaccine protection: Evidence from the US Influenza Vaccine Effectiveness Network, 2011–2012 through 2014–2015. Clin. Infect. Dis. 2017, 64, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Cheong, H.J.; Hwang, I.S.; Choi, W.S.; Jo, Y.M.; Park, D.W.; Cho, G.J.; Hwang, T.G.; Kim, W.J. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine 2010, 28, 3929–3935. [Google Scholar] [CrossRef]
- Erbelding, E.J.; Post, D.J.; Stemmy, E.J.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A universal influenza vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018, 218, 347–354. [Google Scholar] [CrossRef]
- Pesonen, S.; Kangasniemi, L.; Hemminki, A. Oncolytic adenoviruses for the treatment of human cancer: Focus on translational and clinical data. Mol. Pharm. 2011, 8, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Sumida, S.M.; Truitt, D.M.; Lemckert, A.A.; Vogels, R.; Custers, J.H.; Addo, M.M.; Lockman, S.; Peter, T.; Peyerl, F.W.; Kishko, M.G.; et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 2005, 174, 7179–7185. [Google Scholar] [CrossRef]
- Ledgerwood, J.E.; Costner, P.; Desai, N.; Holman, L.; Enama, M.E.; Yamshchikov, G.; Mulangu, S.; Hu, Z.; Andrews, C.A.; Sheets, R.A.; et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine 2010, 29, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Croyle, M.A.; Patel, A.; Tran, K.N.; Gray, M.; Zhang, Y.; Strong, J.E.; Feldmann, H.; Kobinger, G.P. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS ONE 2008, 3, e3548. [Google Scholar] [CrossRef]
- Van Kampen, K.R.; Shi, Z.; Gao, P.; Zhang, J.; Foster, K.W.; Chen, D.T.; Marks, D.; Elmets, C.A.; Tang, D.C. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 2005, 23, 1029–1036. [Google Scholar] [CrossRef]
- Li, Y.; Jin, L.; Chen, T. The effects of secretory IgA in the mucosal immune system. BioMed Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef]
- Kwong, J.C.; Pereira, J.A.; Quach, S.; Pellizzari, R.; Dusome, E.; Russell, M.L.; Hamid, J.S.; Feinberg, Y.; Winter, A.L.; Gubbay, J.B.; et al. Randomized evaluation of live attenuated vs. inactivated influenza vaccines in schools (RELATIVES) cluster randomized trial: Pilot results from a household surveillance study to assess direct and indirect protection from influenza vaccination. Vaccine 2015, 33, 4910–4915. [Google Scholar] [CrossRef]
- King, R.G.; Silva-Sanchez, A.; Peel, J.N.; Botta, D.; Meza-Perez, S.; Allie, R.; Schultz, M.D.; Liu, M.; Bradley, J.E.; Qiu, S.; et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 in mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tan, W.G.; Jin, H.T.; West, E.E.; Penaloza-MacMaster, P.; Wieland, A.; Zilliox, M.J.; McElrath, M.J.; Barouch, D.H.; Ahmed, R. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J. Virol. 2013, 87, 1359–1372. [Google Scholar] [CrossRef]
- Takamura, S.; Kohlmeier, J.E. Establishment and maintenance of conventional and circulation-driven lung-resident memory CD8+ T cells following respiratory virus infections. Front. Immunol. 2019, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, P.; Shen, Y.; Jiang, X.; Xu, F. CD8+ Resident memory T cells and viral infection. Front. Immunol. 2018, 9, 2093. [Google Scholar] [CrossRef]
- Barouch, D.H.; Kik, S.V.; Weverling, G.J.; Dilan, R.; King, S.L.; Maxfield, L.F.; Clark, S.; Ng’ang’a, D.; Brandariz, K.L.; Abbink, P.; et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 2011, 29, 5203–5209. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, A.K.; Machado, H.B.; Herschman, H.R. The influence of innate and pre-existing immunity on adenovirus therapy. J. Cell. Biochem. 2009, 108, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Schafer, S.C.; Zhang, L.; Kobinger, G.P.; Juelich, T.; Freiberg, A.N.; Croyle, M.A. A single sublingual dose of an adenovirus-based vaccine protects against lethal Ebola challenge in mice and guinea pigs. Mol. Pharm. 2012, 9, 156–167. [Google Scholar] [CrossRef]
- Kim, E.H.; Park, H.J.; Han, G.Y.; Song, M.K.; Pereboev, A.; Hong, J.S.; Chang, J.; Byun, Y.H.; Seong, B.L.; Nguyen, H.H. Intranasal adenovirus-vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice. J. Virol. 2014, 88, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | NasoVAX | Placebo N = 15 | Fluzone N = 20 | ||
|---|---|---|---|---|---|
| 1 × 109 vp N = 15 | 1 × 1010 vp N = 15 | 1 × 1011 vp N = 15 | |||
| Sex, n (%) | |||||
| Female | 11 (73.3) | 10 (66.7) | 6 (40.0) | 6 (40.0) | 10 (50.0) |
| Male | 4 (26.7) | 5 (33.3) | 9 (60.0) | 9 (60.0) | 10 (50.0) |
| Age (years) | |||||
| Mean (SD) | 30.1 (8.50) | 31.6 (8.54) | 32.6 (9.35) | 29.3 (6.87) | 34.2 (8.69) |
| Range: minimum, maximum | 19, 47 | 18, 46 | 21, 48 | 20, 45 | 21, 49 |
| Race, n (%) a | |||||
| White | 8 (53.3) | 9 (60.0) | 8 (53.3) | 9 (60.0) | 9 (45.0) |
| Black | 6 (40.0) | 6 (40.0) | 6 (40.0) | 6 (40.0) | 9 (45.0) |
| Asian | 1 (6.7) | 2 (13.3) | 2 (13.3) | 0 | 0 |
| Other | 1 (6.7) | 0 | 0 | 0 | 2 (10.0) |
| Immunogenicity b | |||||
| HAI GMT (95% CI) | 39.1 (19.4, 78.8) | 71.3 (32.6, 156.0) | 35.6 (15.6, 81.4) | 30.3 (14.0, 65.8) | 51.9 (28.9, 93.2) |
| Microneutralization GMT (95% CI) | 21.4 (10.5, 43.7) | 44.9 (21.5, 93.6) | 27.6 (13.3, 57.4) | 16.2 (8.5, 31.2) | 26.4 (14.5, 48.2) |
| ELISpot GM SFU/106 cells (95% CI) | 3.6 (1.2, 11.0) | 1.8 (0.7, 4.4) | 6.9 (2.0, 24.2) | 7.2 (1.8. 28.4) | 2.9 (1.3, 6.3) |
| Event | NasoVAX | Placebo N = 15 n (%) | |||
|---|---|---|---|---|---|
| 1 × 109 vp N = 15 n (%) | 1 × 1010 vp N = 15 n (%) | 1 × 1011 vp N = 15 n (%) | Overall N = 45 n (%) | ||
| Any local or systemic event | 13 (86.7) | 12 (80.0) | 8 (53.3) | 33 (73.3) | 7 (46.6) |
| Any local event | 9 (60.0) | 9 (60.0) | 6 (40.0) | 24 (53.3) | 6 (40.0) |
| Nasal irritation | 3 (20.0) | 5 (33.3) | 1 (6.7) | 9 (20.0) | 2 (13.3) |
| Sneezing | 4 (26.7) | 5 (33.3) | 3 (20.0) | 12 (26.7) | 3 (20.0) |
| Nasal congestion | 6 (40.0) | 7 (46.7) | 2 (13.3) | 15 (33.3) | 4 (26.7) |
| Cough | 3 (20.0) | 1 (6.7) | 2 (13.3) | 6 (13.3) | 3 (20.0) |
| Sore throat | 4 (26.7) | 4 (26.7) | 3 (20.0) | 11 (24.4) | 3 (20.0) |
| Change in smell | 2 (13.3) | 0 | 0 | 2 (4.4) | 1 (6.7) |
| Change in taste | 1 (6.7) | 1 (6.7) | 0 | 2 (4.4) | 2 (13.3) |
| Change in vision | 0 | 0 | 0 | 0 | 0 |
| Eye pain | 2 (13.3) | 3 (20.0) | 0 | 5 (11.1) | 1 (6.7) |
| Any systemic event | 9 (60.0) | 11 (73.3) | 5 (33.3) | 25 (55.6) | 6 (40.0) |
| Headache | 7 (46.7) | 10 (66.7) | 4 (26.7) | 21 (46.7) | 6 (40.0) |
| Fatigue | 5 (33.3) | 7 (46.7) | 3 (20.0) | 15 (33.3) | 4 (26.7) |
| Muscle ache | 2 (13.3) | 1 (6.7) | 3 (20.0) | 6 (13.3) | 1 (6.7) |
| Nausea | 2 (13.3) | 2 (13.3) | 0 | 4 (8.9) | 2 (13.3) |
| Vomiting | 0 | 0 | 0 | 0 | 1 (6.7) |
| Diarrhea | 0 | 0 | 1 (6.7) | 1 (2.2) | 1 (6.7) |
| Chills | 0 | 1 (6.7) | 0 | 1 (2.2) | 0 |
| Fever | 0 | 0 | 0 | 0 | 0 |
| Preferred Term | NasoVAX | Placebo N = 15 n (%) | |||
|---|---|---|---|---|---|
| 1 × 109 vp N = 15 n (%) | 1 × 1010 vp N = 15 n (%) | 1 × 1011 vp N = 15 n (%) | Overall N = 45 n (%) | ||
| Any adverse event | 2 (13.3) | 5 (33.3) | 3 (20.0) | 10 (22.2) | 3 (20.0) |
| Skin abrasion a | 1 (6.7) | 1 (6.7) | 2 (13.3) | 4 (8.9) | 1 (6.7) |
| Epistaxis | 0 | 0 | 2 (13.3) | 2 (4.4) | 0 |
| Rhinorrhoea | 1 (6.7) | 1 (6.7) | 0 | 2 (4.4) | 0 |
| Chills | 0 | 0 | 1 (6.7) | 1 (2.2) | 0 |
| Ear pain | 0 | 0 | 1 (6.7) | 1 (2.2) | 0 |
| Flushing | 0 | 0 | 1 (6.7) | 1 (2.2) | 0 |
| Laryngitis | 0 | 1 (6.7) | 0 | 1 (2.2) | 0 |
| Sinusitis | 0 | 1 (6.7) | 0 | 1 (2.2) | 0 |
| Upper respiratory tract infection | 0 | 1 (6.7) | 0 | 1 (2.2) | 0 |
| Lacrimation increased | 0 | 0 | 0 | 0 | 1 (6.7) |
| Nasal septum ulceration | 0 | 0 | 0 | 0 | 1 (6.7) |
| Statistic | NasoVAX | Placebo | Fluzone | ||
|---|---|---|---|---|---|
| 1 × 109 vp | 1 × 1010 vp | 1 × 1011 vp | |||
| Hemagglutinin Inhibition Response at Day 29 | |||||
| GMT (95% CI) a | 83.8 (42.1, 166.7) | 160.0 (94.8, 270.0) | 152.8 (93.5, 249.7) | 27.6 (13.5, 56.8) | 293.4 (186.7, 461.3) |
| GMR (95% CI) | 2.1 (1.1, 4.2) | 2.2 (1.2, 4.3) | 4.3 (1.5, 12.0) | 0.9 (0.7, 1.2) | 5.7 (2.9, 11.2) |
| SCR b (95% CI) | 13.3% (1.7%, 40.5%) | 26.7% (7.8%, 55.1%) | 33.3% (11.8%, 61.6%) | 0.0% (0.0%, 21.8%) | 50.0% (27.2%, 72.8%) |
| SPR c (95% CI) | 80.0% (51.9%, 95.7%) | 100.0% (78.2%, 100.0%) | 100.0% (78.2%, 100.0%) | 53.3% (26.6%, 78.7%) | 95.0% (75.1%, 99.9%) |
| Microneutralization Response at Day 29 | |||||
| GMT (95% CI) | 44.9 (21.8, 92.3) | 113.1 (58.0, 220.8) | 142.5 (93.6, 217.1) | 17.8 (9.1, 35.0) | 162.8 (95.8, 276.6) |
| Responder rate 4-fold rise (95% CI) | 13.3% (1.7%, 40.5%) | 26.7% (7.8%, 55.1%) | 53.3% (26.6%, 78.7%) | 0.0% (0.0%, 21.8%) | 50.0% (27.2%, 72.8%) |
| Immunogenicity Endpoint | NasoVAX | |||||
|---|---|---|---|---|---|---|
| 1 × 109 vp | 1 × 1010 vp | 1 × 1011 vp | ||||
| Predose Ad5 Serostatus | Positive a N = 7 | Negative N = 8 | Positive a N = 8 | Negative N = 7 | Positive a N = 10 | Negative N = 5 |
| Predose Anti-Ad5 Neutralizing Titer GMT (95% CI) | 5110.7 (1024.9, 25,484.7) | 112.4 (112.4, 112.4) | 3250.1 (827.5, 12764.4) | 112.4 (112.4, 112.4) | 4015.4 (1483.7, 10866.9) | 112.4 (112.4, 112.4) |
| Immunogenicity Endpoint | ||||||
| Day 29 HAI GMR (95% CI) | 1.22 (0.89,1.67) | 3.51 (1.02, 12.12) | 1.61 (0.70, 3.71) | 3.28 (0.95, 11.33) | 4.44 (1.28, 15.38) | 4.00 (0.23, 69.46) |
| Day 29 MN GMR (95% CI) | 1.22 (0.89, 1.67) | 3.36 (0.88, 12.86) | 1.61 (0.84, 3.10) | 4.20 (1.21, 14.63) | 5.46 (1.83, 16.30) | 4.60 (0.58, 36.68) |
| Day 8 change from baseline (SFU/106 cells), median (95% CI) | 0 (−17.2, 17.2) | 89 (−21.5, 198.5) | 0 (−48.1, 48.1) | 43 (−26.8, 112.8) | 267 (46.8, 487.2) | 735 (323.6,1146.4) |
| Day 29 IgA GMR (95% CI) | 1.03 (0.79, 1.35) | 1.72 (0.81, 3.68) | 2.62 (1.64, 4.16) | 1.96 (0.96, 4.00) | 1.87 (1.01, 3.44) | 1.78 (0.55, 5.77) |
| Day 29 anti-Ad5 GMR (95% CI) | 1.09 (0.83, 1.44) | 2.43 (0.84, 7.05) | 1.42 (0.78, 2.56) | 1.79 (0.89, 3.61) | 2.47 (1.25, 4.89) | 2.10 (0.53, 8.38) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasker, S.; Wight O’Rourke, A.; Suyundikov, A.; Jackson Booth, P.-G.; Bart, S.; Krishnan, V.; Zhang, J.; Anderson, K.J.; Georges, B.; Roberts, M.S. Safety and Immunogenicity of a Novel Intranasal Influenza Vaccine (NasoVAX): A Phase 2 Randomized, Controlled Trial. Vaccines 2021, 9, 224. https://doi.org/10.3390/vaccines9030224
Tasker S, Wight O’Rourke A, Suyundikov A, Jackson Booth P-G, Bart S, Krishnan V, Zhang J, Anderson KJ, Georges B, Roberts MS. Safety and Immunogenicity of a Novel Intranasal Influenza Vaccine (NasoVAX): A Phase 2 Randomized, Controlled Trial. Vaccines. 2021; 9(3):224. https://doi.org/10.3390/vaccines9030224
Chicago/Turabian StyleTasker, Sybil, Anna Wight O’Rourke, Anvar Suyundikov, Peta-Gay Jackson Booth, Stephan Bart, Vyjayanthi Krishnan, Jianfeng Zhang, Katie J. Anderson, Bertrand Georges, and M. Scot Roberts. 2021. "Safety and Immunogenicity of a Novel Intranasal Influenza Vaccine (NasoVAX): A Phase 2 Randomized, Controlled Trial" Vaccines 9, no. 3: 224. https://doi.org/10.3390/vaccines9030224
APA StyleTasker, S., Wight O’Rourke, A., Suyundikov, A., Jackson Booth, P.-G., Bart, S., Krishnan, V., Zhang, J., Anderson, K. J., Georges, B., & Roberts, M. S. (2021). Safety and Immunogenicity of a Novel Intranasal Influenza Vaccine (NasoVAX): A Phase 2 Randomized, Controlled Trial. Vaccines, 9(3), 224. https://doi.org/10.3390/vaccines9030224






