Chimeric Measles Virus (MV/RSV), Having Ectodomains of Respiratory Syncytial Virus (RSV) F and G Proteins Instead of Measles Envelope Proteins, Induced Protective Antibodies against RSV
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strains and Cells
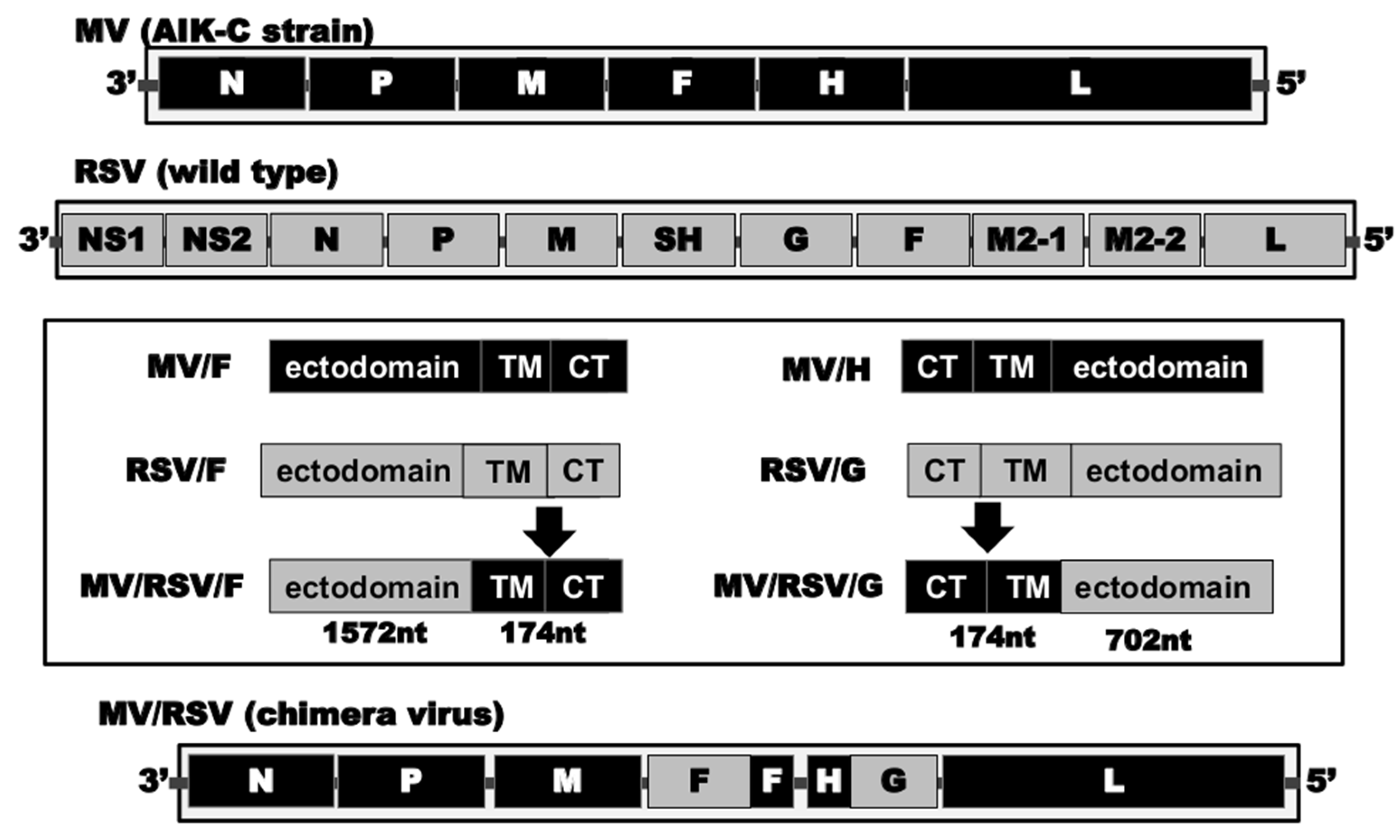
2.2. Construction of Recombinant Chimeric AIK-C (MV/RSV)
2.3. Virus Culture and Purification
2.4. Immunostaining
2.5. Viral Tropism
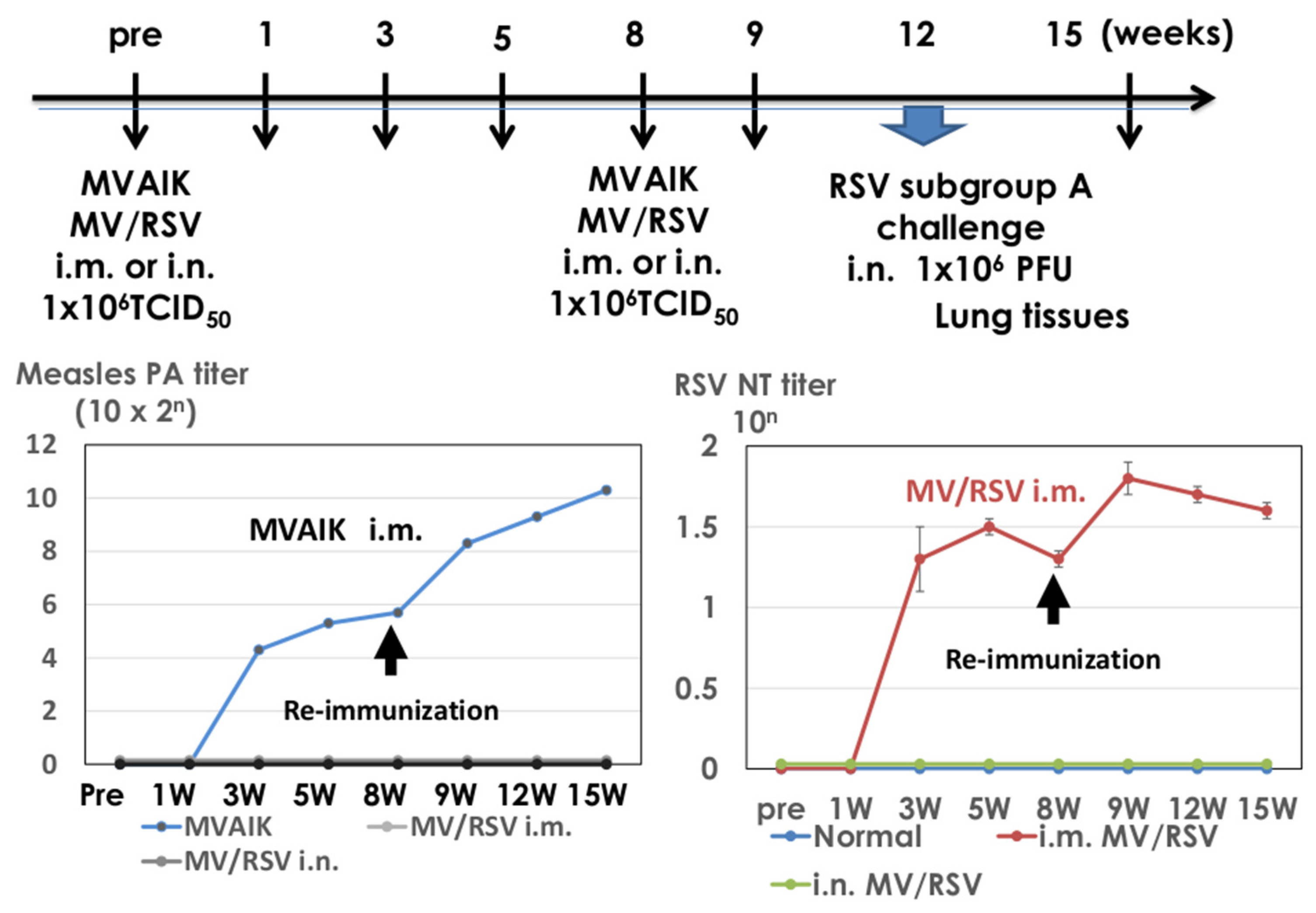
2.6. Immunization and RSV Challenge in Cotton Rats
2.7. Serology
2.8. Statistical Analysis
3. Results
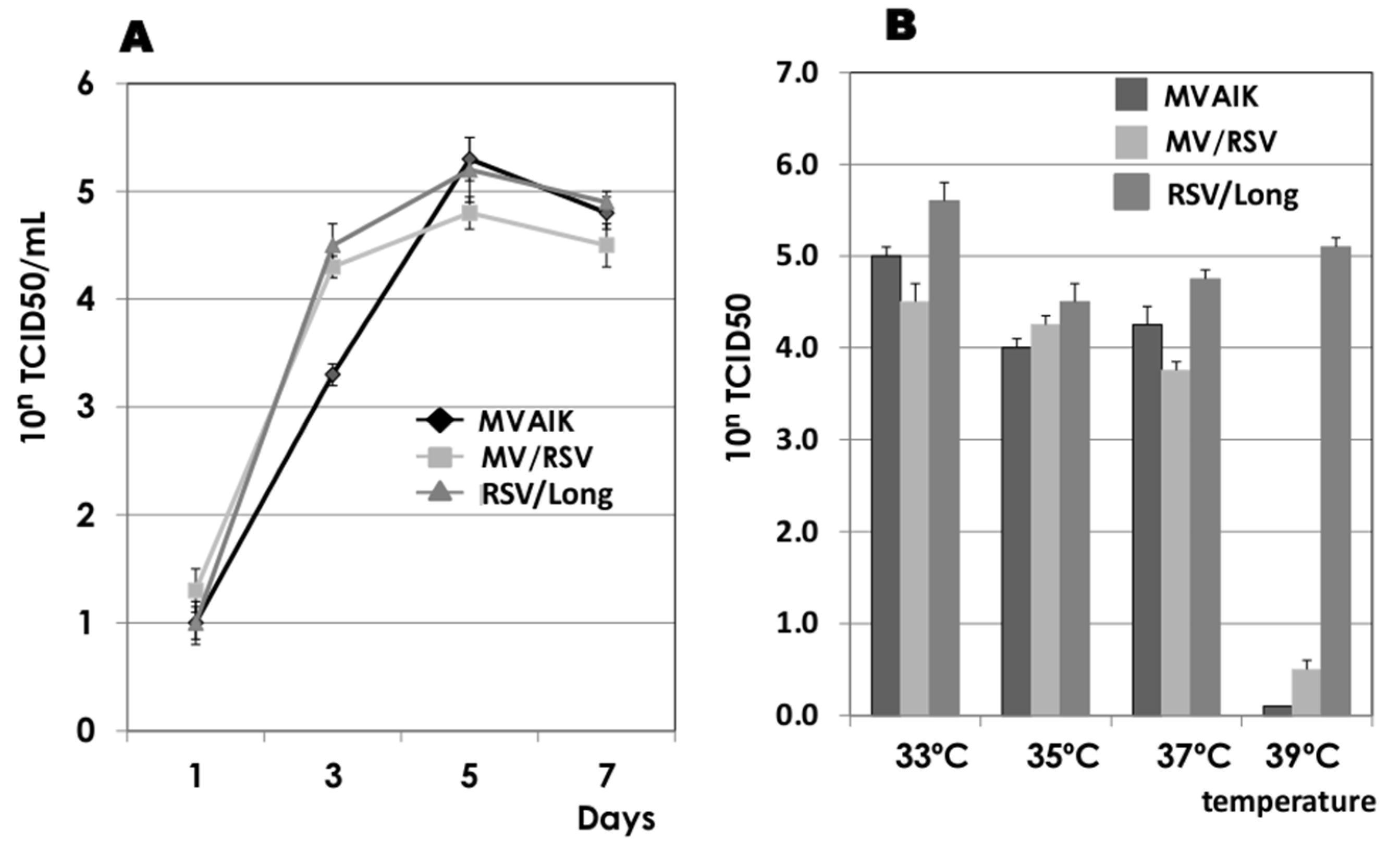
3.1. Virus Growth
3.2. Expression of Envelope Proteins
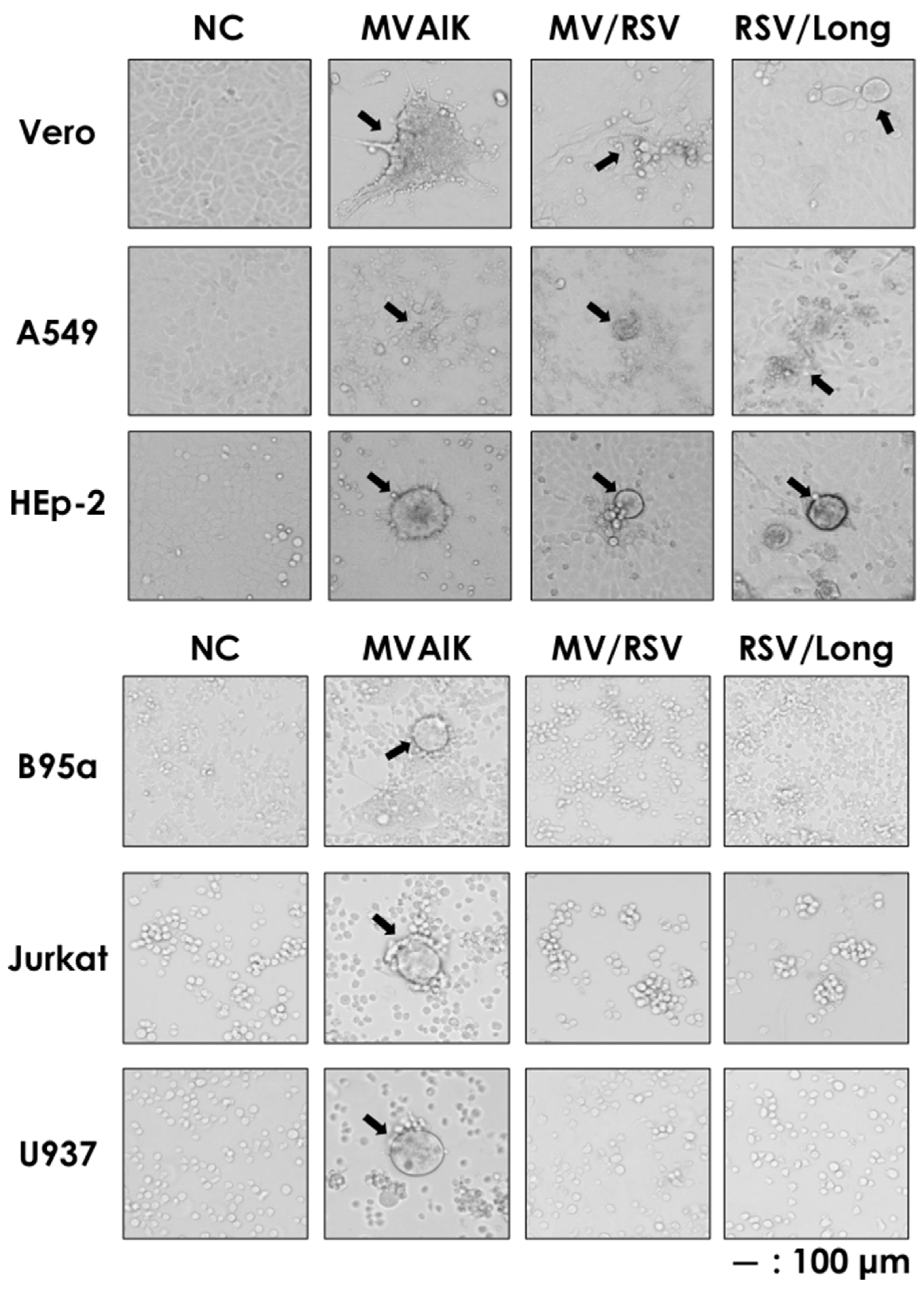
3.3. Different Cellular Tropism
3.4. Immunogenicity of MV/RSV
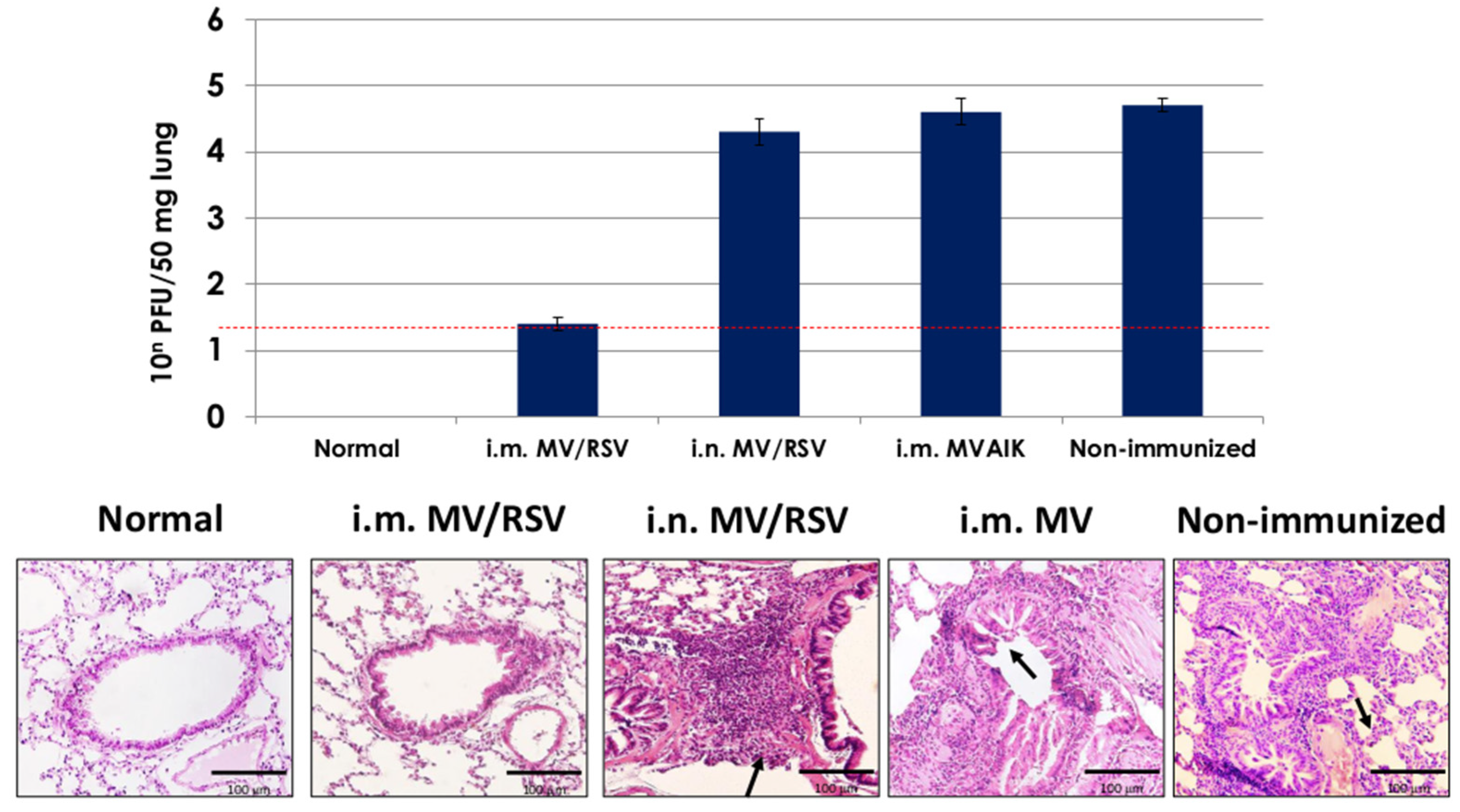
3.5. Protective Effect of MV/RSV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPE | cytopathic effect |
| CT | cytoplasmic |
| CTL | cytotoxic T lymphocytes |
| F | fusion |
| FI-RSV | formalin inactivated RSV |
| G | glyco |
| HA | hemagglutinin |
| i.m. | intramuscularly |
| i.n. | intranasally |
| L | large |
| N | nucleo |
| NT | neutralizing test |
| P | phosphor |
| PA | Particle agglutination |
| RSV | respiratory syncytial virus |
| TM | transmembrane |
| ts | temperature sensitivity |
References
- Oshansky, C.M.; Zhang, W.; Moore, E.; Tripp, R.A. The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiol. 2009, 4, 279–297. [Google Scholar] [CrossRef]
- Figueras-Aloy, J.; Manzoni, P.; Paes, B.; Simões, E.A.F.; Bont, L.; Checchia, P.A.; Fauroux, B.; Carbonell-Estrany, X. Defining the Risk and Associated Morbidity and Mortality of Severe Respiratory Syncytial Virus Infection Among Preterm Infants Without Chronic Lung Disease or Congenital Heart Disease. Infect. Dis. Ther. 2016, 5, 417–452. [Google Scholar] [CrossRef]
- Manzoni, P.; Figueras-Aloy, J.; Simões, E.A.F.; Checchia, P.A.; Fauroux, B.; Bont, L.; Paes, B.; Carbonell-Estrany, X. Defining the Incidence and Associated Morbidity and Mortality of Severe Respiratory Syncytial Virus Infection Among Children with Chronic Diseases. Infect. Dis. Ther. 2017, 6, 383–411. [Google Scholar] [CrossRef]
- Glezen, W.P.; Taber, L.H.; Frank, A.L.; Kasel, J.A. Risk of Primary Infection and Reinfection with Respiratory Syncytial Virus. Am. J. Dis. Child. 1986, 140, 543–546. [Google Scholar] [CrossRef]
- Histoshi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Committee on Infectious Diseases and Bronchiolitis Guidelines Committee; Brady, M.T.; Byington, C.L.; Dele Davies, H.; Edwards, K.M.; Jackson, M.A.; Maldonado, Y.A.; Murray, D.L.; Orenstein, W.A.; Rathore, M.H.; et al. Updated Guidance for Palivizumab Prophylaxis Among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics 2014, 134, 415–420. [Google Scholar] [CrossRef]
- Murphy, B.R.; Prince, G.A.; Walsh, E.E.; Kim, H.W.; Parrott, R.H.; Hemming, V.G.; Rodriguez, W.J.; Chanock, R.M. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J. Clin. Microbiol. 1986, 24, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, J.A.; Rubino, K.L.; Levely, M.E.; Adams, E.G.; Collins, P.L. Cytolytic T-lymphocyte responses to respiratory syncytial virus: Effector cell phenotype and target proteins. J. Virol. 1990, 64, 4232–4241. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Nakayama, T. Experimental animal model for analyzing immunobiological responses following vaccination with formalin-inactivated respiratory syncytial virus. Microbiol. Immunol. 2016, 60, 234–242. [Google Scholar] [CrossRef]
- Christiaansen, A.F.; Knudson, C.J.; Weiss, K.A.; Varga, S.M. The CD4 T cell response to respiratory syncytial virus infection. Immunol. Res. 2014, 59, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Ciarlet, M.; Cooper, B.W.; Dionigi, L.; Keith, P.; O’Brien, K.B.; Rafie-Kolpin, M.; Dormitzer, P.R. The path to an RSV vaccine. Curr. Opin. Virol. 2013, 3, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Modjarrad, K.; Giersing, B.; Kaslow, D.C.; Smith, P.G.; Moorthy, V.S. WHO consultation on Respiratory Syncytial Virus Vaccine Development Report from a World Health Organization Meeting held on 23–24 March 2015. Vaccine 2016, 34, 190–197. [Google Scholar] [CrossRef]
- Anderson, L.; Dormitzer, P.; Nokes, D.; Rappuoli, R.; Roca, A.; Graham, B. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 2013, 31, B209–B215. [Google Scholar] [CrossRef] [PubMed]
- Luongo, C.; Winter, C.C.; Collins, P.L.; Buchholz, U.J. Increased Genetic and Phenotypic Stability of a Promising Live-Attenuated Respiratory Syncytial Virus Vaccine Candidate by Reverse Genetics. J. Virol. 2012, 86, 10792–10804. [Google Scholar] [CrossRef]
- Yang, C.-F.; Wang, C.K.; Malkin, E.; Schickli, J.H.; Shambaugh, C.; Zuo, F.; Galinski, M.S.; Dubovsky, F.; Tang, R.S. Implication of respiratory syncytial virus (RSV) F transgene sequence heterogeneity observed in Phase 1 evaluation of MEDI-534, a live attenuated parainfluenza type 3 vectored RSV vaccine. Vaccine 2013, 31, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Munir, S.; Amaro-Carambot, E.; Surman, S.; Mackow, N.; Yang, L.; Buchholz, U.J.; Collins, P.L.; Schaap-Nutt, A. Chimeric Bovine/Human Parainfluenza Virus Type 3 Expressing Respiratory Syncytial Virus (RSV) F Glycoprotein: Effect of Insert Position on Expression, Replication, Immunogenicity, Stability, and Protection against RSV Infection. J. Virol. 2014, 88, 4237–4250. [Google Scholar] [CrossRef]
- Sawada, A.; Komase, K.; Nakayama, T. AIK-C measles vaccine expressing fusion protein of respiratory syncytial virus induces protective antibodies in cotton rats. Vaccine 2011, 29, 1481–1490. [Google Scholar] [CrossRef]
- Yamaji, Y.; Nakayama, T. Recombinant measles viruses expressing respiratory syncytial virus proteins induced virus-specific CTL responses in cotton rats. Vaccine 2014, 32, 4529–4536. [Google Scholar] [CrossRef]
- Yamaji, Y.; Sawada, A.; Yasui, Y.; Ito, T.; Nakayama, T. Simultaneous Administration of Recombinant Measles Viruses Expressing Respiratory Syncytial Virus Fusion (F) and Nucleo (N) Proteins Induced Humoral and Cellular Immune Responses in Cotton Rats. Vaccines 2019, 7, 27. [Google Scholar] [CrossRef]
- Higuchi, A.; Toriniwa, H.; Komiya, T.; Nakayama, T. Recombinant Measles AIK-C Vaccine Strain Expressing the prM-E Antigen of Japanese Encephalitis Virus. PLoS ONE 2016, 11, e0150213. [Google Scholar] [CrossRef]
- Ito, T.; Kumagai, T.; Yamaji, Y.; Sawada, A.; Nakayama, T. Recombinant Measles AIK-C Vaccine Strain Expressing Influenza HA Protein. Vaccines 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Komase, K.; Nakayama, T.; Iijima, M.; Miki, K.; Kawanishi, R.; Uejima, H. The phosphoprotein of attenuated measles AIK-C vaccine strain contributes to its temperature-sensitive phenotype. Vaccine 2006, 24, 826–834. [Google Scholar] [CrossRef]
- Sato, T.A.; Miyamura, K.; Sakae, K.; Kobune, F.; Inouye, S.; Fujino, R.; Yamazaki, S. Development of a gelatin particle agglutination reagent for measles antibody assay. Arch. Virol. 1997, 142, 1971–1977. [Google Scholar] [CrossRef]
- Olszewska, W.; Suezer, Y.; Sutter, G.; Openshaw, P. Protective and disease-enhancing immune responses induced by recombinant modified vaccinia Ankara (MVA) expressing respiratory syncytial virus proteins. Vaccine 2004, 23, 215–221. [Google Scholar] [CrossRef]
- Kim, E.; Okada, K.; Beeler, J.A.; Crim, R.L.; Piedra, P.A.; Gilbert, B.E.; Gambotto, A. Development of an Adenovirus-Based Respiratory Syncytial Virus Vaccine: Preclinical Evaluation of Efficacy, Immunogenicity, and Enhanced Disease in a Cotton Rat Model. J. Virol. 2014, 88, 5100–5108. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Slobod, K.S.; Jones, B.G.; Sealy, R.E.; Takimoto, T.; Boyd, K.; Surman, S.; Russell, C.J.; Portner, A.; Hurwitz, J.L. Sendai virus recombinant vaccine expressing a secreted, unconstrained respiratory syncytial virus fusion protein protects against RSV in cotton rats. Int. Immunol. 2015, 27, 229–236. [Google Scholar] [CrossRef] [PubMed]
- De Baets, S.; Schepens, B.; Sedeyn, K.; Schotsaert, M.; Roose, K.; Bogaert, P.; Fiers, W.; Saelens, X. Recombinant Influenza Virus Carrying the Respiratory Syncytial Virus (RSV) F85-93 CTL Epitope Reduces RSV Replication in Mice. J. Virol. 2013, 87, 3314–3323. [Google Scholar] [CrossRef] [PubMed]
- Swett-Tapia, C.; Bogaert, L.; De Jong, P.; Van Hoek, V.; Schouten, T.; Damen, I.; Spek, D.; Wanningen, P.; Radošević, K.; Widjojoatmodjo, M.N.; et al. Recombinant measles virus incorporating heterologous viral membrane proteins for use as vaccines. J. Gen. Virol. 2016, 97, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Sawada, A.; Yamaji, Y.; Ito, T. Recombinant measles AIK-C vaccine strain expressing heterologous virus antigens. Vaccine 2016, 34, 292–295. [Google Scholar] [CrossRef]
- Hong, D.T.; Hien, N.D.; Thao, P.T.P.; Anh, D.D.; Mai, H.H.; Huyen, D.T.T.; Huong, N.L.; Phuong, B.H.; Iijima, M.; Ito, T.; et al. Immunogenicity of the AIK-C measles vaccine in infants aged <9 months in Vietnam. Vaccine 2019, 37, 4576–4580. [Google Scholar] [CrossRef]
- Reisinger, E.C.; Tschismarov, R.; Beubler, E.; Wiedermann, U.; Firbas, C.; Loebermann, M.; Pfeiffer, A.; Muellner, M.; Tauber, E.; Ramsauer, K. Immunogenicity, safety, and tolerability of the measles-vectored chikungunya virus vaccine MV-CHIK: A double-blind, randomised, placebo-controlled and active-controlled phase 2 trial. Lancet 2018, 392, 2718–2727. [Google Scholar] [CrossRef]
- Haga, T.; Murayama, N.; Shimizu, Y.; Saito, A.; Sakamoto, T.; Morita, T.; Komase, K.; Nakayama, T.; Uchida, K.; Katayama, T.; et al. Analysis of antibody response by temperature-sensitive measles vaccine strain in the cotton rat model. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 395–406. [Google Scholar] [CrossRef]
- Spielhofer, P.; Bächi, T.; Fehr, T.; Christiansen, G.; Cattaneo, R.; Kaelin, K.; Billeter, M.A.; Naim, H.Y. Chimeric Measles Viruses with a Foreign Envelope. J. Virol. 1998, 72, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Takeda, M.; Yanagi, Y. Altered Interaction of the Matrix Protein with the Cytoplasmic Tail of Hemagglutinin Modulates Measles Virus Growth by Affecting Virus Assembly and Cell-Cell Fusion. J. Virol. 2007, 81, 6827–6836. [Google Scholar] [CrossRef] [PubMed]
- Tidjani, O.; Grunitsky, B.; Guérin, N.; Lèvy-Bruhl, D.; Lecam, N.; Xuereff, C.; Tatagan, K. Serological effects of edmonston-zagreb, schwarz, and AIK-C measles vaccine strains given at ages 4-5 or 8-10 months. Lancet 1989, 334, 1357–1360. [Google Scholar] [CrossRef]
- Bolotovski, V.M.; Zargaryantzs, A.I.; Mikheyeva, I.V.; Grabowsky, M.; Clements, C.J.; Albrecht, P.; Brenner, E.R.; Litvinov, S.K. Immunization of 6 and 9 Month Old Infants with AIK-C, Edmonston-Zagreb, Leningrad-16 and Schwarz Strains of Measles Vaccine. Int. J. Epidemiol. 1994, 23, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Nkrumah, F.K.; Osei-Kwasi, M.; Dunyo, S.K.; Koram, K.A.; Afari, E.A. Comparison of AIK-C measles vaccine in infants at 6 months with Schwarz vaccine at 9 months: A randomized controlled trial in Ghana. Bull. World Health Organ. 1998, 76, 353–359. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, A.; Ito, T.; Yamaji, Y.; Nakayama, T. Chimeric Measles Virus (MV/RSV), Having Ectodomains of Respiratory Syncytial Virus (RSV) F and G Proteins Instead of Measles Envelope Proteins, Induced Protective Antibodies against RSV. Vaccines 2021, 9, 156. https://doi.org/10.3390/vaccines9020156
Sawada A, Ito T, Yamaji Y, Nakayama T. Chimeric Measles Virus (MV/RSV), Having Ectodomains of Respiratory Syncytial Virus (RSV) F and G Proteins Instead of Measles Envelope Proteins, Induced Protective Antibodies against RSV. Vaccines. 2021; 9(2):156. https://doi.org/10.3390/vaccines9020156
Chicago/Turabian StyleSawada, Akihito, Takashi Ito, Yoshiaki Yamaji, and Tetsuo Nakayama. 2021. "Chimeric Measles Virus (MV/RSV), Having Ectodomains of Respiratory Syncytial Virus (RSV) F and G Proteins Instead of Measles Envelope Proteins, Induced Protective Antibodies against RSV" Vaccines 9, no. 2: 156. https://doi.org/10.3390/vaccines9020156
APA StyleSawada, A., Ito, T., Yamaji, Y., & Nakayama, T. (2021). Chimeric Measles Virus (MV/RSV), Having Ectodomains of Respiratory Syncytial Virus (RSV) F and G Proteins Instead of Measles Envelope Proteins, Induced Protective Antibodies against RSV. Vaccines, 9(2), 156. https://doi.org/10.3390/vaccines9020156




