Equity in Vaccine Trials for Higher Weight People? A Rapid Review of Weight-Related Inclusion and Exclusion Criteria for COVID-19 Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Included Studies
2.2. Outcome Measures
2.3. Search Strategy
2.4. Data Extraction (Selection and Coding)
3. Results
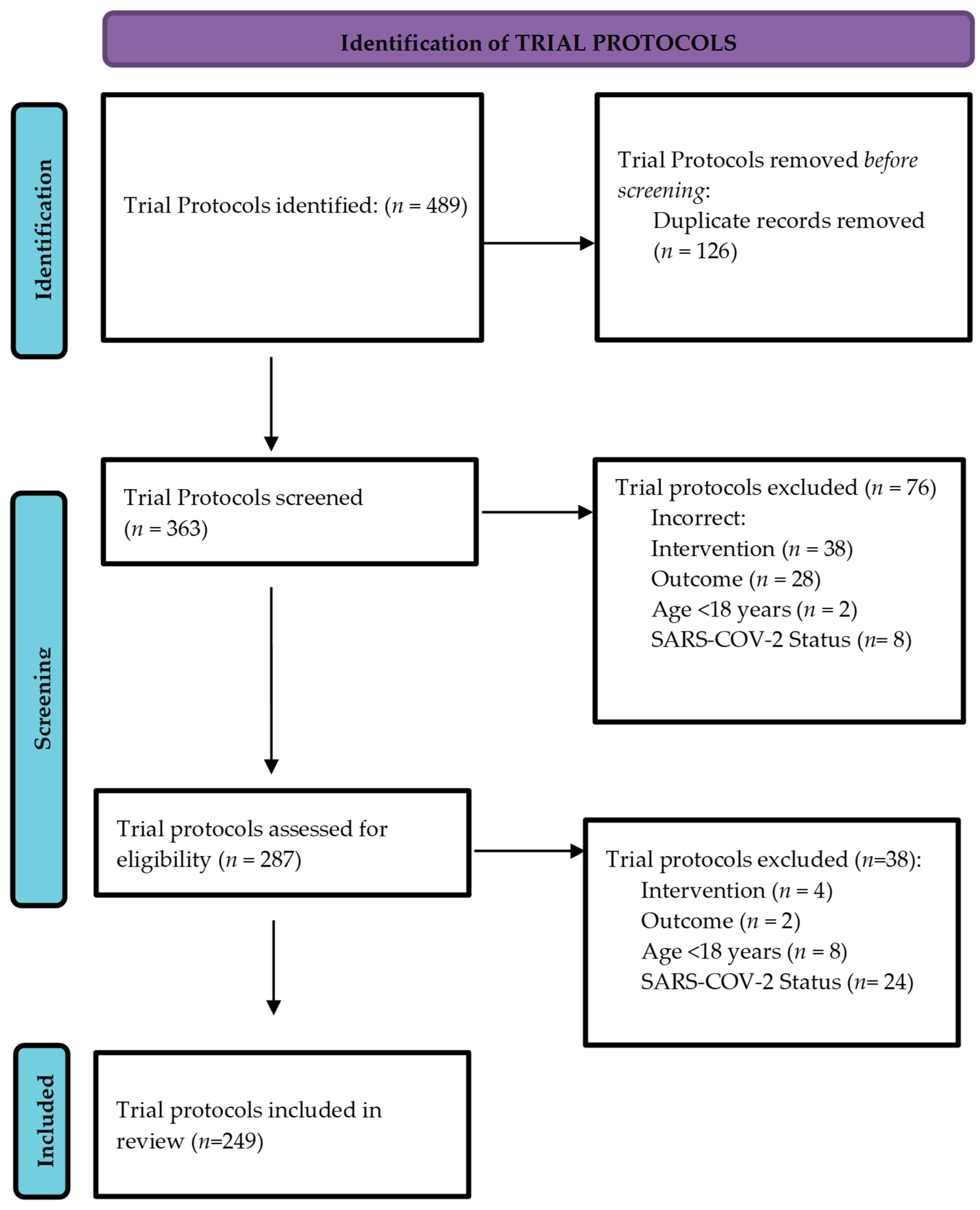
3.1. Search Results
3.2. Trial Characteristics
3.3. Participation of People with Higher Weight in COVID-19 Vaccine Trials
3.4. Participation of People with Higher Weight by Age
3.5. Participation of People with Higher Weight by Health Status
3.6. Number of Higher Weight Individuals Enrolled in COVID-19 Vaccine Trials
3.7. Intention to Analyse Vaccine Efficacy by Weight Status
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Le, T.T.; Andreadakis, Z.; Kumar, A.; Roman, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, J.P.; Gruber, W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diement, D.; Spector, S.A.; Zaks, T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and overweight: Fact Sheet. 9 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 November 2021).
- Wang, M.; Xu, P.S.; Liu, W.; Zhang, C.; Zhang, X.; Wang, L.; Liu, J.; Zhu, Z.; Wang, W. Prevalence and changes of BMI categories in China and related chronic diseases: Cross-sectional National Health Service Surveys (NHSSs) from 2013 to 2018. EClinicalMedicine 2020, 26, 100521. [Google Scholar] [CrossRef]
- Fryar, C.D.; Carroll, M.D.; Afful, J. Prevalence of Overweight, Obesity, and Severe Obesity among Adults Aged 20 and Over: United States, 1960–1962 through 2017–2018. NCHS Heal E-Stats. 2020 Revised 29 January 2021. Available online: https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm (accessed on 13 November 2021).
- Organisation for Economic Co-Operation and Development. Obesity Update 2017. Available online: https://www.oecd.org/health/health-systems/ (accessed on 13 November 2021).
- Pestine, E.; Stokes, A.; Trinquart, L. Representation of obese participants in obesity-related cancer randomized trials. Ann. Oncol. 2018, 29, 1582–1587. [Google Scholar] [CrossRef]
- De Vries, S.T.; Denig, P.; Ekhart, C.; Burgers, J.S.; Kleefstra, N.; Mol, P.G.M.; van Puijenbroek, E.P. Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: An explorative observational study. Br. J. Clin. Pharmacol. 2019, 85, 1507–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapp, N.; Abitbol, J.L.; Mathé, H.; Scherrer, B.; Guillard, H.; Gainer, E.; Ulmann, A. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception 2015, 91, 97–104. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frasca, D.; Blomberg, B.B. The impact of obesity and metabolic syndrome on vaccination success. Vaccines Older Adults 2020, 43, 86–97. [Google Scholar] [CrossRef]
- Banga, N.; Guss, P.; Banga, A.; Rosenman, K.D. Incidence and variables associated with inadequate antibody titers after pre-exposure rabies vaccination among veterinary medical students. Vaccine 2014, 32, 979–983. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Painter, S.D.; Ovsyannikova, I.G.; Poland, G.A. The weight of obesity on the human immune response to vaccination. Vaccine 2015, 33, 4422–4429. [Google Scholar] [CrossRef] [Green Version]
- Eliakim, A.; Schwindt, C.; Zaldivar, F.; Casali, P.; Cooper, D.M. Reduced tetanus antibody titers in overweight children. Autoimmunity 2006, 39, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Eisenberger, N.I.; Moieni, M.; Inagaki, T.K.; Muscatell, K.A.; Irwin, M.R. In sickness and in health: The co-regulation of inflammation and social behavior. Neuropsychopharmacology 2017, 42, 242–253. [Google Scholar] [CrossRef]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic load and its impact on health: A systematic review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, L.; Lyons, B. Health-related shame: An affective determinant of health? Med. Humanit. 2017, 43, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Garritty, C.; Gartlehner, G.; Kamel, C.; King, V.J.; Nussbaumer-Streit, B.; Stevens, A. Cochrane Rapid Reviews Interim Guidance from the Cochrane Rapid Reviews Methods Group. 2020. Available online: https://methods.cochrane.org/rapidreviews/sites/methods.cochrane.org.rapidreviews/files/public/uploads/cochrane_rr_-_guidance-23mar2020-v1.pdf (accessed on 12 November 2021).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, L.; Gregg, J. The war on obesity: A social determinant of health. Health Promot. J. Aust. 2006, 17, 260–263. [Google Scholar] [CrossRef]
- O’Hara, L.; Taylor, J. What’s wrong with the war on obesity? A Narrative Review of the Weight-Centered Health Paradigm and Development of the 3C Framework to Build Critical Competency for a Paradigm Shift. SAGE Open 2018, 8, 1–28. [Google Scholar] [CrossRef]
- Bacon, L.; Aphramor, L. Weight science: Evaluating the evidence for a paradigm shift. Nutr. J. 2011, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Mensinger, J.L.; Tylka, T.L.; Calamari, M.E. Mechanisms underlying weight status and healthcare avoidance in women: A study of weight stigma, body-related shame and guilt, and healthcare stress. Body Image 2018, 25, 139–147. [Google Scholar] [CrossRef]
- Mitchell, R.; Padwal, R.S.; Chuck, A.W.; Klarenbach, S.W. Cancer screening among the overweight and obese in Canada. Am. J. Prev. Med. 2008, 35, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.C.; McCarthy, E.P.; Davis, R.B.; Phillips, R.S. Screening for cervical and breast cancer: Is obesity an unrecognized barrier to preventive care? Ann. Intern. Med. 2000, 132, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Khalatbari-Soltani, S.; Cumming, R.G.; Delpierre, C.; Kelly-Irving, M. Importance of collecting data on socioeconomic determinants from the early stage of the COVID-19 outbreak onwards. J. Epidemiol. Community Health 2020, 74, 620–623. [Google Scholar] [CrossRef]
- Flint, S.W. Stigmatizing Media Portrayal of Obesity during the Coronavirus (COVID-19) Pandemic. Front. Psychol. 2020, 11, 2124. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Tahrani, A.A. COVID-19 and obesity—lack of clarity, guidance, and implications for care. Lancet Diabetes Endocrinol. 2020, 8, 474–475. [Google Scholar] [CrossRef]
- Pausé, C.; Parker, G.; Gray, L. Resisting the problematisation of fatness in COVID-19: In pursuit of health justice. Int. J. Disaster Risk Reduct. 2021, 54, 102021. [Google Scholar] [CrossRef]
| Search Strategy | ||
| Condition or disease: COVID OR Covid-19 OR SARS-Cov-2 OR Coronavirus OR Corona Virus OR nCOV OR novel coronavirus OR severe acute respiratory syndrome coronavirus 2 Intervention/Treatment: vaccine OR vaccines OR vaccination OR Vaccinations OR immunization OR immunizations OR inoculation OR inoculations Study Type: Interventional (Clinical Trials) Applied filters: recruiting, not yet recruiting, active not recruiting, completed, enrolling by invitation, suspended, terminated, withdrawn, unknown status | ||
| Inclusion | Exclusion | |
| Format | Registered Clinical Trial Protocol, in any clinical phase | In-vitro studies, animal studies, non-human trials |
| Intervention | Trials of a novel COVID-19 vaccine evaluating efficacy, safety and/or immunogenicity | Trials evaluating therapies that do not generate active immunity Trials evaluating the efficacy of vaccines designed to protect against other pathogens (e.g., BCG vaccine) |
| SARS-Cov-2 Status | Trials assessing prior history of and exposure to COVID-19, or sero-positivity for COVID-19 | Trials including people infected by COVID-19 |
| Age | ≥18 years | <18 years |
| Language | English language | Non-English language |
| Location | Any | NA |
| Trial Status | |
|---|---|
| Status at Time of Review | Number of Trials n (%) |
| Terminated/Withdrawn/Suspended | 5 (2.01) |
| Active, not-recruiting | 114 (45.78) |
| Recruiting | 107 (42.97) |
| Recruitment complete | 7 (2.81) |
| Completed | 14 (5.62) |
| Unknown | 2 (0.80) |
| Total | 249 |
| Trial Phase | Total Number of Trials n (% of Total Trials) | Trials Including BMI > 30 n (% of Phase) | Trials Excluding BMI > 30 n (% of Phase) | Trials with BMI Not Specified n (% of Phase) |
|---|---|---|---|---|
| I | 80 (32.1) | 26 (32.5) | 43 (53.8) | 11 (13.8) |
| I/II | 59 (23.7) | 14 (23.7) | 22 (37.3) | 23 (39.0) |
| II | 29 (11.6) | 6 (20.7) | 6 (20.7) | 17 (58.6) |
| II/III | 14 (5.6) | 2 (14.3) | 0 (0.00) | 12 (85.7) |
| III | 47 (18.9) | 3 (6.4) | 2 (4.3) | 42 (91.3) |
| IV | 11 (4.4) | 0 (0.0) | 0 (0.0) | 11 (100.0) |
| Not Specified | 9 (3.6) | 0 (0.0) | 0 (0.0) | 9 (100.0) |
| Total | 249 (100) | 51 (20.5) | 73 (29.3) | 125 (50.2) |
| Trial Phase | Number of Trials Including BMI > 30 | BMI Upper Limit | |||||
|---|---|---|---|---|---|---|---|
| BMI = 32 n (% of Phase) | BMI = 34 n (% of Phase) | BMI = 35 n (% of Phase) | BMI = 40 n (% of Phase) | BMI = 50 n (% of Phase) | Upper Limit Not Stated n (% of Phase) | ||
| Phase I | 26 | 3 (11.5) | 1 (3.8) | 15 (57.7) | 4 (15.4) | 1 (3.8) | 2 (7.7) |
| Phase I/II | 14 | 0 (0.0) | 0 (0.0) | 10 (71.4) | 3 (21.4) | 0 (0.0) | 1 (7.14) |
| Phase II | 6 | 2 (33.3) | 0 (0.0) | 3 (50.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) |
| Phase II/III | 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) |
| Phase III | 3 | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 2 (66.7) |
| Phase IV | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not Specified | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total Trials | 51 | 5 (9.8) | 1 (2.0) | 29 (56.9) | 8 (15.7) | 1 (2.0) | 7 (13.7) |
| Age of Trial Participants | Number of Trials n (% of n = 249) | Trials Including BMI > 30 n (% of Age Group) | Trials Excluding BMI > 30 n (% of Age Group) | Trials with BMI Not Specified n (% of Age Group) |
|---|---|---|---|---|
| Age limit ≤45 years | 6 (2.4) | 2 (33.3) | 2 (33.3) | 2 (33.3) |
| Includes adults >45 years | 243 (97.5) | 49 (20.2) | 71 (29.2) | 123 (50.6) |
| Includes adults >65 years | 165 (66.3) | 34 (20.6) | 32 (19.4) | 99 (60.0) |
| Includes adults >85 years | 119 (47.8) | 16 (13.4) | 18 (15.1) | 82 (68.9) |
| No upper age limit specified | 107 (43.0) | 14 (13.1) | 21 (19.6) | 72 (67.3) |
| Health Status of Trial Participants | Total n (% of Total Trials) | Trials Including BMI > 30 n (% of Health Status) | Trials Excluding BMI > 30 n (% of Health Status) | Trials with BMI Not Specified n (% of Health Status) |
|---|---|---|---|---|
| Healthy * | 209 (83.9) | 40 (19.1) | 70 (33.5) | 99 (47.4) |
| Healthy plus mild/moderate comorbidities * | 24 (9.6) | 9 (37.5) | 3 (12.5) | 12 (50) |
| Immunocompromised, not otherwise specified | 4 (1.6) | 0 (0.0) | 0 (0.0) | 4 (100.0) |
| Healthy * plus immunocompromised | 2 (0.8) | 2 (100.0) | 0 (0.0) | 0 (0.0) |
| Specific medical condition (CLL, liver disease, cancer, haemodialysis, and transplant patients) | 8 (3.2) | 0 (0.0) | 0 (0.0) | 8 (100.0) |
| Pregnant | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (100.0) |
| Did not specify | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 249 (100) | 51 (20.5) | 73 (29.3) | 125 (50.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, J.; Sutherland, J.; Bucknall, D.; O’Hara, L.; Heywood, A.; Hobbs, M.; Ballantyne, A.; Gray, L. Equity in Vaccine Trials for Higher Weight People? A Rapid Review of Weight-Related Inclusion and Exclusion Criteria for COVID-19 Clinical Trials. Vaccines 2021, 9, 1466. https://doi.org/10.3390/vaccines9121466
Campbell J, Sutherland J, Bucknall D, O’Hara L, Heywood A, Hobbs M, Ballantyne A, Gray L. Equity in Vaccine Trials for Higher Weight People? A Rapid Review of Weight-Related Inclusion and Exclusion Criteria for COVID-19 Clinical Trials. Vaccines. 2021; 9(12):1466. https://doi.org/10.3390/vaccines9121466
Chicago/Turabian StyleCampbell, Jessica, Juliet Sutherland, Danielle Bucknall, Lily O’Hara, Anita Heywood, Matthew Hobbs, Angela Ballantyne, and Lesley Gray. 2021. "Equity in Vaccine Trials for Higher Weight People? A Rapid Review of Weight-Related Inclusion and Exclusion Criteria for COVID-19 Clinical Trials" Vaccines 9, no. 12: 1466. https://doi.org/10.3390/vaccines9121466
APA StyleCampbell, J., Sutherland, J., Bucknall, D., O’Hara, L., Heywood, A., Hobbs, M., Ballantyne, A., & Gray, L. (2021). Equity in Vaccine Trials for Higher Weight People? A Rapid Review of Weight-Related Inclusion and Exclusion Criteria for COVID-19 Clinical Trials. Vaccines, 9(12), 1466. https://doi.org/10.3390/vaccines9121466







