Model-Based Planning and Delivery of Mass Vaccination Campaigns against Infectious Disease: Application to the COVID-19 Pandemic in the UK
Abstract
:1. Introduction
2. Methods
2.1. Modelling Framework
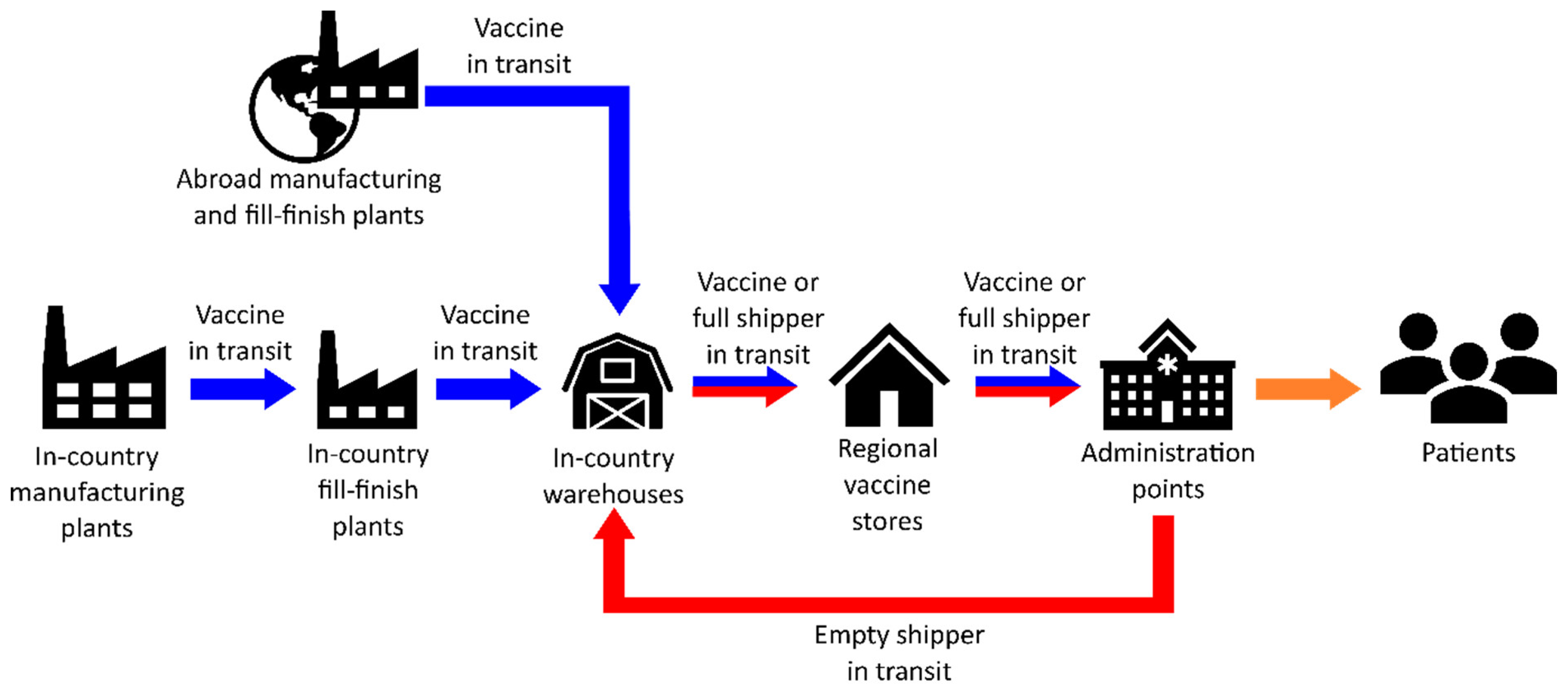
2.2. Vaccine Supply Chain Modelling
- Vaccine demand profile;
- Vaccination timeframe;
- Supply chain superstructure;
- Minimum and maximum inventories (manufacturing and fill-finish, warehouses, regional stores, and administration points);
- Minimum and maximum capacities of manufacturing plants, fill-finish plants, and import rate;
- Minimum and maximum capacities of transportation modes, operating costs and capital cost factors (manufacturing and fill-finish, warehouses, regional stores, and administration points);
- Travel distances and times.
- Optimal supply chain structure;
- Transport mode per route;
- Backlog in each time period;
- Vaccine availability and vaccine wastage at administration points;
- Vaccine supplied to administration points per time period;
- Vaccine import rate and production rates in manufacturing and fill-finish plants;
- Capacity of quality control facilities;
- Capital costs, operating costs, and total annualised cost of supply chain facilities;
- Total transportation costs and transport costs per route;
- Inventories of vaccines in manufacturing facilities, fill-finish facilities, warehouses, regional stores, and administration points;
- Inventories of vaccine thermal shippers (full and empty) in warehouses, regional stores, and administration points (needed only for vaccines stored and transported at ultra-low temperatures).
2.3. Data Sources and Assumptions
2.3.1. Demand Stratification
2.3.2. Vaccine Administration
2.3.3. Vaccine Supply Chain
3. Results and Discussions
3.1. Vaccine Demand Stratification
3.2. Vaccine Administration
3.3. Vaccine Supply Chain Optimisation
3.4. COVID-19 Vaccines Administered at Vaccination Centres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO Timeline—COVID-19. Available online: https://www.who.int/news/item/27-04-2020-who-timeline—covid-19 (accessed on 30 April 2021).
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, Diagnostics and Drug Development. J. Hepatol. 2021, 74, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Kuo, R.L.; Shih, S.R. COVID-19: The First Documented Coronavirus Pandemic in History. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef]
- Cepi Advances 8 COVID-19 Vaccine Programmes. Available online: https://www.glopid-r.org/newsletter-13th-edition/cepi-advances-8-covid-19-vaccine-programmes (accessed on 30 April 2021).
- Why We’re Giving $250 Million More to Fight COVID-19. Available online: https://www.gatesfoundation.org/ideas/articles/coronavirus-funding-additional-250-million-suzman (accessed on 30 April 2021).
- Global Partnership to Make Available 120M Affordable, Quality COVID-19 Rapid Tests for Low- and Middle-Income Countries. Available online: https://www.clintonhealthaccess.org/global-partnership-to-make-available-120m-affordable-quality-covid-19-rapid-tests-for-low-and-middle-income-countries (accessed on 30 April 2021).
- Funding COVID-19 Vaccines: A Timeline. Available online: https://www.devex.com/news/funding-covid-19-vaccines-a-timeline-97950 (accessed on 30 April 2021).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Hung, I.F.N.; Poland, G.A. Single-Dose Oxford—AstraZeneca COVID-19 Vaccine Followed by a 12-Week Booster. Lancet 2021, 397, 854–855. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Tanne, J.H. Covid-19: US Authorises Johnson and Johnson Vaccine Again, Ending Pause in Rollout. BMJ 2021, 373, n1079. [Google Scholar] [CrossRef]
- Livingston, E.H.; Malani, P.N.; Creech, C.B. The Johnson & Johnson Vaccine for COVID-19. JAMA J. Am. Med. Assoc. 2021, 325, 2021. [Google Scholar] [CrossRef]
- Baraniuk, C. Covid-19: What Do We Know about Sputnik v and Other Russian Vaccines? BMJ 2021, 372, 7–8. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and Immunogenicity of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine in Two Formulations: Two Open, Non-Randomised Phase 1/2 Studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and Efficacy of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 MRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 MRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 Vaccine Phase 3 Trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. MRNA-Based SARS-CoV-2 Vaccine Candidate CVnCoV Induces High Levels of Virus-Neutralising Antibodies and Mediates Protection in Rodents. NPJ Vaccines 2021, 6, 57. [Google Scholar] [CrossRef]
- The Sinopharm COVID-19 Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the-sinopharm-covid-19-vaccine-what-you-need-to-know (accessed on 30 April 2021).
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Novavax Vaccine Efficacy Is 86% against UK Variant and 60% against South African Variant. BMJ 2021, 372, n296. [Google Scholar] [CrossRef]
- West, S.; Kis, Z.; Kontoravdi, C.; Papathanasiou, M.; Shah, N.; Chachuat, B. Is the World Ready to Produce a Billion Doses of a COVID-19 Vaccine? Available online: https://www.imperial.ac.uk/news/197321/is-world-ready-produce-billion-doses (accessed on 30 April 2021).
- Q&A: Cold Chains, COVID-19 Vaccines and Reaching Low-Income Countries. Available online: https://www.imperial.ac.uk/news/209993/qa-cold-chains-covid-19-vaccines-reaching (accessed on 30 April 2021).
- Acharya, K.P.; Ghimire, T.R.; Subramanya, S.H. Access to and Equitable Distribution of COVID-19 Vaccine in Low-Income Countries. NPJ Vaccines 2021, 6, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Kaplow, L.; Sun, Y.S.; Holt, T.Z. ‘None Are Safe until All Are Safe’: COVID-19 Vaccine Rollout in Low- and Middle-Income Countries. Available online: https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/none-are-safe-until-all-are-safe-covid-19-vaccine-rollout-in-low-and-middle-income-countries (accessed on 3 November 2021).
- Choi, E.M. COVID-19 Vaccines for Low- and Middle-Income Countries. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in Ensuring Global Access to COVID-19 Vaccines: Production, Affordability, Allocation, and Deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- WHO. Framework for Decision-Making: Implementation of Mass Vaccination Campaigns in the Context of COVID-19; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Becker, A.D.; Grantz, K.H.; Hegde, S.T.; Bérubé, S.; Cummings, D.A.T.; Wesolowski, A. Development and Dissemination of Infectious Disease Dynamic Transmission Models during the COVID-19 Pandemic: What Can We Learn from Other Pathogens and How Can We Move Forward? Lancet Digit. Health 2021, 3, e41–e50. [Google Scholar] [CrossRef]
- Alvarez, M.M.; González-González, E.; Trujillo-de Santiago, G. Modeling COVID-19 Epidemics in an Excel Spreadsheet to Enable First-Hand Accurate Predictions of the Pandemic Evolution in Urban Areas. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, A.L.; Franco, E.; Mohler, G.; Short, M.B.; Sledge, D. The Challenges of Modeling and Forecasting the Spread of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 16732–16738. [Google Scholar] [CrossRef] [PubMed]
- James, L.P.; Salomon, J.A.; Buckee, C.O.; Menzies, N.A. The Use and Misuse of Mathematical Modeling for Infectious Disease Policymaking: Lessons for the COVID-19 Pandemic. Med. Decis. Mak. 2021, 41, 379–385. [Google Scholar] [CrossRef]
- Carcione, J.M.; Santos, J.E.; Bagaini, C.; Ba, J. A Simulation of a COVID-19 Epidemic Based on a Deterministic SEIR Model. Front. Public Health 2020, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.N. Epidemiological Models Are Important Tools for Guiding COVID-19 Interventions. BMC Med. 2020, 18, 10–13. [Google Scholar] [CrossRef]
- Kis, Z.; Kontoravdi, C.; Shattock, R.; Shah, N. Resources, Production Scales and Time Required for Producing RNA Vaccines for the Global Pandemic Demand. Vaccines 2021, 9, 3. [Google Scholar] [CrossRef]
- Van de Berg, D.; Kis, Z.; Behmer, C.F.; Samnuan, K.; Blakney, A.K.; Kontoravdi, C.; Shattock, R.; Shah, N. Quality by Design Modelling to Support Rapid RNA Vaccine Production against Emerging Infectious Diseases. NPJ Vaccines 2021, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kis, Z.; Kontoravdi, C.; Dey, A.K.; Shattock, R.; Shah, N. Rapid Development and Deployment of High-Volume Vaccines for Pandemic Response. J. Adv. Manuf. Process. 2020, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reader, D.; Li, J.; McDonnel, A.; Yadav, P. Modelling the Manufacturing Process for COVID-19 Vaccines: Our Approach. Available online: https://www.cgdev.org/blog/modelling-manufacturing-process-covid-19-vaccines-our-approach (accessed on 30 April 2021).
- Modelling of Manufacturing COVID-19 Vaccines. Available online: https://www.brydenwood.co.uk/projects/modelling-of-manufacturing-covid19-vaccines/s101877 (accessed on 30 April 2021).
- Kis, Z. Enhancing Vaccine Platforms: Computational Models Accelerate Development, Manufacturing, and Distribution. Available online: https://bioprocessintl.com/manufacturing/vaccines/enhancing-vaccine-platforms-a-computational-modeling-framework-accelerates-development-manufacturing-and-distribution (accessed on 30 April 2021).
- Guignard, A.; Praet, N.; Jusot, V.; Bakker, M.; Baril, L. Introducing New Vaccines in Low- and Middle-Income Countries: Challenges and Approaches. Expert Rev. Vaccines 2019, 18, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.Y.; Assi, T.M.; Rajgopal, J.; Norman, B.A.; Chen, S.I.; Brown, S.T.; Slayton, R.B.; Kone, S.; Kenea, H.; Welling, J.S.; et al. Impact of Introducing the Pneumococcal and Rotavirus Vaccines into the Routine Immunization Program in Niger. Am. J. Public Health 2012, 102, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Haidari, L.A.; Wahl, B.; Brown, S.T.; Privor-Dumm, L.; Wallman-Stokes, C.; Gorham, K.; Connor, D.L.; Wateska, A.R.; Schreiber, B.; Dicko, H.; et al. One Size Does Not Fit All: The Impact of Primary Vaccine Container Size on Vaccine Distribution and Delivery. Vaccine 2015, 33, 3242–3247. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.T.; Schreiber, B.; Cakouros, B.E.; Wateska, A.R.; Dicko, H.M.; Connor, D.L.; Jaillard, P.; Mvundura, M.; Norman, B.A.; Levin, C.; et al. The Benefits of Redesigning Benin’s Vaccine Supply Chain. Vaccine 2014, 32, 4097–4103. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.Y.; Schreiber, B.; Wateska, A.R.; Connor, D.L.; Dicko, H.M.; Jaillard, P.; Mvundura, M.; Levin, C.; Avella, M.; Haidari, L.A.; et al. The Benin Experience: How Computational Modeling Can Assist Major Vaccine Policy Changes in Low- and Middle-Income Countries. Vaccine 2015, 33, 2858–2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.T.; Lee, B.Y. Unless Changes Are Made in Benin, Multiple Storage and Transport Bottlenecks May Prevent Vaccines from Reaching the Population. Vaccine 2014, 32, 2518–2519. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.I.; Ribeiro, D.; Barbosa-Povoa, A.P. Design and Planning of Sustainable Vaccine Supply Chain. In Pharmaceutical Supply Chains—Medicines Shortages; Barbosa-Povoa, A., Jenzer, H., de Miranda, J., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Sadjadi, S.J.; Ziaei, Z.; Pishvaee, M.S. The Design of the Vaccine Supply Network under Uncertain Condition: A Robust Mathematical Programming Approach. J. Model. Manag. 2019, 14, 841–871. [Google Scholar] [CrossRef]
- Chen, S.I.; Norman, B.A.; Rajgopal, J.; Assi, T.M.; Lee, B.Y.; Brown, S.T. A Planning Model for the WHO-EPI Vaccine Distribution Network in Developing Countries. IIE Trans. 2014, 46, 853–865. [Google Scholar] [CrossRef]
- Kis, Z.; Papathanasiou, M.; Calvo-Serrano, R.; Kontoravdi, C.; Shah, N. A Model-Based Quantification of the Impact of New Manufacturing Technologies on Developing Country Vaccine Supply Chain Performance: A Kenyan Case Study. J. Adv. Manuf. Process. 2019, 1, e10025. [Google Scholar] [CrossRef]
- Georgiadis, G.P.; Georgiadis, M.C. Optimal Planning of the COVID-19 Vaccine Supply Chain. Vaccine 2021, 39, 5302–5312. [Google Scholar] [CrossRef] [PubMed]
- Office for National Statistics. Available online: https://www.ons.gov.uk (accessed on 30 April 2021).
- Joint Committee on Vaccination and Immunisation: Advice on Priority Groups for COVID-19 Vaccination. 30 December 2020. Available online: https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020 (accessed on 30 April 2021).
- Crocker-Buque, T.; Mohan, K.; Ramsay, M.; Edelstein, M.; Mounier-Jack, S. What Is the Cost of Delivering Routine Vaccinations at GP Practices in England? A Comparative Time-Driven Activity-Based Costing Analysis. Hum. Vaccines Immunother. 2019, 15, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Crocker-Buque, T.; Mounier-Jack, S. Vaccination in England: A Review of Why Business as Usual Is Not Enough to Maintain Coverage. BMC Public Health 2018, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Crocker-Buque, T.; Edelstein, M.; Mounier-Jack, S. A Process Evaluation of How the Routine Vaccination Programme Is Implemented at GP Practices in England. Implement. Sci. 2018, 13, 15–17. [Google Scholar] [CrossRef]
- UK Hits Nearly 500,000 Covid Vaccinations a Day as Three-Quarters of Over-80s Now Jabbed. Available online: https://www.thesun.co.uk/news/uknews/13836401/uk-hits-nearly-500000-covid-vaccinations-a-day (accessed on 30 April 2021).
- Covid Vaccine: How Many People in the UK Have Been Vaccinated So Far? Available online: https://www.bbc.co.uk/news/health-55274833 (accessed on 30 April 2021).
- Coronavirus (COVID-19) in the UK. Available online: https://coronavirus.data.gov.uk/details/vaccinations (accessed on 30 April 2021).
- COVID-19 Vaccination Programme. Available online: https://www.bma.org.uk/advice-and-support/covid-19/vaccines/covid-19-vaccination-programme (accessed on 30 April 2021).
- Vaccination Sites. Available online: https://www.england.nhs.uk/coronavirus/publication/vaccination-sites (accessed on 30 April 2021).
- NIBSC. Statement on Batch Testing for the COVID-19 Vaccine AstraZeneca. Available online: https://www.nibsc.org/about_us/latest_news/batch_testing.aspx (accessed on 1 June 2021).
- Air Freight & Air Cargo Shipping: Air Freight Charges, Rates, Costs & Quotes. Available online: https://www.freightos.com/freight-resources/air-freight-rates-cost-prices (accessed on 30 April 2021).
- World Class Shipping-New York. Available online: http://www.worldclassshipping.com/aircraft.html (accessed on 30 April 2021).
- Department for Transport. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/782192/background-quality-report.pdf (accessed on 30 April 2021).
- COVID-19: UK Set to Reach Herd Immunity “Milestone” Within Days, Say Scientists. Available online: https://news.sky.com/story/covid-19-uk-set-to-reach-herd-immunity-milestone-within-days-say-scientists-12269405 (accessed on 30 April 2021).
- How Many People Have COVID-19 Antibodies in the UK? Available online: https://www.statista.com/chart/23961/uk-share-with-covid-antibodies (accessed on 3 November 2021).
- Herd Immunity: Can the UK Get There? Available online: https://theconversation.com/herd-immunity-can-the-uk-get-there-160026 (accessed on 30 April 2021).
- Emmerich, M.T.M.; Deutz, A.H. A Tutorial on Multiobjective Optimization: Fundamentals and Evolutionary Methods. Nat. Comput. 2018, 17, 585–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Year of Covid Lockdowns Has Cost the UK Economy £251bn, Study Says. Available online: https://www.theguardian.com/business/2021/mar/22/a-year-of-covid-lockdowns-has-cost-the-uk-economy-251bn-study-says (accessed on 27 July 2021).







| Item. | Scenario 1 | Scenario 2 | Units |
|---|---|---|---|
| Cost of vaccine shipper | 7.72 | 7.39 | million $ |
| Cost of dry ice | 20.30 | 19.90 | million $ |
| Cost of vaccinating individuals | 1.88 | 1.85 | billion $ |
| Cost of vaccinating individuals at care home | 24.10 | 24.10 | million $ |
| Cost of vaccine procured | 2.00 | 1.96 | billion $ |
| Cost of quality control checks | 59.90 | 58.70 | million $ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, D.; Kis, Z.; Tak, K.; Papathanasiou, M.M.; Kontoravdi, C.; Chachuat, B.; Shah, N. Model-Based Planning and Delivery of Mass Vaccination Campaigns against Infectious Disease: Application to the COVID-19 Pandemic in the UK. Vaccines 2021, 9, 1460. https://doi.org/10.3390/vaccines9121460
Ibrahim D, Kis Z, Tak K, Papathanasiou MM, Kontoravdi C, Chachuat B, Shah N. Model-Based Planning and Delivery of Mass Vaccination Campaigns against Infectious Disease: Application to the COVID-19 Pandemic in the UK. Vaccines. 2021; 9(12):1460. https://doi.org/10.3390/vaccines9121460
Chicago/Turabian StyleIbrahim, Dauda, Zoltán Kis, Kyungjae Tak, Maria M. Papathanasiou, Cleo Kontoravdi, Benoît Chachuat, and Nilay Shah. 2021. "Model-Based Planning and Delivery of Mass Vaccination Campaigns against Infectious Disease: Application to the COVID-19 Pandemic in the UK" Vaccines 9, no. 12: 1460. https://doi.org/10.3390/vaccines9121460
APA StyleIbrahim, D., Kis, Z., Tak, K., Papathanasiou, M. M., Kontoravdi, C., Chachuat, B., & Shah, N. (2021). Model-Based Planning and Delivery of Mass Vaccination Campaigns against Infectious Disease: Application to the COVID-19 Pandemic in the UK. Vaccines, 9(12), 1460. https://doi.org/10.3390/vaccines9121460








