COVID-19 Vaccination in People Living with HIV (PLWH) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity
Abstract
:1. Introduction
2. Materials and Methods
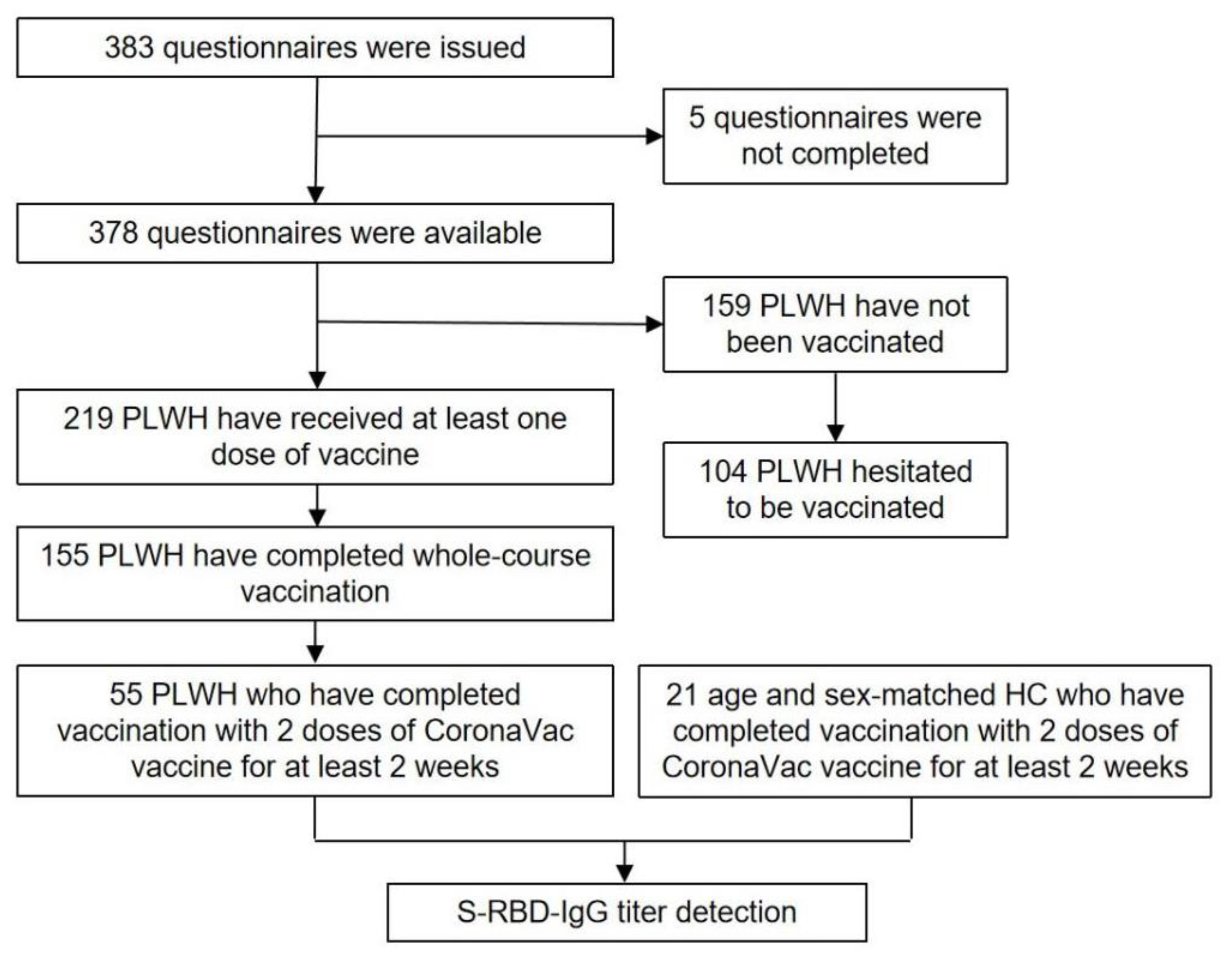
3. Results
3.1. Demographics, HIV Characteristics, and Health Status of PLWH
3.2. COVID-19 Vaccination Status, Intention to Receive COVID-19 Vaccine, and Potential Risk Factors of Vaccine Hesitancy in PLWH
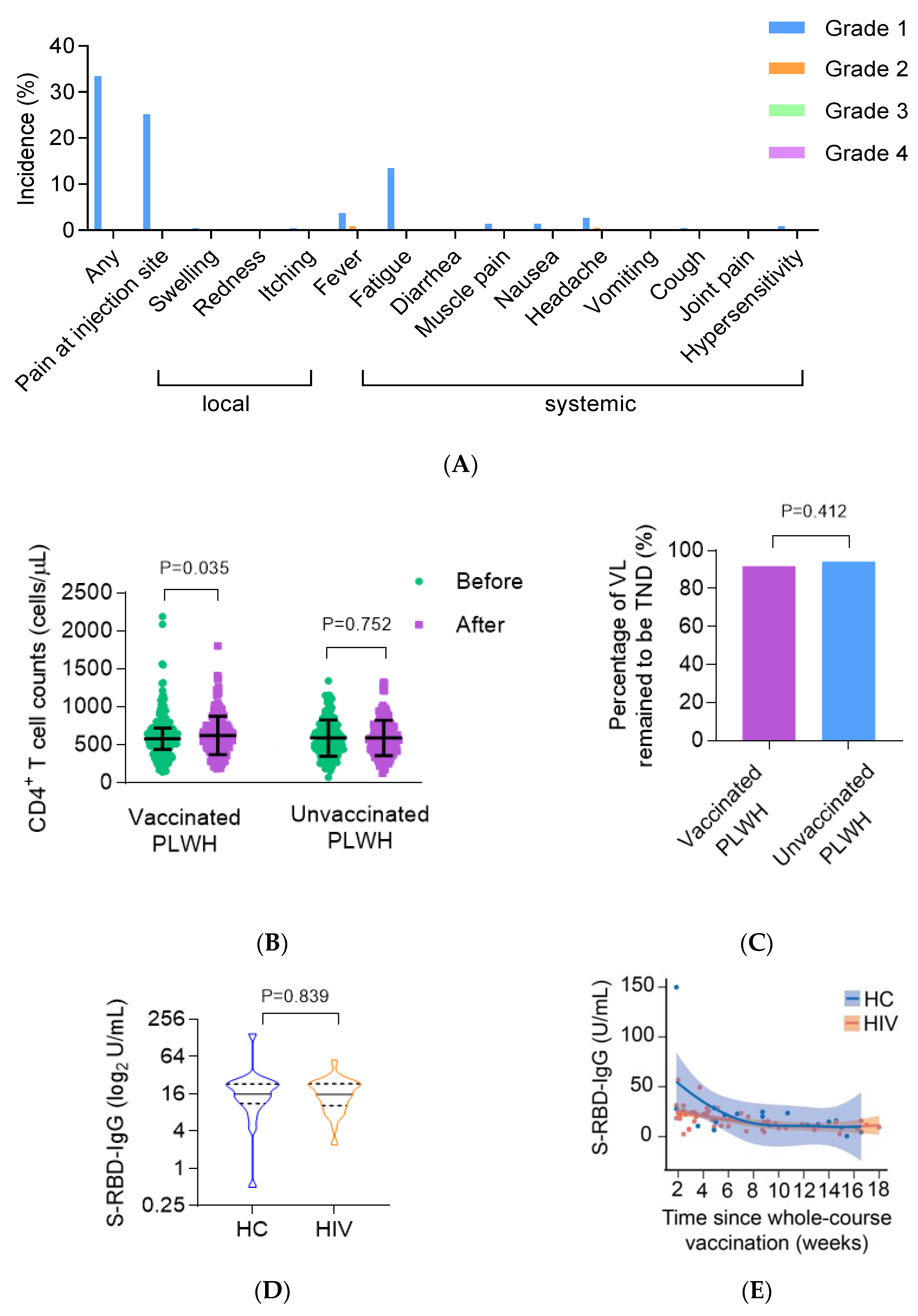
3.3. Safety and the Impact on ART Efficacy of the COVID-19 Vaccine on ART Efficacy in PLWH
3.4. The CoronaVac Vaccine Elicited Comparable Antibody Responses in PLWH Compared with HC
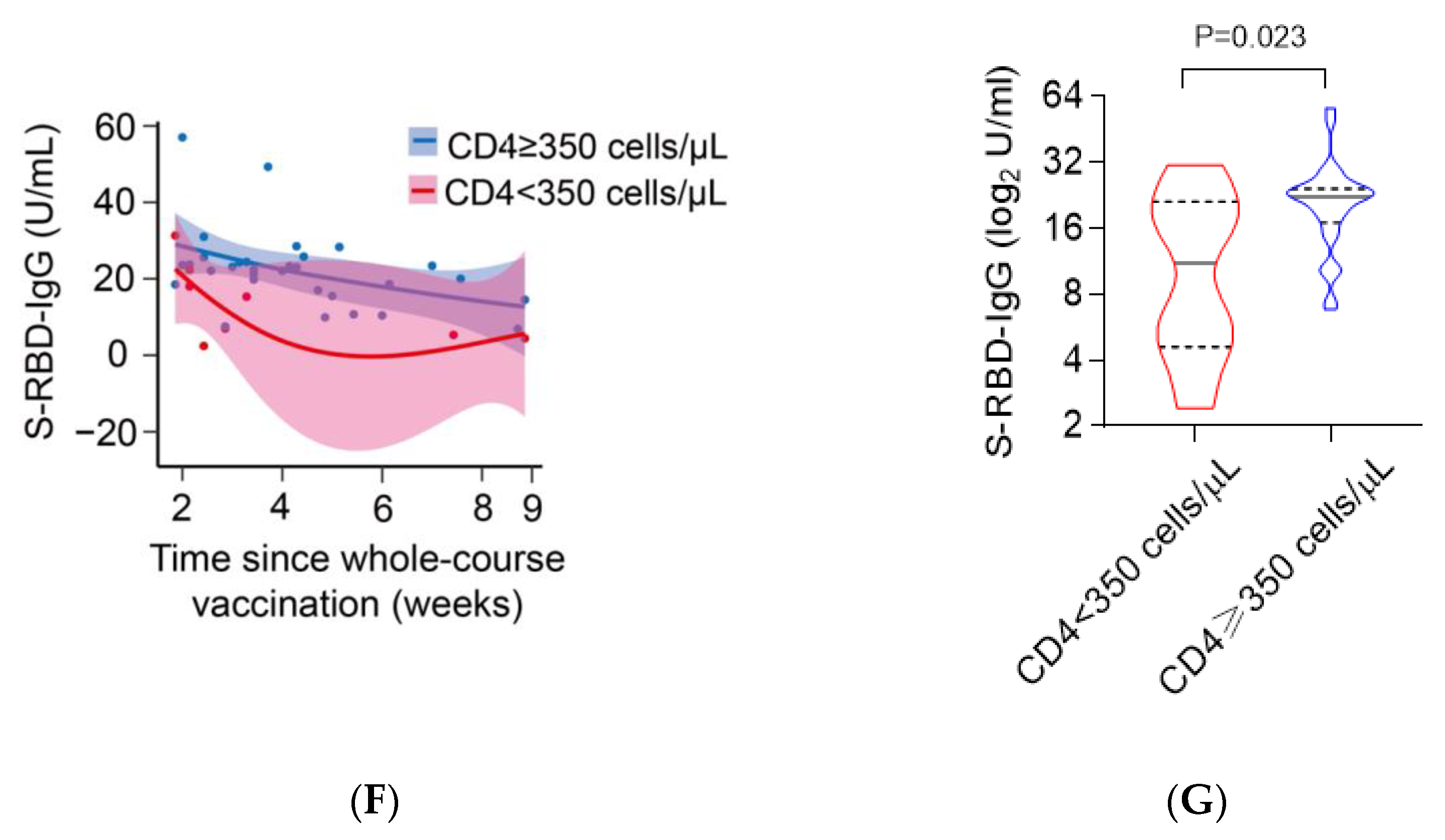
3.5. Poor Immunological Response Was Associated with Impaired Antibody Responses to CoronaVac in PLWH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19). JAMA 2020, 324, 782. [Google Scholar] [CrossRef]
- Brown, L.B.; Spinelli, M.A.; Gandhi, M. The interplay between HIV and COVID-19: Summary of the data and responses to date. Curr. Opin. HIV AIDS 2021, 16, 63–73. [Google Scholar] [CrossRef]
- Kanwugu, O.N.; Adadi, P. HIV/SARS-CoV-2 coinfection: A global perspective. J. Med. Virol. 2021, 93, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Vizcarra, P.; Pérez-Elías, M.J.; Quereda, C.; Moreno, A.; Vivancos, M.J.; Dronda, F.; Casado, J.L.; Moreno, S.; Pérez-Elías, M.J.; Fortún, J.; et al. Description of COVID-19 in HIV-infected individuals: A single-centre, prospective cohort. Lancet HIV 2020, 7, e554–e564. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, R.; Xu, J.; Shi, L.; Feng, H.; Yang, H. An updated meta-analysis on the association between HIV infection and COVID-19 mortality. Aids 2021, 35, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, J.; Patel, R.C.; Zhang, J.; Guo, S.; Zheng, Q.; Olex, A.L.; Olatosi, B.; Weissman, S.B.; Islam, J.Y.; et al. Associations between HIV infection and clinical spectrum of COVID-19: A population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV 2021, 8, e690–e700. [Google Scholar] [CrossRef]
- Rief, W. Fear of Adverse Effects and COVID-19 Vaccine Hesitancy: Recommendations of the Treatment Expectation Expert Group. JAMA Health Forum 2021, 2, e210804. [Google Scholar] [CrossRef]
- Fisher, K.A.; Bloomstone, S.J.; Walder, J.; Crawford, S.; Fouayzi, H.; Mazor, K.M. Attitudes Toward a Potential SARS-CoV-2 Vaccine: A Survey of U.S. Adults. Ann. Intern. Med. 2020, 173, 964–973. [Google Scholar] [CrossRef]
- Kreps, S.; Prasad, S.; Brownstein, J.S.; Hswen, Y.; Garibaldi, B.T.; Zhang, B.; Kriner, D.L. Factors Associated with US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. Open 2020, 3, e2025594. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Nicholas, S.; Leng, A.; Maitland, E.; Wang, J. COVID-19 Vaccination Willingness among Chinese Adults under the Free Vaccination Policy. Vaccines 2021, 9, 292. [Google Scholar] [CrossRef]
- Taylor, S.; Landry, C.A.; Paluszek, M.M.; Groenewoud, R.; Rachor, G.S.; Asmundson, G.J.G. A Proactive Approach for Managing COVID-19: The Importance of Understanding the Motivational Roots of Vaccination Hesitancy for SARS-CoV2. Front. Psychol. 2020, 11, 575950. [Google Scholar] [CrossRef]
- Vallée, A.; Fourn, E.; Majerholc, C.; Touche, P.; Zucman, D. COVID-19 Vaccine Hesitancy among French People Living with HIV. Vaccines 2021, 9, 302. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- AIDS and Hepatitis C Professional Group. Expert recommendation for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in patients with HIV infection. Chin. J. Int. Med. 2021, 7, 615–618. [Google Scholar]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef]
- Guidelines for Standard Criteria for Adverse Events in Clinical Trials of Vaccines. Available online: https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20191231111901460.html (accessed on 26 December 2019).
- Rubin, D.B. Inference and missing data. Biometrika 1976, 63, 581–592. [Google Scholar] [CrossRef]
- Gianotti, N.; Galli, L.; Poli, A.; Torre, L.D.; Vinci, C.; Carini, E.; Galli, A.; Nozza, S.; Spagnuolo, V.; Muccini, C.; et al. Residual viremia in HIV-infected patients who continue a two-drug or switch to a three-drug integrase strand transfer inhibitor based regimen. Aids 2021, 35, 1513–1516. [Google Scholar] [CrossRef]
- Complete Course COVID-19 Vaccinal Rate in Beijing Exceeded 90%. Available online: https://bjrbdzb.bjd.com.cn/bjrb/mobile/2021/20210717/20210717_m.html#page4 (accessed on 17 July 2021).
- Grace, D.; Gaspar, M.; Paquette, R.; Rosenes, R.; Burchell, A.N.; Grennan, T.; Salit, I.E. HIV-positive gay men’s knowledge and perceptions of Human Papillomavirus (HPV) and HPV vaccination: A qualitative study. PLoS ONE 2018, 13, e207953. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.; Poeppl, W.; Herkner, H.; Tillhof, K.D.; Grabmeier-Pfistershammer, K.; Rieger, A.; Forstner, C.; Burgmann, H.; Lagler, H. Predictors for and coverage of influenza vaccination among HIV-positive patients: A cross-sectional survey. HIV Med. 2017, 18, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Chen, Q.; Li, D.; Wang, T.; Gou, Y.; Wei, B.; Tao, C. High prevalence of syphilis, HBV, and HCV co-infection, and low rate of effective vaccination against hepatitis B in HIV-infected patients in West China hospital. J. Med. Virol. 2018, 90, 101–108. [Google Scholar] [CrossRef]
- Ekstrand, M.L.; Heylen, E.; Gandhi, M.; Steward, W.T.; Pereira, M.; Srinivasan, K. COVID-19 Vaccine Hesitancy Among PLWH in South India: Implications for Vaccination Campaigns. J. Acquir. Immune Defic. Syndr. 2021, 88, 421–425. [Google Scholar] [CrossRef]
- Bogart, L.M.; Ojikutu, B.O.; Tyagi, K.; Klein, D.J.; Mutchler, M.G.; Dong, L.; Lawrence, S.J.; Thomas, D.R.; Kellman, S. COVID-19 Related Medical Mistrust, Health Impacts, and Potential Vaccine Hesitancy Among Black Americans Living With HIV. J. Acquir. Immune Defic. Syndr. 2021, 86, 200–207. [Google Scholar] [CrossRef]
- Jones, D.L.; Salazar, A.S.; Rodriguez, V.J.; Balise, R.R.; Starita, C.U.; Morgan, K.; Raccamarich, P.D.; Montgomerie, E.; Nogueira, N.F.; Barreto, O.I.; et al. Severe Acute Respiratory Syndrome Coronavirus 2: Vaccine Hesitancy Among Underrepresented Racial and Ethnic Groups with HIV in Miami, Florida. Open Forum Infect. Dis. 2021, 8, b154. [Google Scholar] [CrossRef]
- Lu, P.J.; Srivastav, A.; Amaya, A.; Dever, J.A.; Roycroft, J.; Kurtz, M.S.; O’Halloran, A.; Williams, W.W. Association of provider recommendation and offer and influenza vaccination among adults aged >/=18years—United States. Vaccine 2018, 36, 890–898. [Google Scholar] [CrossRef]
- Paul, E.; Steptoe, A.; Fancourt, D. Attitudes towards vaccines and intention to vaccinate against COVID-19: Implications for public health communications. Lancet Reg. Health Eur. 2021, 1, 100012. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, Y.; He, Z.; Huang, H.; Tian, X.; Wang, G.; Chen, D.; Ren, Y.; Jia, L.; Wang, W.; et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine in people living with HIV-1. medRxiv 2021. [Google Scholar] [CrossRef]
- Madhi, S.A.; Koen, A.L.; Izu, A.; Fairlie, L.; Cutland, C.L.; Baillie, V.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: An interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021, 8, e568–e580. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Karaba, A.H.; Garliss, C.C.; Beck, E.J.; Wang, K.H.; Laeyendecker, O.; Cox, A.L.; Blankson, J.N. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living with Human Immunodeficiency Virus (HIV). Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Vigezzi, G.P.; Lume, A.; Minerva, M.; Nizzero, P.; Biancardi, A.; Gianfredi, V.; Odone, A.; Signorelli, C.; Moro, M. Safety surveillance after BNT162b2 mRNA COVID-19 vaccination: Results from a cross-sectional survey among staff of a large Italian teaching hospital. Acta Biomed 2021, 92, e2021450. [Google Scholar]
- Skiest, D.J.; Machala, T. Comparison of the effects of acute Influenza infection and Influenza vaccination on HIV viral load and CD4 cell counts. J. Clin. Virol. 2003, 26, 307–315. [Google Scholar] [CrossRef]
- Mondi, A.; Cimini, E.; Colavita, F.; Cicalini, S.; Pinnetti, C.; Matusali, G.; Casetti, R.; Maeurer, M.; Vergori, A.; Mazzotta, V.; et al. COVID-19 in people living with HIV: Clinical implications of dynamics of the immune response to SARS-CoV-2. J. Med. Virol. 2021, 93, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Rossheim, A.E.B.; Young, A.M.P.; Siik, J.; Cunningham, T.D.; Troy, S.B. Association of time since pneumococcal polysaccharide vaccine receipt and CD4 count with antibody response to the 13-valent pneumococcal conjugate vaccine in HIV-infected adults. Hum. Vacc. Immunother. 2016, 12, 2117–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeger-Madiot, R.; Heredia, M.; Graff-Dubois, S. Germinal centers B-cell reaction and T follicular helper cells in response to HIV-1 infection. Curr. Opin. HIV AIDS 2019, 14, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, N.; Kuo, H.H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 2020, 183, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Yorsaeng, R.; Suntronwong, N.; Phowatthanasathian, H.; Assawakosri, S.; Kanokudom, S.; Thongmee, T.; Vichaiwattana, P.; Auphimai, C.; Wongsrisang, L.; Srimuan, D.; et al. Immunogenicity of a third dose viral-vectored COVID-19 vaccine after receiving two-dose inactivated vaccines in healthy adult. medRxiv 2021. [Google Scholar] [CrossRef]
| Variables | Statistic Value |
|---|---|
| Sex (n, %) | |
| Male | 374 (98.9%) |
| Female | 4 (1.1%) |
| Age (years, n, %) | |
| 18–30 | 100 (26.7%) |
| 31–40 | 192 (51.3%) |
| 41–50 | 56 (15.0%) |
| 51–60 | 26 (7.0%) |
| Marital status (n, %) | |
| Unmarried | 279 (76.2%) |
| Married | 69 (18.8%) |
| Divorced/widowed | 18 (4.9%) |
| Educational background (n, %) | |
| High school and below | 70 (19.1%) |
| College/undergraduate | 246 (67.2%) |
| Postgraduate and above | 50 (13.7%) |
| Occupation (n, %) | |
| Business/service staff | 129 (35.1%) |
| Professional and technical personnel | 76 (20.7%) |
| Public official | 36 (9.8%) |
| Farmer/industrial worker | 21 (5.7%) |
| Student | 6 (1.6%) |
| Unemployed | 12 (3.2%) |
| Others | 88 (23.9%) |
| Duration of ART treatment (years, median and IQR) | 4.3 (2.8–6.0) |
| ART regimen (n, %) | |
| EFV + TDF + 3TC | 378 (100%) |
| CD4+ T cell counts prior to ART initiation (cells/L, median and IQR) | 305 (203–433) |
| VL prior to ART initiation (log10 copies/mL, median and IQR) | 4.67 (4.16–5.01) |
| CD4+ T cell counts before 6 months (cells/L, n, %) | |
| <200 | 10 (2.6%) |
| 200–349 | 52 (13.8%) |
| 350–500 | 70 (18.5%) |
| >500 | 246 (65.1%) |
| Mode of HIV transmission (n, %) | |
| MSM | 275 (72.7%) |
| Heterosexual sex | 33 (8.7%) |
| Transfusion | 9 (2.3%) |
| Others/unknown | 61 (16.1%) |
| Scores of SF-12 (median and IQR) | |
| PCS | 53 (47–55) |
| MCS | 53 (46–56) |
| Patients Received at Least One Dose | n (%) |
| Manufacturers | |
| CoronaVac vaccine (Sinovac Life Sciences, Beijing, China) | 128 (58.4%) |
| BBIBP-CorV vaccine (Beijing Institute of Biological Products, Beijing, China) | 87 (39.7%) |
| Recombinant protein subunit vaccine (Anhui Zhifei Longcom Biopharmaceutical, Anhui, China) | 3 (1.4%) |
| Recombinant adenovirus type-5 vectored vaccine (CanSino Biologics, Tianjin, China) | 1 (0.5%) |
| Vaccinees who do not have concerns about vaccine | 202 (92.2%) |
| Vaccinees who completed whole-course vaccination | 155 (70.7%) |
| The Reasons for Not Having Been Vaccinated | n(%) |
| Concerns about side effects and/or poor efficacy | 89 (56.0%) |
| Waiting to be scheduled. | 31 (19.5%) |
| Contraindications for the vaccine | 21 (13.8%) |
| No perceived need for vaccination | 15 (9.4%) |
| Scheduling conflicts | 3 (1.9%) |
| Vaccine Acceptance (n = 274) | Vaccine Hesitancy (n = 104) | p Value | |
|---|---|---|---|
| Age (n, %) | 0.625 | ||
| 21–30 | 25 (24.3%) | 75 (27.7%) | |
| 31–40 | 5 (54.4%) | 136 (50.2%) | |
| 41–50 | 13 (12.6%) | 43 (15.9%) | |
| 51–60 | 9 (8.7%) | 17 (6.3%) | |
| Marital status (n, %) | 0.560 | ||
| Unmarried | 78 (77.2%) | 201 (75.8%) | |
| Married | 20 (19.8%) | 49 (18.5%) | |
| Divorced/widowed | 3 (3.0%) | 15 (5.7%) | |
| Educational background (n, %) | 0.648 | ||
| High school and below | 21 (20.6%) | 201 (75.8%) | |
| College/undergraduate | 68 (66.7%) | 49 (18.5%) | |
| Postgraduate and above | 13 (12.7%) | 15 (5.7%) | |
| Occupation (n, %) | 0.621 | ||
| Business/service staff | 29 (28.4%) | 100 (37.6%) | |
| Professional and technical personnel | 24 (23.5%) | 52 (19.5%) | |
| Public officials | 9 (8.8%) | 27 (10.2%) | |
| Farmer/worker | 7 (6.9%) | 14 (5.3%) | |
| Students | 1 (1.0%) | 5 (1.9%) | |
| Unemployed | 3 (2.9%) | 9 (3.4%) | |
| Others | 29 (28.4%) | 59 (22.2%) | |
| Duration of ART treatment (years, median and IQR) | 4.6 (2.9–5.9) | 4.1 (2.8–6.1) | 0.696 |
| CD4+ T cell counts prior to ART initiation (cells/μL, median and IQR) | 305 (183–454) | 306 (213.5–425.5) | 0.567 |
| VL load prior to ART initiation (log10 copies/mL, median and IQR) | 4.72 (4.39–5.16) | 4.65 (3.95–4.97) | 0.103 |
| CD4+ T cell counts before 6 months (n, %) | 0.505 | ||
| <200 | 2 (1.9%) | 2 (0.7%) | |
| 200–349 | 16 (15.4%) | 32 (11.7%) | |
| 350–500 | 23 (22.1%) | 57 (20.8%) | |
| >500 | 63 (60.6%) | 183 (66.8%) | |
| Mode of HIV transmission (n, %) | 0.094 | ||
| MSM | 72 (69.2%) | 203 (74.1%) | |
| Heterosexual sex | 7 (6.7%) | 26 (9.5%) | |
| Transfusion | 1 (1%) | 8 (2.9%) | |
| Others/unknown | 24 (23.1%) | 37 (13.5%) | |
| Scores of SF-12 (median and IQR) | |||
| PCS | 53 (48.5–55) | 54 (47–55) | 0.159 |
| MCS | 53 (47.5–56) | 53 (45–56) | 0.593 |
| Consulted physicians (n, %) | 38 (36.5%) | 148 (54%) | 0.002 |
| HC (n = 21) | PLWH (n = 55) | p Value | |
|---|---|---|---|
| Age (years, mean ± SD) | 35 ± 8 | 36 ± 11 | 0.681 |
| Male (n, %) | 17 (100%) | 55 (100%) | 1.000 |
| Time since whole-course vaccination (weeks, median and IQR) | 4.86 (3–9.14) | 5 (3.71–8.71) | 0.921 |
| Vaccination interval (weeks, mean ± SD) | 3.67 ± 0.8 | 3.64 ± 0.84 | 0.969 |
| CD4+ T cell counts after vaccination (cells/L, mean ± SD) | 769 ± 262 | 572 ± 203 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Han, J.; Li, X.; Chen, D.; Zhao, X.; Qiu, Y.; Zhang, L.; Xiao, J.; Li, B.; Zhao, H. COVID-19 Vaccination in People Living with HIV (PLWH) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity. Vaccines 2021, 9, 1458. https://doi.org/10.3390/vaccines9121458
Liu Y, Han J, Li X, Chen D, Zhao X, Qiu Y, Zhang L, Xiao J, Li B, Zhao H. COVID-19 Vaccination in People Living with HIV (PLWH) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity. Vaccines. 2021; 9(12):1458. https://doi.org/10.3390/vaccines9121458
Chicago/Turabian StyleLiu, Ying, Junyan Han, Xin Li, Danying Chen, Xuesen Zhao, Yaruo Qiu, Leidan Zhang, Jing Xiao, Bei Li, and Hongxin Zhao. 2021. "COVID-19 Vaccination in People Living with HIV (PLWH) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity" Vaccines 9, no. 12: 1458. https://doi.org/10.3390/vaccines9121458
APA StyleLiu, Y., Han, J., Li, X., Chen, D., Zhao, X., Qiu, Y., Zhang, L., Xiao, J., Li, B., & Zhao, H. (2021). COVID-19 Vaccination in People Living with HIV (PLWH) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity. Vaccines, 9(12), 1458. https://doi.org/10.3390/vaccines9121458






