Vaccine Procurement: A Conceptual Framework Based on Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Reporting
3. Results
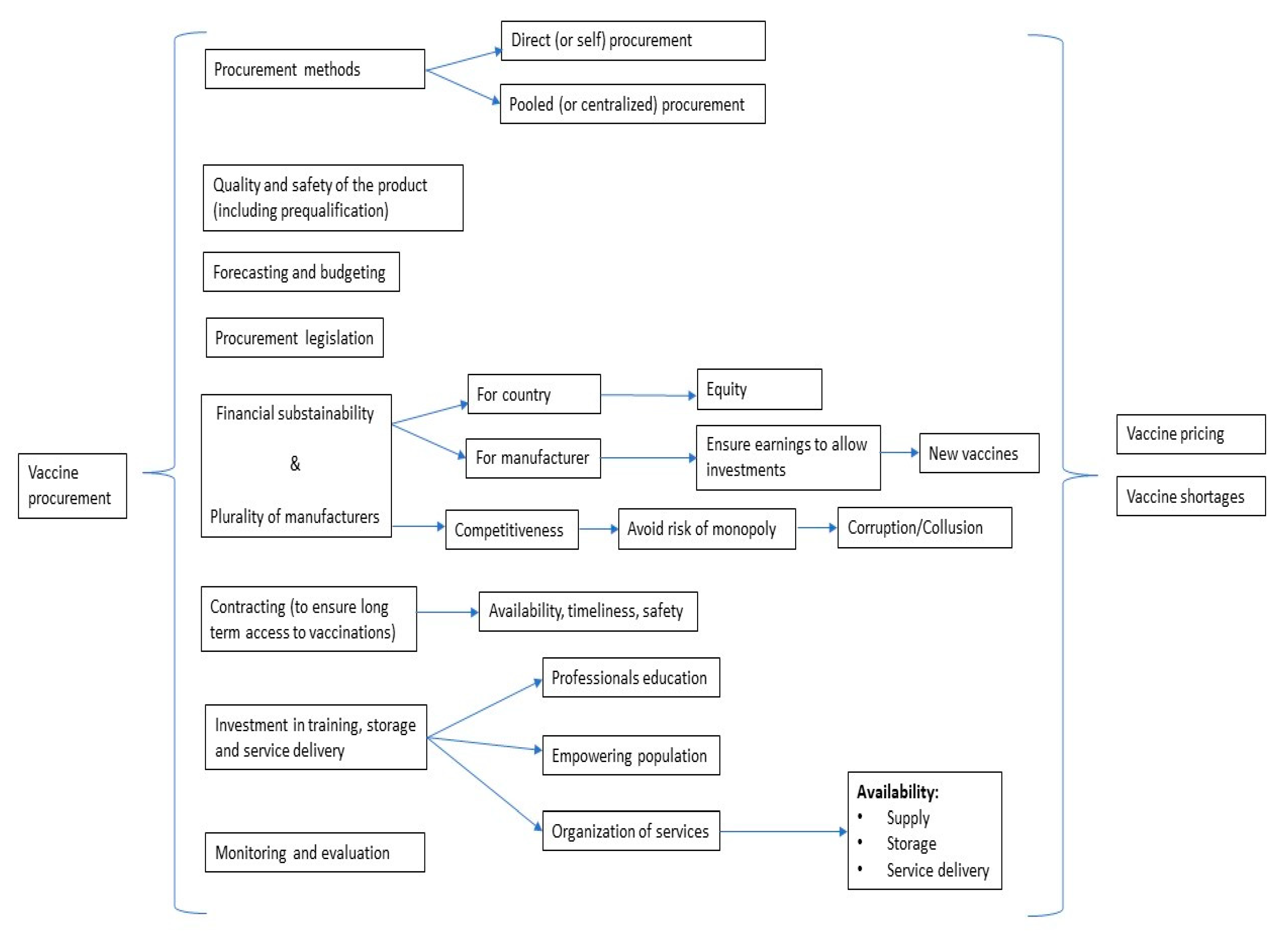
3.1. Conceptual Framework for Vaccine Procurement
3.2. Relevant Definitions of Procurement Methods and Purchase Mechanisms
- Direct (or self) procurement: countries are autonomous in their decisions and do not have to comply with WHO prequalification requisites. Three different main purchase mechanisms are used, depending on the country size, needs/shortages, and bargaining power: competitive bidding, request for quotation, and sole-source procurement. Competitive bidding is a “procurement process in which clearly stated product specifications and contract requirements are issued to multiple suppliers to solicit pricing and performance responses”; request for quotation is a process where “offers (quotations) are requested from several prospective suppliers without employing formal sealed bidding procedures”; sole source procurement refers to “purchasing from a single manufacturer without competition among potential suppliers”.
- Pooled (or centralized) procurement: refers to a mechanism by which several countries (buyers) combine into a single entity that purchases vaccines on their behalf. The WHO has identified four levels of pooled procurement [8]: in level 1 (information sharing and individual informed buying), procurement is conducted by each country, but information about suppliers and products is shared. Benchmarks and best practices are identified. In level 2 (coordinated informed buying), procurement is conducted by each country. Market research is jointly performed. Supplier performance and prices are monitored via shared information, which may also involve the national political level. In level 3 (group contracting), procurement is conducted individually. Still, vaccines are prequalified, prices set, suppliers selected, and products purchased by the process of joint actions and negotiations. In this context, legal framework, procedures, and policies need to be completely harmonized. Group contracting is a useful example of economies of scale. Lastly, in level 4 (central contracting), tendering, awarding contracts and delivery are coordinated by a single representative organization, which can also implement supplementary technical and functional roles. Centralized procurement is traditionally performed within supranational entities (e.g., PAHO) [9], while decentralized procurement “occurs when administrative responsibility, authority, and discretion are delegated to service and sub-service delivery personnel” [10].
3.3. Quality and Safety of Vaccines (including Prequalification)
3.4. Forecasting and Budgeting
3.5. Procurement Legislation
3.6. Financial Sustainability for Both, Countries and Manufacturers (including Plurality of Manufacturers and Domestic Production)
3.7. Contracting
3.8. Investment in Training, Storage and Service Delivery
3.9. Monitoring and Evaluation
3.10. Vaccine Shortages
4. Discussion
Limits and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008, 86, 81–160. [Google Scholar]
- Robson, M.; Andrus, J.K.; Toscano, C.M.; Lewis, M.; Oliveira, L.; Ropero, A.M.; Davila, M.; Fitzsimmons, J.W. A model for enhancing evidence-based capacity to make informed policy decisions on the introduction of new vaccines in the Americas: PAHO’s ProVac initiative. Public Health Rep. 2007, 122, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Huff-Rousselle, M. The logical underpinnings and benefits of pooled pharmaceutical procurement: A pragmatic role for our public institutions? Soc. Sci. Med. 2012, 75, 1572–1580. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Pubmed/National Library of Medicine’s. Publication Characteristics (Publication Types) with Scope Notes. 2020. Available online: https://www.nlm.nih.gov/mesh/pubtypes.html (accessed on 3 March 2020).
- World Health Organization. Immunization Programmes. Procurement Mechanisms. 2018. Available online: https://www.who.int/immunization/programmes_systems/procurement/mechanisms_systems/en/ (accessed on 3 March 2020).
- World Health Organization. Procurement Mechanisms and Systems. 2020. Available online: https://www.who.int/immunization/programmes_systems/procurement/mechanisms_systems/pooled_procurement/en/ (accessed on 3 March 2020).
- World Health Organization. Vaccine Product, Price and Procurement (V3P). 2016. Available online: https://www.who.int/immunization/programmes_systems/procurement/en/ (accessed on 3 March 2020).
- Operating Procedures of the PAHO Revolving Fund for the Purchase of Vaccines, Syringes, and Other Related Supplies; PAHO: Washingotn, DC, USA, 2011.
- McCue Clifford, P.; Pitzer Jack, T. Centralized vs. decentralized purchasing: Current trends in governmental procurement practices. J. Public Budg. Account. Financ. Manag. 2000, 12, 400–420. [Google Scholar]
- Lee, B.Y.; Haidari, L.A.; Prosser, W.; Connor, D.L.; Bechtel, R.; Dipuve, A.; Kassim, H.; Khanlawia, B.; Brown, S.T. Re-designing the Mozambique vaccine supply chain to improve access to vaccines. Vaccine 2016, 34, 4998–5004. [Google Scholar] [CrossRef] [PubMed]
- Lydon, P.; Schreiber, B.; Gasca, A.; Dumolard, L.; Urfer, D.; Senouci, K. Vaccine stockouts around the world: Are essential vaccines always available when needed? Vaccine 2017, 35, 2121–2126. [Google Scholar] [CrossRef]
- Wilsdon, T.; Lawlor, R.; Li, L.; Rafila, A.; Garcia Rojas, A. The impact of vaccine procurement methods on public health in selected European countries. Expert Rev. Vaccines 2020, 19, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Milstien, J.; Dellepiane, N.; Lambert, S.; Belgharbi, L.; Rolls, C.; Knezevic, I.; Fournier-Caruana, J.; Wood, D.; Griffiths, E. Vaccine quality—Can a single standard be defined? Vaccine 2002, 20, 1000–1003. [Google Scholar] [CrossRef]
- Lopalco, P.L.; DeStefano, F. The complementary roles of Phase 3 trials and post-licensure surveillance in the evaluation of new vaccines. Vaccine 2015, 33, 1541–1548. [Google Scholar] [CrossRef]
- Kartoglu, U.; Milstien, J. Tools and approaches to ensure quality of vaccines throughout the cold chain. Expert Rev. Vaccines 2014, 13, 843–854. [Google Scholar] [CrossRef]
- Bumpas, J. Study on Comparative Efficiencies in Vaccine Procurement Mechanisms; World Bank: Washington, DC, USA, 2008. [Google Scholar]
- Dellepiane, N.; Wood, D. Twenty-five years of the WHO vaccines prequalification programme (1987–2012): Lessons learned and future perspectives. Vaccine 2015, 33, 52–61. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Committee on Specifications for Pharmaceutical Preparations, 5th ed.; Worl Bank: Geneva, Switzerland, 2016. [Google Scholar]
- Coyne, P.E. The World Health Organization Prequalification Programme—Playing an essential role in assuring quality medical products. Int. Health 2019, 11, 79–80. [Google Scholar] [CrossRef]
- Ethgen, O.; Baron-Papillon, F.; Cornier, M. How much money is spent on vaccines across Western European countries? Hum. Vaccines Immunother. 2016, 12, 2038–2045. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Centers for Disease Control and Prevention. Vaccine Storage and Handling Toolkit. 2019. Available online: https://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/index.html (accessed on 3 March 2020).
- Shamsi, G.N.; Torabi, S.A. Vaccine Supply Management. In Operations Research Applications in Health Care Management; Kahraman, C.T.Y., Ed.; Springer: Cham, Switzerland, 2018; Volume 262. [Google Scholar]
- Thompson, K.M.; Duintjer Tebbens, R.J. Framework for Optimal Global Vaccine Stockpile Design for Vaccine-Preventable Diseases: Application to Measles and Cholera Vaccines as Contrasting Examples. Risk Anal. 2016, 36, 1487–1509. [Google Scholar] [CrossRef] [PubMed]
- Duclos, P.; Dumolard, L.; Abeysinghe, N.; Adjagba, A.; Janusz, C.B.; Mihigo, R.; Mosina, L.; Takashima, Y.; Öztürk, M.H. Progress in the establishment and strengthening of national immunization technical advisory groups: Analysis from the 2013 WHO/UNICEF joint reporting form, data for 2012. Vaccine 2013, 31, 5314–5320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrett, A.D. Yellow Fever in Angola and Beyond—The Problem of Vaccine Supply and Demand. N. Engl. J. Med. 2016, 375, 301–303. [Google Scholar] [CrossRef] [PubMed]
- GAVI Board. Meningococcal Outbreak Response Supply and Procurement Roadmap; GAVI: Geneva, Switzerland, 2019. [Google Scholar]
- Johansen, K.; Jønsrud, K.; Greve-Isdahl, M.; Rydland, K.; Wolden, B.; Aaberge, I. Report on Understanding Mechanisms for Defining the Anticipated Needs to Ensure Sufficient Size of Supply and Stockpiles, Including Their Sustainability; European Union: Brussels, Belgien, 2019. [Google Scholar]
- World Health Organization. Procurement of Vaccines for Public-Sector Programmes: A Reference Manual; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- De Roeck, D. Group Procurement of Vaccines for Central/Eastern Europe and Newly Independent States: Feasibility, Issues, and Options; Children’s Vaccine Program at PATH and the department of Immunization, Vaccines and Biologicals of the World Health Organization; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Tafuri, S.; Stefanizzi, P. Italy, where a judge could decide the future of immunization policies. Ann. Igiene Med. Prev. Comunita 2020, 32, 430–432. [Google Scholar]
- GAVI Board. Lessons Learned: New Procurement Strategies for Vaccines; Final Report to the GAVI Board; GAVI: Geneva, Switzerland, 2002. [Google Scholar]
- GAVI the Vaccine Alliance. Supply and Procurement Strategy 2016–2020; GAVI: Geneva, Switzerland, 2016. [Google Scholar]
- Rey-Jurado, E.; Tapia, F.; Munoz-Durango, N.; Lay, M.K.; Carreno, L.J.; Riedel, C.A.; Bueno, S.M.; Genzel, Y.; Kalergis, A.M. Assessing the Importance of Domestic Vaccine Manufacturing Centers: An Overview of Immunization Programs, Vaccine Manufacture, and Distribution. Front. Immunol. 2018, 9, 26. [Google Scholar] [CrossRef]
- Stuurman, A.L.; Rizzo, C.; Haag, M.D.M. Seasonal influenza vaccine procurement systems in Europe. Eur. J. Public Health 2018, 28 (Suppl. S4), 82. [Google Scholar] [CrossRef]
- Pagliusi, S.; Che, Y.; Dong, S. The art of partnerships for vaccines. Vaccine 2019, 37, 5909–5919. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Review of Vaccine Price Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2013. [Google Scholar]
- World Health Organization. MI4A: Market Information for Access to Vaccines. 2019. Available online: https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/en/ (accessed on 3 March 2020).
- World Health Organization. Factors to Consider When Comparing Vaccine Prices; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Malhame, M.; Baker, E.; Gandhi, G.; Jones, A.; Kalpaxis, P.; Iqbal, R.; Momeni, Y.; Nguyen, A. Shaping markets to benefit global health—A 15-year history and lessons learned from the pentavalent vaccine market. Vaccine X 2019, 2, 100033. [Google Scholar] [CrossRef]
- European Commission. World Trends in R&D Private Investment. Facts and Figures; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Vaccines Europe. The EU Vaccine Industry in Figures; Vaccines Europe: Brussels, Belgium, 2016. [Google Scholar]
- Fadeyi, I.; McLean, T.; Tavella, F.; Heron, L. Differences In Vaccine Pricing Between High-Income And Low-Income Markets. Value Health 2017, 20, 411. [Google Scholar] [CrossRef][Green Version]
- Hinsch, M.; Kaddar, M.; Schmitt, S. Enhancing medicine price transparency through price information mechanisms. Glob. Health 2014, 10, 34. [Google Scholar] [CrossRef]
- World Health Organization. WHO/UNICEF Joint Reporting Process. 2019. Available online: https://www.who.int/immunization/monitoring_surveillance/routine/reporting/en/ (accessed on 3 March 2020).
- Fifty-Fourth World Health Assembly. WHO Medicines Strategy; WHO: Geneva, Switzerland, 2001.
- World Health Organization. Severnty-Second World Health Assembly: Improving the Transparency of Markets for Medicines, Vaccines, and Other Health Products; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Myths and Facts about Vaccine Product Price and Procurement; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Multi-Country Regional Pooled Procurement of Medicines. Identifying Key Principles for Enabling Regional Pooled Procurement and a Framework for Inter-Regional Collaboration in the African, Caribbean, and Pacific Island Countries; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Dimitri, N.; Piga, G.; Spagnolo, G. Handbook of Procurement; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Terms OGos. Cartel. 2020. Available online: https://stats.oecd.org/glossary/detail.asp?ID=3157#:~:text=A%20cartel%20is%20a%20formal,profits%20or%20combination%20of%20these (accessed on 3 March 2020).
- Zaffran, M.; Vandelaer, J.; Kristensen, D.; Melgaard, B.; Yadav, P.; Antwi-Agyei, K.O.; Lasher, H. The imperative for stronger vaccine supply and logistics systems. Vaccine 2013, 31 (Suppl. S2), B73–B80. [Google Scholar] [CrossRef] [PubMed]
- Odone, A.; Signorelli, C. When vaccine hesitancy makes headlines. Vaccine 2017, 35, 1209–1210. [Google Scholar] [CrossRef]
- European Commission. The Organization and Delivery of Vaccination Services in the European Union; Bernd, E.R., McKee, M., Eds.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Nelson, E.A.; Bloom, D.E.; Mahoney, R.T. Monitoring what governments “give for” and “spend on” vaccine procurement: Vaccine Procurement Assistance and Vaccine Procurement Baseline. PLoS ONE 2014, 9, e89593. [Google Scholar] [CrossRef] [PubMed]
- Filia, A.; Grossi, A.; Rota, M.C.; Rezza, G. Report on Previous Experiences with Vaccine Shortages in EU Countries (and non-EU Consortium Member Countries), and Responses at National and European Levels; European Union: Brussels, Belgien, 2019. [Google Scholar]
- Centers for Disease Control and Prevention. Current Vaccine Shortages and Delays. 2020. Available online: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html (accessed on 3 March 2020).
- Saitoh, A.; Okabe, N. Progress and challenges for the Japanese immunization program: Beyond the “vaccine gap”. Vaccine 2018, 36, 4582–4588. [Google Scholar] [CrossRef]
- McCurry, J. Flu jab shortage in Japan puts elderly at risk. Lancet Med. Health Policy 2004, 363, 134. [Google Scholar] [CrossRef]
- New Zealand Ministry of Health. BCG Vaccine and Vaccinator Endorsement. 2019. Available online: https://www.health.govt.nz/our-work/preventative-health-wellness/immunisation/immunisation-programme-decisions/bcg-vaccine-and-vaccinator-endorsement (accessed on 3 March 2020).
- Drug Shortages Canada. Drug Shortage Report for ENGERIX-B. 2019. Available online: https://www.drugshortagescanada.ca/shortage/6511 (accessed on 3 March 2020).
- Drug Shortages Canada. Reports for AVAXIM. 2019. Available online: https://www.drugshortagescanada.ca/drug/8884 (accessed on 3 March 2020).
- Australian Governement Department of Health. Medicine Shortages Information Initiative. 2020. Available online: https://apps.tga.gov.au/prod/MSI/search (accessed on 3 March 2020).
- Prevenzione e Controllo Dell’influenza: Raccomandazioni per la Stagione 2020–2021; Italian Ministry of Health: Rome, Italiy, 2020.
- Zerhouni, E. GAVI, the vaccine alliance. Cell 2019, 179, 13–17. [Google Scholar] [CrossRef]
- Burioni, R.; Odone, A.; Signorelli, C. Lessons from Italy’s policy shift on immunization. Nature 2018, 555, 30. [Google Scholar] [CrossRef] [PubMed]
- Burgess, R.A.; Osborne, R.H.; Yongabi, K.A.; Greenhalgh, T.; Gurdasani, D.; Kang, G.; Falade, A.G.; Odone, A.; Busse, R.; Martin-Moreno, J.M.; et al. The COVID-19 vaccines rush: Participatory community engagement matters more than ever. Lancet 2021, 397, 8–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianfredi, V.; Filia, A.; Rota, M.C.; Croci, R.; Bellini, L.; Odone, A.; Signorelli, C. Vaccine Procurement: A Conceptual Framework Based on Literature Review. Vaccines 2021, 9, 1434. https://doi.org/10.3390/vaccines9121434
Gianfredi V, Filia A, Rota MC, Croci R, Bellini L, Odone A, Signorelli C. Vaccine Procurement: A Conceptual Framework Based on Literature Review. Vaccines. 2021; 9(12):1434. https://doi.org/10.3390/vaccines9121434
Chicago/Turabian StyleGianfredi, Vincenza, Antonietta Filia, Maria Cristina Rota, Roberto Croci, Lorenzo Bellini, Anna Odone, and Carlo Signorelli. 2021. "Vaccine Procurement: A Conceptual Framework Based on Literature Review" Vaccines 9, no. 12: 1434. https://doi.org/10.3390/vaccines9121434
APA StyleGianfredi, V., Filia, A., Rota, M. C., Croci, R., Bellini, L., Odone, A., & Signorelli, C. (2021). Vaccine Procurement: A Conceptual Framework Based on Literature Review. Vaccines, 9(12), 1434. https://doi.org/10.3390/vaccines9121434






