Trusted Sources of COVID-19 Vaccine Information among Hesitant Adopters in the United States
Abstract
:1. Introduction
2. Methods
2.1. Study Design, Aims, and Approach
2.2. Participant Recruitment
2.3. Consent and Remuneration
2.4. Data Collection
2.5. Instruments
2.6. Study Sample
2.7. Data Analysis
3. Results
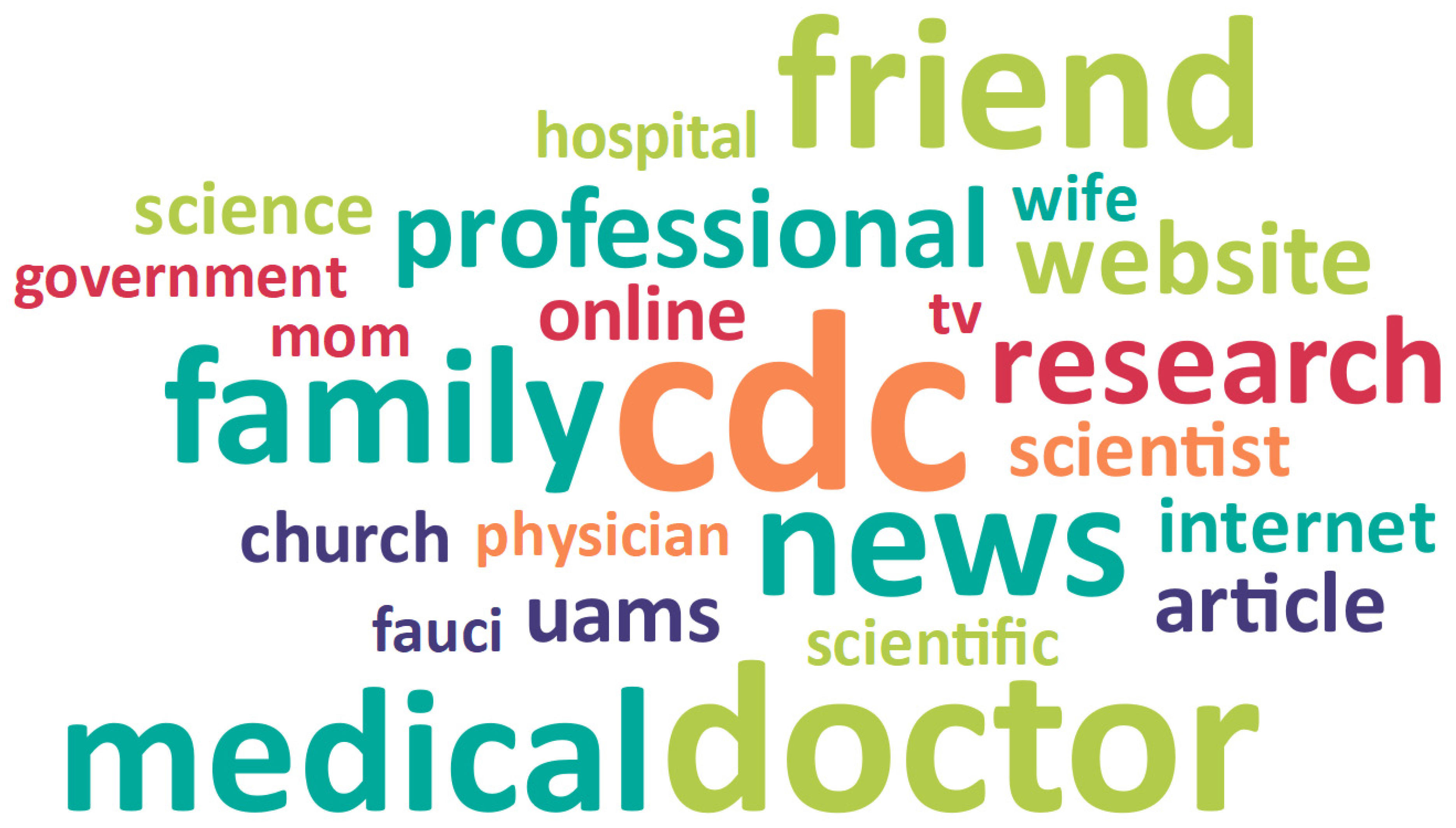
3.1. Trusted Sources of Information
3.1.1. Health Care/Medical Science
Health Care Providers
Public Health Organizations and Leaders
3.1.2. Personal Relationships
Family and Friends
Vaccinated Individuals
Religious Institutions and Leaders
Employer and Coworkers
3.1.3. News and Social Media
News Media
Internet
3.1.4. Individual/Myself
Trusted My Gut
Did My Own Research
3.2. Do Not Trust Any Sources of Information
3.2.1. Do Not Trust Any Sources
3.2.2. Lack of Trust in Traditional Sources
4. Discussion
4.1. Strengths and Limitations
4.2. Recommendations and Implications for Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; El Burai Felix, S.; Tie, Y.; Fullerton, K.E. Coronavirus Disease 2019 Case Surveillance–United States, 22 January–30 May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. CDC COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days (accessed on 24 August 2021).
- Hughes, M.M.; Wang, A.; Grossman, M.K.; Pun, E.; Whiteman, A.; Deng, L.; Hallisey, E.; Sharpe, J.D.; Ussery, E.N.; Stokley, S.; et al. County-Level COVID-19 Vaccination Coverage and Social Vulnerability–United States, 14 December 2020–1 March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 431–436. [Google Scholar] [CrossRef]
- Hassan, A.; Weiland, N. Some Mass Vaccination Sites in U.S. Close as Demand Begins to Fall. The New York Times. 23 April 2021. Available online: https://www.nytimes.com/2021/04/23/us/some-mass-vaccination-sites-in-us-close-as-demand-begins-to-fall.html (accessed on 15 September 2021).
- Centers for Disease Control and Prevention. COVID-19 Vaccinations in the United States. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccinations (accessed on 24 August 2021).
- Ritchie, H.; Mathieu, E.; Rodes-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Our World in Data, 2020. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 15 September 2021).
- Willis, D.E.; Andersen, J.A.; Bryant-Moore, K.; Selig, J.P.; Long, C.R.; Felix, H.C.; Curran, G.M.; McElfish, P.A. COVID-19 vaccine hesitancy: Race/ethnicity, trust, and fear. Clin. Transl. Sci. 2021, 14, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://www.who.int/vietnam/news/feature-stories/detail/ten-threats-to-global-health-in-2019 (accessed on 24 August 2021).
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J. Vaccine hesitancy: An overview. Hum. Vaccines Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.C.; Jamison, A.M.; An, J.; Hancock, G.R.; Freimuth, V.S. Measuring vaccine hesitancy, confidence, trust and flu vaccine uptake: Results of a national survey of White and African American adults. Vaccine 2019, 37, 1168–1173. [Google Scholar] [CrossRef]
- Kirzinger, A.; Sparks, G.; Brodie, M. KFF COVID-19 Vaccine Monitor: In Their Own Words, Six Months Later. Available online: https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-in-their-own-words-six-months-later/ (accessed on 24 August 2021).
- Enkel, S.; Attwell, K.; Snelling, T.; Christian, H. ‘Hesitant compliers’: Qualitative analysis of concerned fully-vaccinating parents. Vaccine 2018, 36, 6459–6463. [Google Scholar] [CrossRef]
- Latkin, C.A.; Dayton, L.; Miller, J.R.; Yi, G.; Jaleel, A.; Nwosu, C.C.; Yang, C.; Falade-Nwulia, O. Behavioral and Attitudinal Correlates of Trusted Sources of COVID-19 Vaccine Information in the US. Behav. Sci. 2021, 11, 56. [Google Scholar] [CrossRef]
- Piltch-Loeb, R.; Savoia, E.; Goldberg, B.; Hughes, B.; Verhey, T.; Kayyem, J.; Miller-Idriss, C.; Testa, M. Examining the effect of information channel on COVID-19 vaccine acceptance. PLoS ONE 2021, 16, e0251095. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Friedman, D.B.; Tam, C.C.; Zeng, C.; Li, X. Vaccine acceptance among college students in South Carolina: Do information sources and trust in information make a difference? medRxiv 2020. [Google Scholar] [CrossRef]
- Bergman, M. Advances in Mixed Methods Research: Theories and Applications; SAGE Publications Ltd.: London, UK, 2008. [Google Scholar]
- Creswell, J.W. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches, 4th ed.; SAGE: Thousand Oaks, CA, USA, 2013; p. 273. [Google Scholar]
- Creswell, J.W.; Plano Clark, V.L. Designing and Conducting Mixed Methods Research, 2nd ed.; SAGE: Thousand Oaks, CA, USA, 2010; p. 488. [Google Scholar]
- Creswell, J.; Plano Clark, V.; Gutmann, M.; Hanson, W. Advanced mixed methods research designs. In Handbook of Mixed Methods in Social and Behavioral Research; Tashakkori, A., Teddlie, C., Eds.; SAGE: Thousand Oaks, CA, USA, 2003; pp. 209–240. [Google Scholar]
- Johnson, R.; Onweugbuzie, A.; Turner, L. Toward a definitions of mixed methods research. J. Mix. Methods Res. 2007, 1, 112–133. [Google Scholar] [CrossRef]
- Johnson, R.; Onwuegbuzie, A. Mixed methods research: A research paradigm whose time has come. Educ. Res. 2004, 33, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Sale, J.E.; Lohfeld, L.H.; Brazil, K. Revisiting the Quantitative-Qualitative Debate: Implications for Mixed-Methods Research. Qual. Quant. 2002, 36, 43–53. [Google Scholar] [CrossRef] [PubMed]
- McElfish, P.; Cleek, A.; Willis, D.; Purvis, R.; James, L. Leveraging community engagement capacity to address COVD-19 disparities among Pacific Islander and Latinx Communities in Arkansas. J. Clin. Transl. Sci. 2020, 5, e81. [Google Scholar] [CrossRef]
- Harris, P.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- PhenX Toolkit. PhenX Toolkit: News. Available online: https://www.phenxtoolkit.org/news/statistics (accessed on 15 April 2021).
- Centers for Disease Control and Prevention. 2019 BRFSS Questionnaire. Available online: https://www.cdc.gov/brfss/questionnaires/pdf-ques/2019-BRFSS-Questionnaire-508.pdf (accessed on 5 May 2021).
- Quinn, S.C.; Jamison, A.; Freimuth, V.S.; An, J.; Hancock, G.R.; Musa, D. Exploring racial influences on flu vaccine attitudes and behavior: Results of a national survey of White and African American adults. Vaccine 2017, 35, 1167–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neergaard, M.A.; Olesen, F.; Andersen, R.S.; Sondergaard, J. Qualitative description—The poor cousin of health research? BMC Med. Res. Methodol. 2009, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuckartz, U.; Radiker, S. Analyzing Qualitative Data with MAXQDA; Springer: Basel, Switzerland, 2019. [Google Scholar]
- United States Census Bureau. QuickFacts: Arkansas. United States. Available online: https://www.census.gov/quickfacts/fact/table/AR,US/PST045219 (accessed on 7 October 2021).
- Earnshaw, V.; Eaton, L.; Kalichman, S.; Brousseau, N.; Hill, E.; Fox, A. COVID-19 conspiracy beliefs, health behaviors, and policy support. Transl. Behav. Med. 2020, 10, 850–856. [Google Scholar] [CrossRef]
- McFadden, S.M.; Malik, A.A.; Aguolu, O.G.; Willebrand, K.S.; Omer, S.B. Perceptions of the adult US population regarding the novel coronavirus outbreak. PLoS ONE 2020, 15, e0231808. [Google Scholar] [CrossRef]
- Fridman, I.; Lucas, N.; Henke, D.; Zigler, C.K. Association Between Public Knowledge About COVID-19, Trust in Information Sources, and Adherence to Social Distancing: Cross-Sectional Survey. JMIR Public Health Surveill. 2020, 6, e22060. [Google Scholar] [CrossRef]
- Boyle, J.; Brassell, T.; Dayton, J. Who Do Americans Trust for COVID-19 News and Information? ICF International, 2020. Available online: https://www.icf.com/insights/health/covid-19-public-health-findings-mitigation (accessed on 15 September 2021).
- Malik, A.A.; McFadden, S.M.; Elharake, J.; Omer, S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 2020, 26, 100495. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; Foreman, J.; Tozan, Y.; Capasso, A.; Jones, A.M.; DiClemente, R.J. Trends and Predictors of COVID-19 Information Sources and Their Relationship With Knowledge and Beliefs Related to the Pandemic: Nationwide Cross-Sectional Study. JMIR Public Health Surveill. 2020, 6, e21071. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.; Brassell, T.; Dayton, J. American Trust in COVID-19 Information from Federal and State/Local Government Is Trending Downward. ICF International, 2020. Available online: https://www.icf.com/insights/health/covid-19-survey-trust-government-response-erodes (accessed on 15 September 2021).
- Boyle, J.; Brassell, T.; Dayton, J. As Cases Increase, American Trust in COVID-19 Information from Federal, State, and Local Governments Continues to Decline. ICF International, 2020. Available online: https://www.icf.com/insights/health/covid-19-survey-american-trust-government-june (accessed on 15 September 2021).
- Brewer, N.; Chapman, G.; Rothman, A.; Leask, J.; Kempe, A. Increasing Vaccination: Putting Psychological Science Into Action. Psychol. Sci. Public Interest 2017, 18, 149–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrich, N.; Holmes, B. Communicating during a pandemic: Information the public wants about the disease and new vaccines and drugs. Health Promot. Pract. 2011, 12, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Sam, I. Public sources of information and information needs for pandemic influenza A(H1N1). J. Community Health 2010, 35, 676–682. [Google Scholar] [CrossRef]
- Pew Research Center. Americans’ Views of the News Media During COVID-19 Outbreak. Pew Research Center, 8 May 2020. Available online: https://www.journalism.org/2020/05/08/americans-views-of-the-news-media-during-the-covid-19-outbreak/ (accessed on 15 September 2021).
- Mitchell, A.; Oliphant, J. Americans Immersed in COVID-19 News; Most Think Media Are Doing Fairly Well Covering It; Pew Research Center: 18 March 2020. Available online: https://www.pewresearch.org/journalism/2020/03/18/americans-immersed-in-covid-19-news-most-think-media-are-doing-fairly-well-covering-it/ (accessed on 15 September 2021).
| Frequency | % or | |
|---|---|---|
| Age | 867 | 37.21 |
| 18–24 | 178 | 20.53% |
| 25–34 | 219 | 25.26% |
| 35–44 | 227 | 26.18% |
| 45–54 | 136 | 15.69% |
| 55–64 | 84 | 9.69% |
| 65+ | 23 | 2.65% |
| Sex | 862 | |
| Female | 519 | 60.21% |
| Male | 343 | 39.79% |
| Race/Ethnicity | 851 | |
| American Indian/Alaska Native | 8 | 0.94% |
| Asian | 40 | 4.70% |
| Black/African American | 55 | 6.46% |
| Native Hawaiian or Pacific Islander | 72 | 8.46% |
| White | 379 | 44.54% |
| Hispanic/Latinx | 277 | 32.55% |
| Multiracial | 20 | 2.35% |
| Education | 840 | |
| Less than High School | 110 | 13.10% |
| High School or GED | 212 | 25.24% |
| Some College | 200 | 23.81% |
| Four-Year Degree or More | 318 | 37.86% |
| Marital Status | 838 | |
| Married | 397 | 47.37% |
| Not Married | 441 | 52.63% |
| Employment Status | 816 | |
| Full Time | 413 | 50.61% |
| Part Time | 82 | 10.05% |
| Other | 321 | 39.34% |
| COVID-19 Vaccine Hesitancy | 867 | |
| A Little Hesitant | 448 | 51.67% |
| Somewhat Hesitant | 269 | 31.03% |
| Very Hesitant | 150 | 17.30% |
| Very Likely | Somewhat Likely | Not too Likely | Not at All Likely | PNA * | |
|---|---|---|---|---|---|
| Health Care Provider | 558 (64.88%) | 210 (24.42%) | 54 (6.28%) | 23 (2.67%) | 15 (1.74%) |
| CDC | 444 (51.87%) | 251 (29.32%) | 89 (10.40%) | 55 (6.43%) | 17 (1.99%) |
| Family | 368 (42.64%) | 318 (36.85%) | 130 (15.06%) | 35 (4.06%) | 12 (1.39%) |
| Health Department | 352 (40.88%) | 331 (38.44%) | 106 (12.31%) | 56 (6.50%) | 16 (1.86%) |
| Pharmacist | 326 (37.95%) | 274 (31.90%) | 164 (19.09%) | 77 (8.96%) | 18 (2.10%) |
| Religious Leader | 162 (18.86%) | 176 (20.49%) | 173 (20.14%) | 320 (37.25%) | 28 (3.26%) |
| Social Media | 129 (15.00%) | 153 (17.79%) | 222 (25.81%) | 337 (39.19%) | 19 (2.21%) |
| Primary Themes | Subthemes |
|---|---|
| Trusted sources of information (92.5%) | Health care/Medical science (50.2%) Health care providers Academic medicine and health science research Local medical institutions Health care providers they know personally (i.e., PCP, family member, friends) Public health organizations and leaders CDC Arkansas Department of Health Dr. Fauci |
| Personal relationships (21.8%) Family and friends Vaccinated individuals Religious institutions and leaders Employer and coworkers | |
| News and social media (16.4%) News media Internet | |
| Individual/Myself (4.2%) Trusted my gut Did my own research | |
| Do not trust any sources of information (7.5%) | Do not trust any sources Lack of trust in traditional sources News media Government |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purvis, R.S.; Hallgren, E.; Moore, R.A.; Willis, D.E.; Hall, S.; Gurel-Headley, M.; McElfish, P.A. Trusted Sources of COVID-19 Vaccine Information among Hesitant Adopters in the United States. Vaccines 2021, 9, 1418. https://doi.org/10.3390/vaccines9121418
Purvis RS, Hallgren E, Moore RA, Willis DE, Hall S, Gurel-Headley M, McElfish PA. Trusted Sources of COVID-19 Vaccine Information among Hesitant Adopters in the United States. Vaccines. 2021; 9(12):1418. https://doi.org/10.3390/vaccines9121418
Chicago/Turabian StylePurvis, Rachel S., Emily Hallgren, Ramey A. Moore, Don E. Willis, Spencer Hall, Morgan Gurel-Headley, and Pearl A. McElfish. 2021. "Trusted Sources of COVID-19 Vaccine Information among Hesitant Adopters in the United States" Vaccines 9, no. 12: 1418. https://doi.org/10.3390/vaccines9121418
APA StylePurvis, R. S., Hallgren, E., Moore, R. A., Willis, D. E., Hall, S., Gurel-Headley, M., & McElfish, P. A. (2021). Trusted Sources of COVID-19 Vaccine Information among Hesitant Adopters in the United States. Vaccines, 9(12), 1418. https://doi.org/10.3390/vaccines9121418






