Protection of Vaccine Preventable Diseases in a Population of HIV-Infected Children: A 3 Years Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Seroprevalence Study
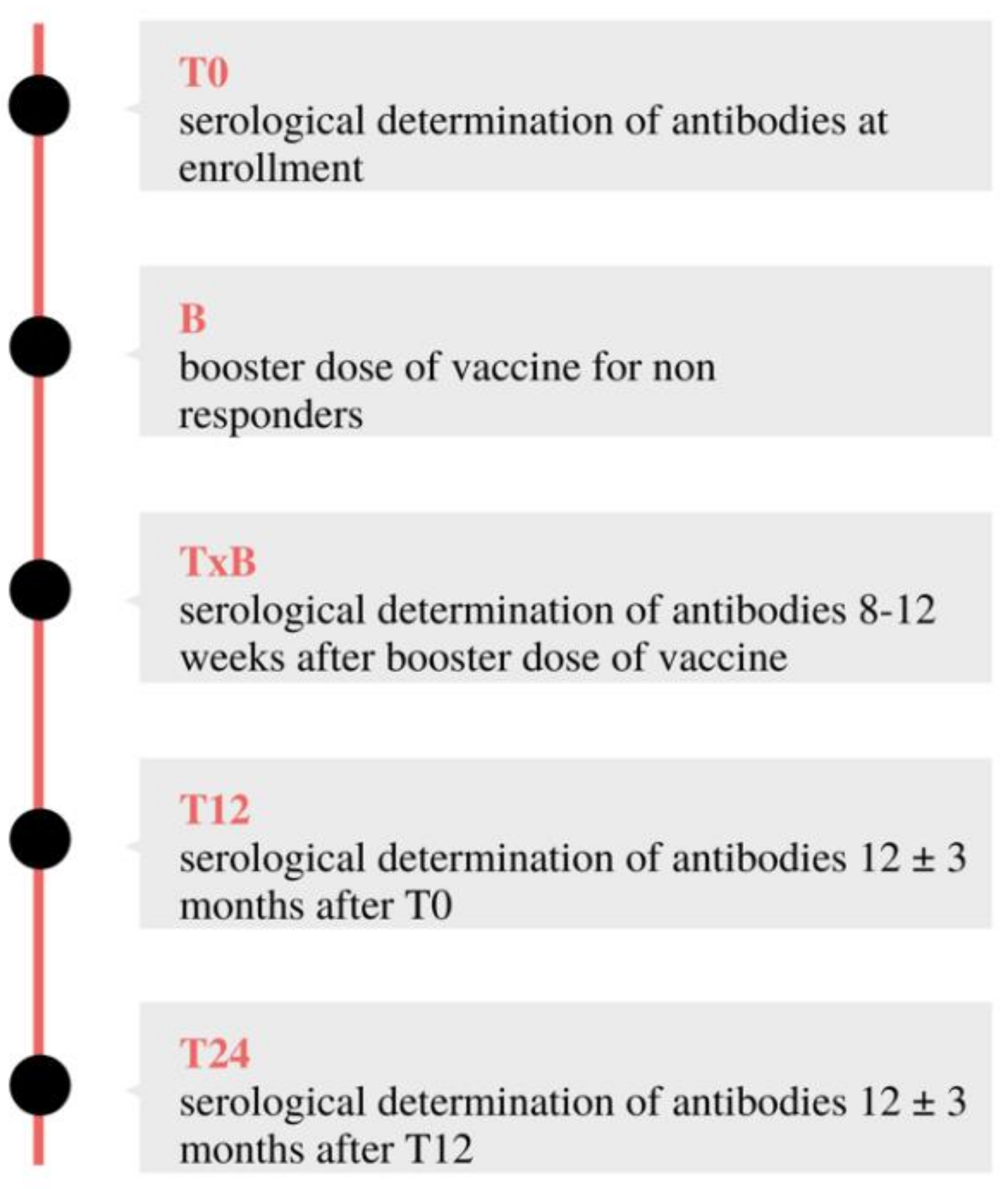
2.2. Prospective Cohort Study
- T12: 12 ± three months after T0
- T24: 12 ± three months after T12
2.3. Data Collection and Laboratory Testing
- anti-measles IgG: AU/mL <13.5: Negative; 13.5–16.5: Undetermined; >16.5: Positive.
- anti-mumps IgG: AU/mL <9: Negative; 9–11: Undetermined; >11: Positive.
- anti-rubella IgG: IU/mL <7: Negative; 7–10: Undetermined; >10: Positive.
- anti-HBV IgG: mIU/mL <10: Negative; >10: Positive.
2.4. Sample Size and Statistical Analysis
3. Results
3.1. Population Description
3.2. Seroprotection Rate in HIV Infected and Uninfected Children
3.3. Evaluation of Vaccine Booster Dose Effect in HIV Infected Patients
3.4. Long Term Follow-Up of Seroprotective Rate in HIV Infected Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Progress toward global measles control and elimination, 1990–1996. MMWR 1997, 46, 893–897. [Google Scholar]
- Centers for Disease Control and Prevention. Update: Global measles control and mortality reduction worldwide 1991–2001. MMWR 2003, 52, 471–475. [Google Scholar]
- Shapiro, E.D.; Vazquez, M.; Esposito, D.; Holabird, N.; Steinberg, S.P.; Dziura, J.; La Russa, P.S.; Gershon, A.A. Effectiveness of 2 doses of varicella vaccine in children. J. Infect. Dis. 2011, 203, 312–315. [Google Scholar] [CrossRef]
- Bhattacharya, S.D.; Bhattacharyya, S.; Chatterjee, D.; Niyogi, S.; Chauhan, N.; Sudar, A. Risk factors for incomplete immunization in children with HIV infection. Indian J. Pediatr. 2014, 81, 850. [Google Scholar] [CrossRef] [PubMed]
- Tchidjou, H.K.; Sobze, M.S.; Souleyman, A.; Stefanelli, P.; Mbabia, A.; Moussa, I.; Gentile, B.; Colizzi, V.; Rezza, G. Low vaccine coverage among children born to HIV infected women in Niamey, Niger. Hum. Vaccin Immunother. 2016, 12, 540–544. [Google Scholar] [CrossRef]
- Shen, R.; Wang, A.; Pan, X.; Qiao, Y.; Wang, Q.; Wang, X.; Qu, S.; Zhang, T. Levels of vaccination coverage among HIV-exposed children in China: A retrospective study. Infect. Dis. Poverty 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Slogrove, A.L.; Goetghebuer, T.; Cotton, M.F.; Singer, J.; Bettinger, J.A. Pattern of infectious morbidity in HIV-exposed uninfected infants and children. Front. Immunol. 2016, 7, 164. [Google Scholar] [CrossRef]
- Bekker, V.; Scherpbier, H.; Pajkrt, D.; Jurriaans, S.; Zaaijer, H.; Kuijpers, T.W. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: Loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics 2006, 118, e315–e322. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Iwajomo, O.H.; Moons, P.; Nkhata, R.; Mzinza, D.; Ogunniyi, A.D.; Williams, N.A.; Heyderman, R.; Finn, A. Delayed reconstitution of B cell immunity to pneumococcus in HIV-infected Malawian children on antiretroviral therapy. J. Infect. 2015, 70, 616–623. [Google Scholar] [CrossRef]
- Moir, S.; Buckner, C.M.; Ho, J.; Wang, W.; Chen, J.; Waldner, A.J.; Posada, J.G.; Kardava, L.; O’Shea, M.A.; Kottilil, S.; et al. B cells in early and chronic HIV infection: Evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood 2010, 116, 5571–5579. [Google Scholar] [CrossRef]
- Moss, W.J.; Fisher, C.; Scott, S. HIV type 1 infection is a risk factor for mortality in hospitalized Zambian children with measles. Clin. Infect. Dis. 2008, 46, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Su, J.R.; Ng, C.; Lewis, P.W.; Cano, M.V. Cano Adverse events after vaccination among HIV positive persons, 1990–2016. PLoS ONE 2018, 13, e0199229. [Google Scholar] [CrossRef]
- Jallow, S.; Cutland, C.L.; Masbou, A.K.; Adrian, P.; Madhi, S.A. Maternal HIV infection associated with reduced transplacental transfer of measles antibodies and increased susceptibility to disease. J. Clin. Virol. 2017, 94, 50–56. [Google Scholar] [CrossRef]
- Sutcliffe, C.G.; Moss, W.J. Do children infected with HIV receiving HAART need to be revaccinated? Lancet Infect. Dis. 2010, 10, 630–642. [Google Scholar] [CrossRef]
- Helfand, R.F.; Witte, D.; Fowlkes, A. Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi. J. Infect. Dis. 2008, 198, 1457–1465. [Google Scholar] [CrossRef]
- Stermole, B.M.; Grandits, G.A.; Roediger, M.P.; Clark, B.M.; Ganesan, A.; Weintrob, A.C.; Crum-Cianflone, N.F.; Ferguson, T.M.; Macalino, G.E.; Landrum, M.L. Long-term safety and serologic response to measles, mumps, and rubella vaccination in HIV-1 infected adults. Vaccine 2011, 29, 2874–2880. [Google Scholar] [CrossRef]
- Chaiwarith, R.; Praparattanapan, J.; Nuket, K.; Kotarathitithum, W.; Supparatpinyo, K. Seroprevalence of antibodies to measles, mumps, and rubella, and serologic responses after vaccination among human immunodeficiency virus (HIV)-1 infected adults in Northern Thailand. BMC Infect. Dis. 2016, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Simani, O.E.; Izu, A.; Nunes, M.C.; Violari, A.; Cotton, M.F.; Van Niekerk, N.; Adrian, P.V.; Madhi, S.A. Effect of HIV exposure and timing of antiretroviral therapy initiation on immune memory responses to diphtheria, tetanus, whole cell pertussis and hepatitis B vaccines. Expert Rev. Vaccines 2019, 18, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Mutsaerts, E.A.; Nunes, M.C.; van Rijswijk, M.N.; Klipstein-Grobusch, K.; Grobbee, D.E.; Madhi, S.A. Safety and Immunogenicity of Measles Vaccination in HIV-Infected and HIV-Exposed Uninfected Children: A Systematic Review and Meta-Analysis. EClinicalMedicine 2018, 1, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Department of Health and Human Services. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4169043/ (accessed on 14 November 2021).
- Menson, E.N.; Mellado, M.J.; Bamford, A.; Castelli, G.; Duiculescu, D.; Marczyńska, M.; Navarro, M.; Scherpbier, H.J.; Heath, P.T.; Paediatric European Network for Treatment of AIDS (PENTA) Vaccines Group; et al. Guidance on vaccination of HIV-infected children in Europe. HIV Med. 2012, 13, 333–336. [Google Scholar] [CrossRef]
- Bayhan, G.I.; Balli, S.E.; Demir, H.; Baydar, Z. How does the immunogenicity of hepatitis B vaccine change over the years in childhood? Hum. Vaccin Immunother. 2021, 17, 2768–2772. [Google Scholar] [CrossRef]
- Simani, O.E.; Izu, A.; Violari, A.; Cotton, M.F.; Van Niekerk, N.; Adrian, P.V.; Madhi, S.A. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS 2014, 28, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Pensieroso, S.; Cagigi, A.; Palma, P.; Nilsson, A.; Capponi, C.; Freda, E.; Bernardi, S.; Thorstensson, R.; Chiodi, F.; Rossi, P. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc. Natl. Acad. Sci. USA 2009, 106, 7939–7944. [Google Scholar] [CrossRef]
- Succi, R.C.M.; Krauss, M.R.; Harris, D.R.; Machado, D.; de Moraes-Pinto, M.I.; Mussi-Pinhata, M.M.; Ruz, N.P.; Pierre, R.B.; Roca, L.A.K.; João, E.; et al. Immunity After Childhood Vaccinations in Perinatally HIV-exposed Children With and Without HIV Infection in Latin America. Pediatr. Infect. Dis. J. 2018, 37, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.A.; Matin, F. Subnormal and waning immunity to tetanus toxoid in previously vaccinated HIV-infected children and response to booster doses of the vaccine. Int. J. Infect. Dis. 2013, 17, e1249–e1251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siberry, G.K.; Patel, K.; Bellini, W.J.; Karalius, B.; Purswani, M.U.; Burchett, S.K.; Meyer, W.A.; Sowers, S.B.; Ellis, A.; Van Dyke, R.B.; et al. Immunity to Measles, Mumps, and Rubella in US children with perinatal HIV infection or perinatal HIV exposure without Infection. Clin. Infect. Dis. 2015, 61, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Adetokunboh, O.O.; Awotiwon, A.; Ndwandwe, D.; Uthman, O.A.; Wiysonge, C.S. The burden of vaccine-preventable diseases among HIV- infected and HIV-exposed children in sub-Saharan Africa: A systematic review and meta-analysis. Hum. Vaccines Immunother. 2019, 15, 2590–2605. [Google Scholar] [CrossRef]


| Characteristic | All 63 (100) | HIV-Uninfected 37 (58.73) | HIV-Infected 26 (41.27) | |
|---|---|---|---|---|
| Sex n (%) | Female | 34 (53.97) | 18 (48.65) | 16 (61.54) |
| Male | 29 (46.03) | 19 (51.35) | 10 (38.46) | |
| Age, y; median (range) | 10.08 (1.68–22.20) | 7.09 (2.00–17.38) | 13.89 (1.82–22.20) | |
| Country of origin n (%) | Italy | 55 (87.30) | 35 (94.59) | 20 (76.92) |
| Other, EU | 1 (1.59) | 0 (0) | 1 (3.85) | |
| Other, non-EU | 7 (11.11) | 2 (5.41) | 5 (19.23) |
| Characteristic | HIV-Infected (26) |
|---|---|
| HIV disease duration, months; median (range) | 18 (1–151) |
| CD4 cells/mL; median (range) | 795 (144–2358) |
| Patients with HIV RNA <40 copies/mL; n (%) | 23 (88) |
| ART; n (%) | 26 (100) |
| 2NRTI + IP | 15 (57.69) |
| 2NRTI + IT | 8 (30.77) |
| 2NRTI + 1NNRTI | 1 (3.85) |
| 1NNRTI + 1IP | 2 (7.69) |
| Vertical transmission route; n (%) | 26 (100%) |
| Vaccine | |||
|---|---|---|---|
| HIV-Uninfected 37 | HIV-Infected 26 | p | |
| Measles | |||
| IgG positive %, n | 83.8 (31) | 65 (17) | 0.1 |
| Mumps | |||
| IgG positive %, n | 78.4 (29) | 61 (16) | 0.1 |
| Rubella | |||
| IgG positive %, n | 92 (34) | 65 (17) | 0.02 |
| Hepatitis B | |||
| IgG positive %, n | 78.4 (29) | 45.4 (10) 1 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruzzese, E.; Pagano, F.; Diana, A.; Punzi, L.; Guarino, A. Protection of Vaccine Preventable Diseases in a Population of HIV-Infected Children: A 3 Years Prospective Study. Vaccines 2021, 9, 1331. https://doi.org/10.3390/vaccines9111331
Bruzzese E, Pagano F, Diana A, Punzi L, Guarino A. Protection of Vaccine Preventable Diseases in a Population of HIV-Infected Children: A 3 Years Prospective Study. Vaccines. 2021; 9(11):1331. https://doi.org/10.3390/vaccines9111331
Chicago/Turabian StyleBruzzese, Eugenia, Federica Pagano, Alfredo Diana, Liana Punzi, and Alfredo Guarino. 2021. "Protection of Vaccine Preventable Diseases in a Population of HIV-Infected Children: A 3 Years Prospective Study" Vaccines 9, no. 11: 1331. https://doi.org/10.3390/vaccines9111331
APA StyleBruzzese, E., Pagano, F., Diana, A., Punzi, L., & Guarino, A. (2021). Protection of Vaccine Preventable Diseases in a Population of HIV-Infected Children: A 3 Years Prospective Study. Vaccines, 9(11), 1331. https://doi.org/10.3390/vaccines9111331





