A Systematic Review of the Sex and Gender Reporting in COVID-19 Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. General Principles of Sex and Gender Terminology
3.2. Recognizing the Importance of Sex and Gender in Abstracts and Introductions
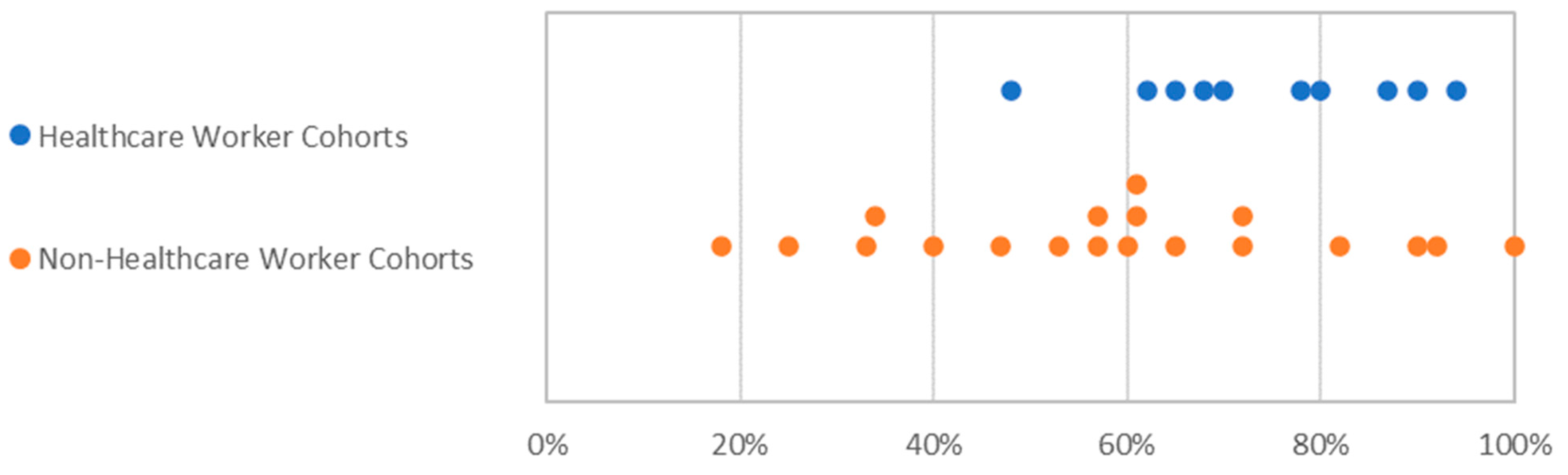
3.3. Gender Balance in Clinical Trials
3.4. Pregnant Women
3.5. Trial Design
3.6. Sex-Disaggregation of Data
3.7. Sex- and Gender-Based Analysis
3.8. Sex and Gender Implications in the Discussion Section
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Boon, A.; Michelson, A.; Foraker, R.; Zhan, M.; Payne, P. Estrogen hormone is an essential sex factor inhibiting in ammation and immune response. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, R.; Klein, S.L. The intersection of sex and gender in the treatment of influenza. Curr. Opin. Virol. 2019, 35, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Voigt, E.A.; Ovsyannikova, I.G.; Kennedy, R.B.; Grill, D.E.; Goergen, K.M.; Schaid, D.J.; Poland, G.A. Sex differences in older adults’ immune responses to seasonal influenza vaccination. Front. Immunol. 2019, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Somiya, M.; Mine, S.; Yasukawa, K.; Ikeda, S. Sex differences in the incidence of anaphylaxis to LNP-mRNA COVID-19 vaccines. Vaccine 2021, 39, 3313–3314. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S.; Abdullah, R.; Vered, S.; Nitzan, D. A study of ethnic, gender and educational differences in attitudes toward COVID-19 vaccines in Israel—Implications for vaccination implementation policies. Isr. J. Health Policy Res. 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Latkin, C.A.; Dayton, L.; Yi, G.; Colon, B.; Kong, X. Mask usage, social distancing, racial, and gender correlates of COVID-19 vaccine intentions among adults in the US. PLoS ONE 2021, 16, e0246970. [Google Scholar] [CrossRef]
- Heidari, S.; Durrheim, D.N.; Faden, R.; Kochhar, S.; MacDonald, N.; Olayinka, F.; Goodman, T.S. Time for action: Towards an intersectional gender approach to COVID-19 vaccine development and deployment that leaves no one behind. BMJ Glob. Health 2021, 6, e006854. [Google Scholar] [CrossRef]
- Palmer-Ross, A.; Ovseiko, P.V.; Heidari, S. Inadequate reporting of COVID-19 clinical studies: A renewed rationale for the Sex and Gender Equity in Research (SAGER) guidelines. BMJ Glob. Health 2021, 6, e004997. [Google Scholar] [CrossRef]
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and Gender Equity in Research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Curno, M.J.; Rossi, S.; Hodges-Mameletzis, I.; Johnston, R.; Price, M.; Heidari, S. A systematic review of the inclusion (or exclusion) of women in HIV research: From clinical studies of antiretrovirals and vaccines to cure strategies. J. Acquir. Immune Defic. Syndr. 2016, 71, 181–188. [Google Scholar] [CrossRef]
- Easy Fisher Exact Test Calculator. 2021. Available online: https://www.socscistatistics.com/tests/fisher/default2.aspx (accessed on 3 June 2021).
- WHO. Draft Landscape and Tracker of COVID-19 Candidate Vaccines; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Juster, R.-P.; Lupien, S. What a Difference Sex and Gender Make; Ottawa, ON, Canada, 2012; Available online: https://cihr-irsc.gc.ca/e/documents/What_a_Difference_Sex_and_Gender_Make-en.pdf (accessed on 8 November 2021).
- Richie, C. Sex, not gender. A plea for accuracy. Exp. Mol. Med. 2019, 51, 1. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef] [PubMed]
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. 2021, 27, 790–792. [Google Scholar] [CrossRef] [PubMed]
- European Union. European Comission—Funding and tender opportunities. Gender Equality. 2020. Available online: https://ec.europa.eu/research/participants/docs/h2020-funding-guide/cross-cutting-issues/gender_en.htm (accessed on 8 August 2021).
- Yakerson, A. Women in clinical trials: A review of policy development and health equity in the Canadian context. Int. J. Equity Health 2019, 18, 56. [Google Scholar] [CrossRef] [Green Version]
- NIH. NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. In Policy & Compliance; 2017. Available online: https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm (accessed on 15 September 2021).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Gajula, V.; Madathala, R.R.; Chennaiahgari, N.; Malayala, S.V. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J. Med. Virol. 2021, 93, 4420–4429. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Padoan, A.; Dall’Olmo, L.; della Rocca, F.; Barbaro, F.; Cosma, C.; Basso, D.; Cattelan, A.; Cianci, V.; Plebani, M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta 2021, 519, 60–63. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021, 21, 950–961. [Google Scholar] [CrossRef]
- Ording, A.; Cronin-Fenton, D.; Ehrenstein, V.; Lash, T.; Acquavella, J.; Rørth, M.; Sørensen, H.T. Challenges in translating end points from trials to observational cohort studies in oncology. Clin. Epidemiol. 2016, 8, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Gartlehner, G.; Hansen, R.; Nissman, D.; Lohr, K.; Carey, T.S. Criteria for distinguishing effectiveness from efficacy trials in systematic reviews. Agency Healthc. Res. Qual. 2006, 12, 1–28. [Google Scholar]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Ríos, S.S.; Romero, M.M.; Zamora, E.B.C.; Sahuquillo, M.T.T.; Rizos, L.R.; Sánchez-Jurado, P.M.; Sánchez-Nievas, G.; Señalada, J.J.B.; Nogueras, I.G.; Cazalla, J.D.D.E.; et al. Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study. J. Am. Geriatr. Soc. 2021, 69, 1441–1447. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021, 325, 1784. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. Am. J. Transplant. 2021, 21, 1332–1337. [Google Scholar] [CrossRef]
- Lacson, E.; Argyropoulos, C.P.; Manley, H.J.; Aweh, G.; Chin, A.I.; Salman, L.H.; Hsu, C.M.; Johnson, D.S.; Weiner, D.E. Immunogenicity of SARS-CoV-2 vaccine in dialysis. medRxiv 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, 14–23 December 2020. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm (accessed on 8 November 2021).
- Ou, M.T.; Boyarsky, B.J.; Motter, J.D.; Greenberg, R.S.; Teles, A.T.; Ruddy, J.A.; Krach, M.R.; Jain, V.S.; Werbel, W.A.; Avery, R.K.; et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation 2021, 105, 2170–2174. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv-Baran, T.; Arbel, Y.S.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8061088/ (accessed on 8 November 2021). [CrossRef] [PubMed]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef]
- Avery, E.; Clark, J. Sex-related reporting in randomised controlled trials in medical journals. Lancet 2016, 388, 2839–2840. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Fink, A.; Klein, S. Sex and gender impact immuneresponses to vaccines among the elderly. Physiology 2015, 30, 408–416. [Google Scholar] [CrossRef]
- Engler, R.J.M.; Nelson, M.R.; Klote, M.M.; VanRaden, M.J.; Huang, C.-Y.; Cox, N.J.; Klimov, A.; Keitel, W.A.; Nichol, K.L.; Carr, W.W.; et al. Half- vs. full-dose trivalent inactivated influenza vaccine (2004–2005). Arch. Intern. Med. 2008, 168, 2405–2414. [Google Scholar] [CrossRef]
- Bischof, E.; Wolfe, J.; Klein, S.L. Clinical trials for COVID-19 should include sex as a variable. J. Clin. Investig. 2020, 130, 3350–3352. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, V.M.; Janssen, E.B.; van Bussel, B.C.; Jorissen, L.L.; Tas, J.; Sels, J.-W.E.; Bergmans, D.C.; Dinh, T.H.; van Kuijk, S.M.; Hana, A.; et al. The “sex gap” in COVID-19 trials: A scoping review. EClinicalMedicine 2020, 29–30, 100652. [Google Scholar] [CrossRef] [PubMed]
- Brady, E.; Nielsen, M.W.; Andersen, J.P.; Oertelt-Prigione, S. Lack of consideration of sex and gender in COVID-19 clinical studies. Nat. Commun. 2021, 12, 4015. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Sze, S.; Minhas, J.; Bangash, M.N.; Pareek, N.; Divall, P.; Williams, C.M.; Oggioni, M.R.; Squire, I.B.; Nellums, L.; et al. The impact of ethnicity on clinical outcomes in COVID-19: A systematic review. EClinicalMedicine 2020, 23, 100404. [Google Scholar] [CrossRef] [PubMed]
- EudraVigilance. European Database of Suspected Adverse Drug Reaction Reports. Available online: http://www.adrreports.eu/en/index.html. (accessed on 12 April 2021).
- Centers for Disease Control and Prevention. Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines Among Adolescents and Young Adults. In Vaccines and Immunizations; 2021. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html (accessed on 27 August 2021).
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ 2020, 369, m2107. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. COVID Unlocking Will Create ‘Perfect Storm’ for Pregnant Women, Say Maternity Colleges. 2021. Available online: https://www.rcog.org.uk/en/news/covid-unlocking-will-create-perfect-storm-for-pregnant-women-say-maternity-colleges/ (accessed on 8 September 2021).
- Campbell, D. One in Six Most Critically Ill NHS Covid Patients Are Unvaccinated Pregnant Women. The Guardian. 11 October 2021. Available online: https://www.theguardian.com/lifeandstyle/2021/oct/11/one-in-six-most-critically-ill-patients-are-unvaccinated-pregnant-women-with-covid (accessed on 12 October 2021).
- Haverfield, J.; Tannenbaum, C. A 10-year longitudinal evaluation of science policy interventions to promote sex and gender in health research. Health Res. Policy Syst. 2021, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Canadian Institutes of Health Research. Science Is Better with Sex and Gender. Strategic Plan 2018–2023. 2020. Available online: https://cihr-irsc.gc.ca/e/51310.html (accessed on 27 August 2021).
- Bignucolo, A.; Scarabel, L.; Mezzalira, S.; Polesel, J.; Cecchin, E.; Toffoli, G. Sex Disparities in efficacy in COVID-19 vaccines: A systematic review and meta-analysis. Vaccines 2021, 9, 825. [Google Scholar] [CrossRef]
- Bueno, S.M.; Abarca, K.; González, P.A.; Gálvez, N.M.; Soto, J.A.; Duarte, L.F.; Schultz, B.M.; Pacheco, G.A.; González, L.A.; Vázquez, Y.; et al. Interim Report: Safety and Immunogenicity of an Inactivated Vaccine. 2021. (in press) [Google Scholar]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.J.; Bibi, S. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 VOC 202012/01 (B.1.1.7). SSRN Electron. J. 2021, 19, 1–40. [Google Scholar] [CrossRef]
- Simonson, M.; Perlis, R.H.; Baum, M.; Lazer, D.; Druckman, J.; Santillana, M.; Della Volpe, J. The Covid states project: A 50-state COVID-19 survey report # 47: Update on COVID-19 vaccine attitudes among healthcare workers. OSF Prepr. 2021. Available online: https://osf.io/a352z/ (accessed on 9 September 2021).
- Fairleigh Dickinson University. FDU Poll Finds Masculinity Is a Major Risk Factor for COVID-19. 2021. Available online: https://www.fdu.edu/news/fdu-poll-finds-masculinity-is-a-major-risk-factor-for-covid-19/ (accessed on 28 August 2021).
- Male, V. Menstrual changes after covid-19 vaccination. BMJ 2021, 374, 2211. [Google Scholar] [CrossRef]


| Citation | Phase | Primary End-Points * | Outcome Data by Sex ** | Vaccine | Quotes |
|---|---|---|---|---|---|
| Ewer et al., 2021 [26] | Phase I/II | Efficacy and safety | Immune response | ChAdOx1 nCoV-19 (AZD1222) | “We found no sex difference in vaccine response at any of the time points measured” |
| Zhu et al., 2021 [27] | Phase II | Adverse reactions and antibody response | Antibody response | Adenovirus type-5 (Ad5)-vectored | “Sex and age of the participants did not differentiate their IFNγ-ELISpot T-cell responses induced by vaccination” |
| Logunov et al., 2021 [28] | Phase III | Efficacy | Immunogenicity | rAd26 and rAd5 vector-based heterologous prime-boost | “Antibody levels did not differ significantly between men (n = 179) and women (n = 159; p = 0.258).” |
| Baden et al., 2021 [29] | Phase III | Efficacy and safety | Efficacy | mRNA-1273 | “The vaccine efficacy to prevent COVID-19 was consistent across subgroups stratified by demographic and baseline characteristics: age groups (18 to <65 years of age and ≥65 years), presence of risk for severe COVID-19, sex […]” |
| Polack et al., 2020 [24] | Phase III | Efficacy and adverse events | Efficacy | BNT162b2 mRNA | “Similar vaccine efficacy (generally 90–100%) was observed across subgroups defined by age, sex, race, ethnicity, […]” |
| Ella et al., 2021 [30] | Phase I/II | Efficacy and adverse events | Efficacy | BBV152 | “Seroconversion rates and GMTs across three age groups (≥12 to <18 years, ≥18 to <55 years, and ≥55 to ≤65 years) and between both sexes were similar, but only small numbers of participants were included in the youngest and oldest age groups“ |
| Sadoff et al., 2021 [16] | Phase III | Efficacy | Efficacy | Ad26.COV2.S | “No meaningful differences in vaccine efficacy were observed among subgroups defined according to sex, race, or ethnicity.” |
| Citation | Objective | Primary Outcome | Quotes | Statistically Significant Difference? |
|---|---|---|---|---|
| McMahon et al., 2021 [33] | Cutaneous reactions to vaccine | Adverse events | “Ninety per cent of the vaccine reactions were reported in female patients.” | Yes |
| Greinacher et al., 2021 [34] | Thrombotic thrombocytopenia after vaccination | Adverse events | “Among these patients, the median age was 36 years (range, 22–49); 9 of 11 were women. All the patients presented with concomitant thrombocytopenia (median nadir of platelet count, approximately 20,000 per cubic millimetre; range, 9000–107,000).” | Yes |
| Salmerón Ríos et al., 2021 [35] | Vaccine efficacy in frail or disabled nursing home residents | Effectiveness | “Frailty, disability, older age, sex, cognitive impairment, and comorbidities were not associated with different antibody titres.” | No |
| Boyarsky et al., 2021 [36] | Immunogenicity in solid organ transplant patients | Effectiveness | “The immune response to the vaccine by sex was found to have a p value = 0.60 and therefore sex is not statistically significant.” | No |
| Shimabukuro, 2021 [37] | Allergic reactions after the Moderna vaccine (December–January) | Adverse events | “The clinical and epidemiological characteristics of anaphylaxis case reports after receipt of the Moderna COVID-19 vaccine are similar to those reported after receipt of the Pfizer-BioNTech COVID-19 vaccine (5). […] A strong female predominance of anaphylaxis case reports exists for both vaccines.” | Yes |
| Lacson et al., 2021 [38] | Immunogenicity in patients undergoing dialysis | Effectiveness | “Factors associated with poor seroconversion in our cohort include female sex, younger vintage, potential immunosuppression from diseases, transplant, or medications, [Congestive Heart Failure], and covaccination and hospitalization during the peri-vaccination period.” | Yes |
| Shimabukuro, 2021 [39] | Allergic reactions after the Pfizer-BioNTech vaccine (December only) | Adverse events | “A strong female predominance of anaphylaxis case reports exists for both vaccines.” | Yes |
| Ou et al., 2021 [40] | Immunogenicity in solid organ transplant patients | Adverse events | “Females were more likely to experience systemic symptoms after either dose.” | Yes |
| Street et al., 2021 [41] | Efficacy in patients with chronic lymphocytic leukaemia | Effectiveness | “In a univariate analysis (this table), the variables found to be significantly associated with response included: younger age (≤65 years), female sex, early disease stage (Binet stage A), mutated IGHV, b2-microglobulin (≤3.5 mg/L), untreated/off-therapy ≤ 12 months from the last anti-CD20 therapy, IgG levels ≤ 550 mg/dL, IgM levels ≤ 40 mg/dL, and IgA levels ≤ 80 mg/dL.” | Yes |
| Padoan et al., 2021 [25] | Antibody response in a cohort of characterized healthcare workers | Effectiveness | “No significant anti-S-RBD level differences were found between males and females in any of the studied conditions.” | No |
| Scheme | No. of Interventional Vaccine Trials Complying with the Recommendation | No. of Observational Vaccine Trials Complying with the Recommendation | Overall % |
|---|---|---|---|
| 1. Introduction: Sex and gender differences in the infection, manifestation, or outcomes of COVID-19 should be acknowledged in the introduction. | 0/42 | 2/33 | 3% |
| (2a) Methodology: Papers should report how sex and gender were taken into account in the design of the study. | 0/42 | 2/33 | 3% |
| (2b) Methodology: Papers should justify reasons for the exclusion, or differing numbers, of males or females. | 1/42 | 1/33 | 3% |
| (3a) Results: “Sex- and gender-based analyses should be reported regardless of positive or negative outcome” [10]. Articles should note if there is a difference between sexes or genders, or if there is no difference, in their results. | 7/41 | 10/29 | 24% |
| (3b) Results: Articles should report all their data disaggregated by sex. | 7/41 | 10/29 | 24% |
| (4) Discussion: What the results of the study mean for women and men should be analysed in the discussion section. | 2/42 | 8/33 | 13% |
| (5) Generalizability: If a sex and gender analysis is not done, this should be justified or addressed in relation to the generalizability of the results. | 2/42 | 3/33 | 7% |
| Name of Vaccine (Ref.) | Phase | Introduction | Methodology | Sex Disaggregation | Discussion | Generalizability | No. of Doses * |
|---|---|---|---|---|---|---|---|
| CoronaVac [59] | III | No | No | No | No | No | 367 million |
| AstraZeneca ** [60] | II/III | No | No | No | No | No | 3009 million |
| Sputnik V [28] | III | No | No | Yes (immunogenicity) | No | No | 765 million |
| Moderna [29] | III | No | No | Yes (efficacy) | No | No | 816 million |
| Pfizer-BioNTech [24] | III | No | No | Yes (efficacy) | No | Yes | 1220 million |
| Janssen [16] | III | No | No | Yes (efficacy) | No | No | 368 million |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, S.; Palmer-Ross, A.; Goodman, T. A Systematic Review of the Sex and Gender Reporting in COVID-19 Clinical Trials. Vaccines 2021, 9, 1322. https://doi.org/10.3390/vaccines9111322
Heidari S, Palmer-Ross A, Goodman T. A Systematic Review of the Sex and Gender Reporting in COVID-19 Clinical Trials. Vaccines. 2021; 9(11):1322. https://doi.org/10.3390/vaccines9111322
Chicago/Turabian StyleHeidari, Shirin, Alice Palmer-Ross, and Tracey Goodman. 2021. "A Systematic Review of the Sex and Gender Reporting in COVID-19 Clinical Trials" Vaccines 9, no. 11: 1322. https://doi.org/10.3390/vaccines9111322
APA StyleHeidari, S., Palmer-Ross, A., & Goodman, T. (2021). A Systematic Review of the Sex and Gender Reporting in COVID-19 Clinical Trials. Vaccines, 9(11), 1322. https://doi.org/10.3390/vaccines9111322






