Prevalence of Pneumococcal Carriage among Jordanian Infants in the First 6 Months of Age, 2008–2016
Abstract
1. Introduction
2. Materials and Methods
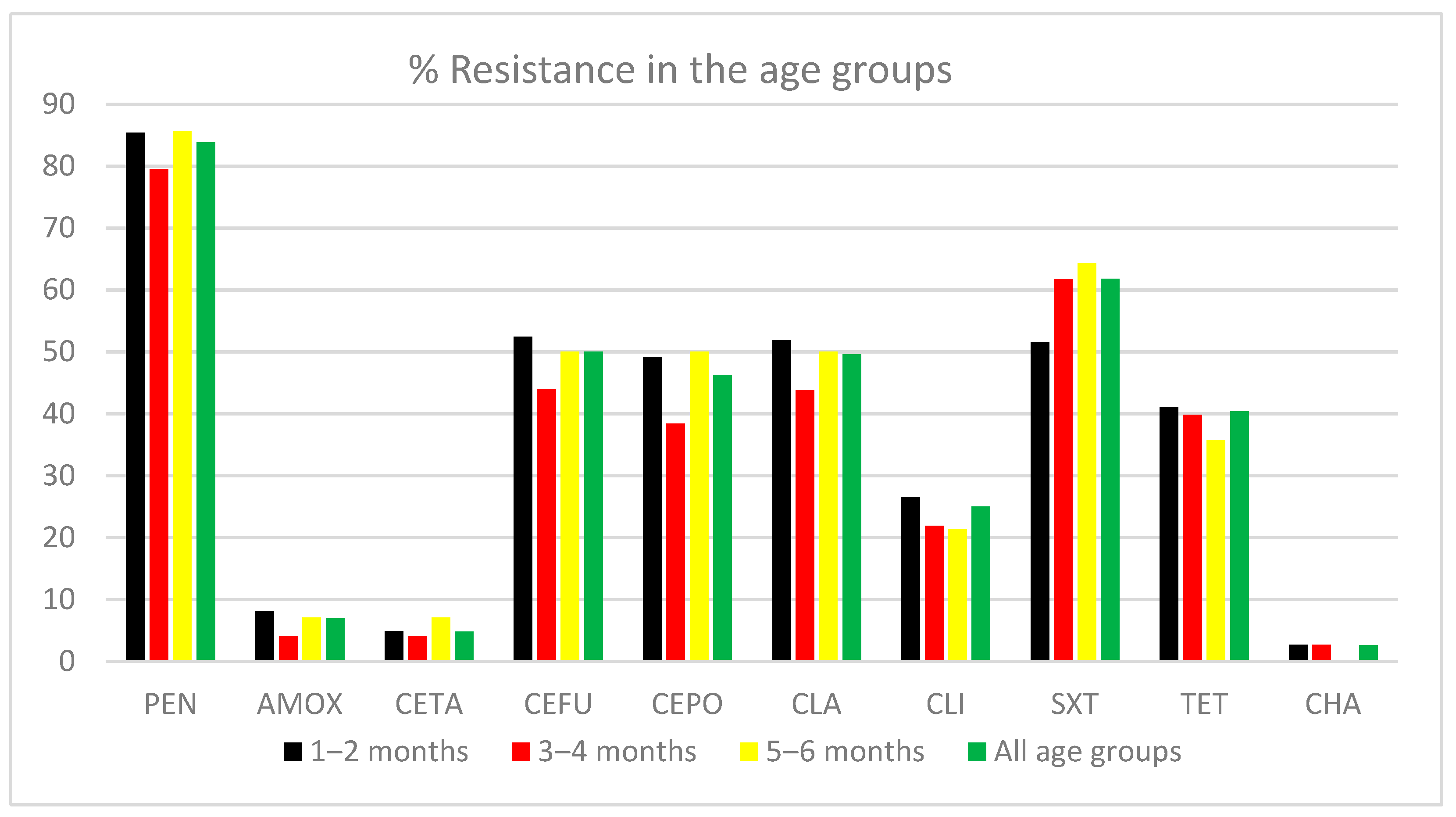
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marangu, D.; Zar, H.J. Childhood pneumonia in low-and-middle-income countries: An update. Paediatr. Respir. Rev. 2019, 32, 3–9. [Google Scholar] [CrossRef]
- Mulholland, K. Childhood pneumonia mortality-a permanent global emergency. Lancet 2007, 21, 285–289. [Google Scholar] [CrossRef]
- Al-Lahham, A.; Van der Linden, M. Streptococcus pneumoniae carriage, resistance and serotypes among Jordanian children from Wadi Al Seer District, JORDAN. Int. Arab. J. Antimicrob. Agents 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Schaumburg, F.; Alabi, A.; von Eiff, C.; Flamen, A.; Traore, H.; Grobusch, M.P.; Peters, G.; Kremsner, P.G.; van der Linden, M. Streptococcus pneumoniae colonization in remote African Pygmies. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 105–109. [Google Scholar] [CrossRef]
- Simell, B.; Auranen, K.; Kayhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L.; Pneumococcal Carriage, G. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef]
- Bogaert, D.; De Groot, R.; Hermans, P.W. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Al-Lahham, A. Multicenter study of pneumococcal carriage in children 2 to 4 years of age in the winter seasons of 2017–2019 in Irbid and Madaba governorates of Jordan. PLoS ONE 2020, 15, e0237247. [Google Scholar] [CrossRef]
- Shak, J.R.; Vidal, J.E.; Klugman, K.P. Influence of bacterial interactions on pneumococcal colonization of the nasopharynx. Trends Microbiol. 2013, 21, 129–135. [Google Scholar] [CrossRef]
- Ekdahl, K.; Ahlinder, I.; Hansson, H.B.; Melander, E.; Molstad, S.; Soderstrom, M.; Persson, K. Duration of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae: Experiences from the South Swedish Pneumococcal Intervention Project. Clin. Infect. Dis. 1997, 25, 1113–1117. [Google Scholar] [CrossRef]
- Otsuka, T.; Chang, B.; Shirai, T.; Iwaya, A.; Wada, A.; Yamanaka, N.; Okazaki, M.; SADO-Study Working Group. Individual Risk Factors Associated With Nasopharyngeal Colonization With Streptococcus pneumoniae and Haemophilus influenzae: A Japanese Birth Cohort Study. Pediatr. Infect. Dis. J. 2013, 32, 709–714. [Google Scholar] [CrossRef]
- Collaborators, G.L.R.I. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systemic analysis for the global burden of disease study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Wardlaw, T.M.; Johansson, E.W.; Hodge, M.J. Pneumonia: The Forgotten Killer of Children; World Health Organization, UNICEF, Eds.; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Croucher, N.J.; Lochen, A.; Bentley, S.D. Pneumococcal Vaccines: Host Interactions, Population Dynamics, and Design Principles. Annu. Rev. Microbiol. 2018, 72, 521–549. [Google Scholar] [CrossRef]
- Ganaie, F.; Saad, J.S.; McGee, L.; van Tonder, A.J.; Bentley, S.D.; Lo, S.W.; Gladstone, R.A.; Turner, P.; Keenan, J.D.; Breiman, R.F.; et al. A New Pneumococcal Capsule Type, 10D, is the 100th Serotype and Has a Large cps Fragment from an Oral Streptococcus. mBio 2020, 11. [Google Scholar] [CrossRef]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef]
- Jacobs, M.R.; Dagan, R. Antimicrobial resistance among pediatric respiratory tract infections: Clinical challenges. Semin. Pediatr. Infect. Dis. 2004, 15, 5–20. [Google Scholar] [CrossRef]
- Pallares, R.; Fenoll, A.; Linares, J. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides and quinolones. Int. J. Antimicrob. Agents 2003, 22 (Suppl. S1), S15–S24, discussion S25–S16. [Google Scholar] [CrossRef]
- Schranz, J. Pneumococcal conjugate vaccines: What do we know and what do we need? Procedia Vaccinol. 2009, 1, 189–205. [Google Scholar] [CrossRef][Green Version]
- Adegbola, R.A.; DeAntonio, R.; Hill, P.C.; Roca, A.; Usuf, E.; Hoet, B.; Greenwood, B.M. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: A systematic review and meta-analysis. PLoS ONE 2014, 9, e103293. [Google Scholar] [CrossRef]
- Verani, J.R.; Massora, S.; Acacio, S.; Dos Santos, R.T.; Vubil, D.; Pimenta, F.; Moura, I.; Whitney, C.G.; Costa, M.H.; Macete, E.; et al. Nasopharyngeal carriage of Streptococcus pneumoniae among HIV-infected and -uninfected children <5 years of age before introduction of pneumococcal conjugate vaccine in Mozambique. PLoS ONE 2018, 13, e0191113. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2021; ISBN 978-1-68440-104-8. [Google Scholar]
- Malfroot, A.; Verhaegen, J.; Dubru, J.M.; Van Kerschaver, E.; Leyman, S. A cross-sectional survey of the prevalence of Streptococcus pneumoniae nasopharyngeal carriage in Belgian infants attending day care centres. Clin. Microbiol. Infect. 2004, 10, 797–803. [Google Scholar] [CrossRef]
- Al-Lahham, A.; Khanfar, N.; Albataina, N.; Al Shwayat, R.; Altwal, R.; Abulfeilat, T.; Alawneh, G.; Khurd, M.; Alqadi Altamimi, A. Urban and Rural Disparities in Pneumococcal Carriage and Resistance in Jordanian Children, 2015–2019. Vaccines 2021, 9, 789. [Google Scholar] [CrossRef]
- Dunne, E.M.; Choummanivong, M.; Neal, E.F.G.; Stanhope, K.; Nguyen, C.D.; Xeuatvongsa, A.; Satzke, C.; Sychareun, V.; Russell, F.M. Factors associated with pneumococcal carriage and density in infants and young children in Laos PDR. PLoS ONE 2019, 14, e0224392. [Google Scholar] [CrossRef]
- Huang, W.H.; Fang, S.Y. High prevalence of antibiotic resistance in isolates from the middle meatus of children and adults with acute rhinosinusitis. Am. J. Rhinol. 2004, 18, 387–391. [Google Scholar] [CrossRef]
- Gámez, G.; Rojas, J.P.; Cardona, S.; Noreña, J.D.C.; Palacio, M.A.; Mejía, L.F.; Torres, J.L.; Contreras, J.; Muñoz, L.M.; Criales, J.; et al. Factors Associated with Streptococcus pneumoniae Nasopharyngeal Carriage and Antimicrobial Susceptibility among Children Under the Age of 5 Years in the Southwestern Colombia. Thieme 2021. [Google Scholar] [CrossRef]
- Johnson, H.L.; Deloria-Knoll, M.; Levine, O.S.; Stoszek, S.K.; Freimanis Hance, L.; Reithinger, R.; Muenz, L.R.; O’Brien, K.L. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: The pneumococcal global serotype project. PLoS Med. 2010, 7. [Google Scholar] [CrossRef]
- Chaguza, C.; Senghore, M.; Bojang, E.; Lo, S.W.; Ebruke, C.; Gladstone, R.A.; Tientcheu, P.E.; Bancroft, R.E.; Worwui, A.; Foster-Nyarko, E.; et al. Carriage Dynamics of Pneumococcal Serotypes in Naturally Colonized Infants in a Rural African Setting During the First Year of Life. Front. Pediatr. 2021, 8, 587730. [Google Scholar] [CrossRef]
- Voysey, M.; Pollard, A.J.; Sadarangani, M.; Fanshawe, T.R. Prevalence and decay of maternal pneumococcal and meningococcal antibodies: A meta-analysis of type-specific decay rates. Vaccine 2017, 35, 5850–5857. [Google Scholar] [CrossRef]
- Weinberger, D.M.; Dagan, R.; Givon-Lavi, N.; Regev-Yochay, G.; Malley, R.; Lipsitch, M. Epidemiologic evidence for serotype-specific acquired immunity to pneumococcal carriage. J. Infect. Dis. 2008, 197, 1511–1518. [Google Scholar] [CrossRef]
- Darboe, M.K.; Fulford, A.J.; Secka, O.; Prentice, A.M. The dynamics of nasopharyngeal Streptococcus pneumoniae carriage among rural Gambian mother-infant pairs. BMC Infect. Dis. 2010, 10, 195. [Google Scholar] [CrossRef]
- Bosch, A.A.; van Houten, M.A.; Bruin, J.P.; Wijmenga-Monsuur, A.J.; Trzcinski, K.; Bogaert, D.; Rots, N.Y.; Sanders, E.A. Nasopharyngeal carriage of Streptococcus pneumoniae and other bacteria in the 7th year after implementation of the pneumococcal conjugate vaccine in the Netherlands. Vaccine 2016, 34, 531–539. [Google Scholar] [CrossRef]
- Gray, B.M.; Converse, G.M., 3rd; Dillon, H.C., Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: Acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 1980, 142, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Apte, A.; Dayma, G.; Nazyat, H.; Williams, I.; Sangavi, S.; Uddin, J.; Kawade, A.; Islam, M.; Kar, S.; Li, Y.; et al. Nasopharyngeal pneumococcal carriage in South Asian infants: Results of observational cohort studies in vaccinated and unvaccinated populations. J. Glob. Health 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Nisar, M.I.; Nayani, K.; Akhund, T.; Riaz, A.; Irfan, O.; Shakoor, S.; Muneer, S.; Muslim, S.; Hotwani, A.; Kabir, F.; et al. Nasopharyngeal carriage of Streptococcus pneumoniae in children under 5 years of age before introduction of pneumococcal vaccine (PCV10) in urban and rural districts in Pakistan. BMC Infect. Dis. 2019, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Swedan, S.F.; Hayajneh, W.A.; Bshara, G.N. Genotyping and serotyping of macrolide and multidrug resistant Streptococcus pneumoniae isolated from carrier children. Indian J. Med. Microbiol. 2016, 34, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kayhty, H.; Auranen, K.; Nohynek, H.; Dagan, R.; Makela, H. Nasopharyngeal colonization: A target for pneumococcal vaccination. Expert Rev. Vaccines 2006, 5, 651–667. [Google Scholar] [CrossRef]
- Goldblatt, D.; Hussain, M.; Andrews, N.; Ashton, L.; Virta, C.; Melegaro, A.; Pebody, R.; George, R.; Soininen, A.; Edmunds, J.; et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: A longitudinal household study. J. Infect. Dis. 2005, 192, 387–393. [Google Scholar] [CrossRef]
- Laval, C.B.; de Andrade, A.L.; Pimenta, F.C.; de Andrade, J.G.; de Oliveira, R.M.; Silva, S.A.; de Lima, E.C.; Fabio, J.L.; Casagrande, S.T.; Brandileone, M.C. Serotypes of carriage and invasive isolates of Streptococcus pneumoniae in Brazilian children in the era of pneumococcal vaccines. Clin. Microbiol. Infect. 2006, 12, 50–55. [Google Scholar] [CrossRef]
- Marchisio, P.; Esposito, S.; Schito, G.C.; Marchese, A.; Cavagna, R.; Principi, N. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children: Implications for the use of heptavalent pneumococcal conjugate vaccine. Emerg. Infect. Dis. 2002, 8, 479–484. [Google Scholar] [CrossRef]
- Goyal, R.; Singh, N.P.; Kaur, M.; Talwar, V. Antimicrobial resistance in invasive and colonising Streptococcus pneumoniae in North India. Indian J. Med. Microbiol. 2007, 25, 256–259. [Google Scholar] [CrossRef]
- Al-Bakri, A.G.; Bustanji, Y.; Yousef, A.-M. Community consumption of antibacterial drugs within the Jordanian population: Sources, patterns and appropriateness. Int. J. Antimicrob. Agents. 2005, 26, 389–395. [Google Scholar] [CrossRef] [PubMed]
| Age (M) | No. Cases | Carriers n (%) | Cases Received PCV7; No. of Doses n (%) | VT-Carriage for Vaccinated Cases n (%) | VT-Carriage for Non-Vaccinated Cases n (%) |
|---|---|---|---|---|---|
| 1 M a | 4 | 2 (50.0%) | 1 (25%); 1 dose | 1/1 (100%) | 0/3 (0.0%) |
| 2 M b | 315 | 183 (58.1%) | 306 (97.1%); 1 dose | 46/306 (15.0%) | 6/9 (66.7%) |
| 3 M c | 104 | 59 (56.7%) | 93 (89.4%); 2 doses | 17/93 (18.3%) | 2/11 (18.2%) |
| 4 M d | 29 | 14 (48.3%) | 10 (34.5%); 2 doses 2 (6.9%); 1 dose | 2/10 (20%) 0/2 (0.0%) | 2/17 (11.7%) |
| 5 M e | 16 | 8 (50.0%) | 2 (12.5%); 1 dose | 0/2 (0.0%) | 3/14 (21.4%) |
| 6 M f | 16 | 6 (37.5%) | 1 (6.3%); 3 doses 1 (6.3%); 2 doses | 0/1 (0.0%) 0/1 (0.0%) | 4/14 (28.6%) |
| Serotype | All Age Groups n (%) | Age 1–2 M n (%) | Age 3–4 M n (%) | Age 5–6 M n (%) | % Vaccinated * |
|---|---|---|---|---|---|
| 19F | 34 (12.5%) | 22 (12.4%) | 10 (13.7%) | 2 (14.3%) | 85.3% |
| 6A | 31 (11.4%) | 24 (13.0%) | 5 (6.8%) | 2 (14.3%) | 96.8% |
| 11A | 23 (8.4%) | 14 (7.5%) | 8 (10.9%) | 1 (7.1%) | 91.3% |
| 19A | 19 (7.0%) | 17 (9.2%) | 2 (2.7%) | 0 (0.0%) | 100% |
| 6B | 18 (6.6%) | 13 (7.0%) | 3 (4.1%) | 2 (14.3%) | 77.8% |
| 23F | 16 (5.9%) | 10 (5.4%) | 5 (6.8%) | 1 (7.1%) | 68.85 |
| 15B | 14 (5.1%) | 12 (6.5%) | 2 (2.7%) | 0 (0.0%) | 92.8% |
| 15A | 11 (4.0%) | 7 (3.8%) | 3 (4.1%) | 1 (7.1%) | 90.9% |
| 23A | 11 (4.0%) | 7 (3.8%) | 4 (5.5%) | 0 (0.0%) | 81.8% |
| 35B | 10 (3.7%) | 9 (4.8%) | 1 (1.4%) | 0 (0.0%) | 80.0% |
| 14 | 10 (3.7%) | 5 (2.7%) | 4 (5.5%) | 1 (7.1%) | 80.0% |
| NT | 8 (2.9%) | 5 (2.7%) | 3 (4.1%) | 0 (0.0%) | 87.5% |
| 33F | 7 (2.6%) | 4 (2.2%) | 2 (2.7%) | 1 (7.1%) | 85.7% |
| 16F | 7 (2.6%) | 2 (1.1%) | 5 (6.8%) | 0 (0.0%) | 100% |
| 15C | 6 (2.2%) | 5 (2.7%) | 1 (1.4%) | 0 (0.0%) | 100% |
| 24F | 6 (2.2%) | 4 (2.2%) | 2 (2.7%) | 0 (0.0%) | 100% |
| 17F | 5 (1.8%) | 3 (1.6%) | 1 (1.4%) | 1 (7.1%) | 80.0% |
| 9V | 4 (1.5%) | 3 (1.6%) | 0 (0.0%) | 1 (7.1%) | 75.0% |
| 3 | 4 (1.5%) | 2 (1.1%) | 2 (2.7%) | 0 (0.0%) | 100% |
| 10A | 4 (1.5%) | 3 (1.6%) | 1 (1.4%) | 0 (0.0%) | 100% |
| 9N | 3 (1.1%) | 2 (1.1%) | 1 (1.4%) | 0 (0.0%) | 100% |
| 33A | 3 (1.1%) | 1 (0.54%) | 2 (2.7%) | 0 (0.0%) | 66.7% |
| 34 | 3 (1.1%) | 1 (0.54%) | 1 (1.4%) | 1 (7.1%) | 66.7% |
| 7B | 3 (1.1%) | 2 (1.1%) | 1 (1.4%) | 0 (0.0%) | 66.7% |
| 35F | 2 (0.7%) | 1 (0.54%) | 1 (1.4%) | 0 (0.0%) | 100% |
| 4 | 1 (0.36%) | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 100% |
| 13 | 1 (0.36%) | 1 (0.54%) | 0 (0.0%) | 0 (0.0%) | 100% |
| 21 | 1 (0.36%) | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 100% |
| 42 | 1 (0.36%) | 1 (0.54%) | 0 (0.0%) | 0 (0.0%) | 100% |
| 10F | 1 (0.36%) | 1 (0.54%) | 0 (0.0%) | 0 (0.0%) | 100% |
| 16B | 1 (0.36%) | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 100% |
| 28A | 1 (0.36%) | 1 (0.54%) | 0 (0.0%) | 0 (0.0%) | 100% |
| 35C | 1 (0.36%) | 1 (0.54%) | 0 (0.0%) | 0 (0.0%) | 100% |
| 7C | 1 (0.36%) | 1 (0.54%) | 0 (0.0%) | 0 (0.0%) | 100% |
| 7F | 1 (0.36%) | 1 (0.54%) | 0 (0.0%) | 0 (0.0%) | 100% |
| Total | 272 (100%) | 185 (100%) | 73 (100%) | 14 (100%) | 88.2% |
| Age Group | PCV7 n (%) | PCV13 n (%) | PCV20 n (%) |
|---|---|---|---|
| 1–2 Months | 53/185 (28.6%) | 97/185 (52.4%) | 135/185 (73.0%) |
| 3–4 Months | 23/73 (31.5%) | 32/73 (43.8%) | 46/73 (63.0%) |
| 5–6 Months | 7/14 (50.0%) | 9/14 (64.3%) | 11/14 (78.6%) |
| All age groups | 83/272 (30.5%) | 138/272 (50.7%) | 192/272 (70.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Lahham, A. Prevalence of Pneumococcal Carriage among Jordanian Infants in the First 6 Months of Age, 2008–2016. Vaccines 2021, 9, 1283. https://doi.org/10.3390/vaccines9111283
Al-Lahham A. Prevalence of Pneumococcal Carriage among Jordanian Infants in the First 6 Months of Age, 2008–2016. Vaccines. 2021; 9(11):1283. https://doi.org/10.3390/vaccines9111283
Chicago/Turabian StyleAl-Lahham, Adnan. 2021. "Prevalence of Pneumococcal Carriage among Jordanian Infants in the First 6 Months of Age, 2008–2016" Vaccines 9, no. 11: 1283. https://doi.org/10.3390/vaccines9111283
APA StyleAl-Lahham, A. (2021). Prevalence of Pneumococcal Carriage among Jordanian Infants in the First 6 Months of Age, 2008–2016. Vaccines, 9(11), 1283. https://doi.org/10.3390/vaccines9111283






