Predictors of the Therapeutic Response to Intralesional Bivalent HPV Vaccine in Wart Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Blood Sampling
2.3. Preparation of Cultures
2.4. Measurement of IL-4 and IFN-γ
2.5. Statistical Analysis
3. Results
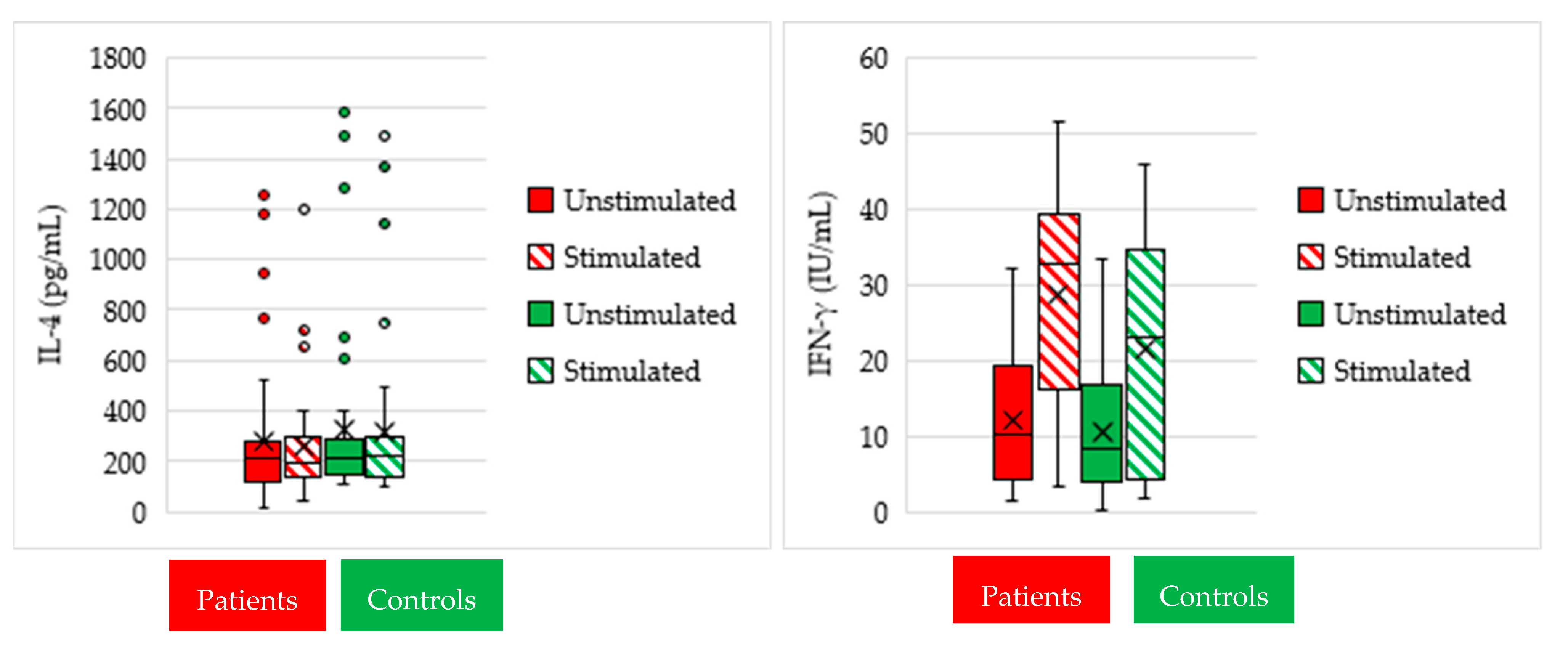
3.1. Cytokine Measurement in Culture Supernatants
3.2. IFN-γ and IL-4 Responses as Predictors of Therapeutic Outcome
3.3. Correlation of IFN-γ and IL-4 Responses with Age in Patients
3.4. Correlation of Clinical Variables with Therapeutic Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leto, M.d.G.P.; Santos Júnior, G.F.d.; Porro, A.M.; Tomimori, J. Human papillomavirus infection: Etiopathogenesis, molecular biology and clinical manifestations. An. Bras. De Dermatol. 2011, 86, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M. Immune responses to human papilloma viruses. Indian J. Med. Res. 2009, 130, 266. [Google Scholar] [PubMed]
- Choi, Y.J.; Park, J.S. Clinical significance of human papillomavirus genotyping. J. Gynecol. Oncol. 2016, 27, e21. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Lambert, P.F. Human papillomavirus and the stroma: Bidirectional crosstalk during the virus life cycle and carcinogenesis. Viruses 2017, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Renoux, V.M.; Bisig, B.; Langers, I.; Dortu, E.; Clémenceau, B.; Thiry, M.; Deroanne, C.; Colige, A.; Boniver, J.; Delvenne, P. Human papillomavirus entry into NK cells requires CD16 expression and triggers cytotoxic activity and cytokine secretion. Eur. J. Immunol. 2011, 41, 3240–3252. [Google Scholar] [CrossRef]
- Amador-Molina, A.; Hernández-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses 2013, 5, 2624–2642. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Nakagawa, M.; Moscicki, A.-B. Cell-mediated immune response to human papillomavirus infection. Clin. Diagn. Lab. Immunol. 2001, 8, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Relhan, V.; Garg, V.K. Immunomodulators in warts: Unexplored or ineffective? Indian J. Dermatol. 2015, 60, 118. [Google Scholar]
- Aldahan, A.S.; Mlacker, S.; Shah, V.V.; Kamath, P.; Alsaidan, M.; Samarkandy, S.; Nouri, K. Efficacy of intralesional immunotherapy for the treatment of warts: A review of the literature. Dermatol. Ther. 2016, 29, 197–207. [Google Scholar] [CrossRef]
- Nofal, A.; Salah, E.; Nofal, E.; Yosef, A. Intralesional antigen immunotherapy for the treatment of warts: Current concepts and future prospects. Am. J. Clin. Dermatol. 2013, 14, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Human papillomavirus vaccine for the treatment of recalcitrant extragenital warts. J. Am. Acad. Dermatol. 2017, 76, AB159. [CrossRef]
- Amer, A.; Nassar, A.; Gamal, D.; Marei, A. Effect of varicella zoster vaccine vs candida antigen injection in treatment of warts. Dermatol. Ther. 2021, 34, e14667. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.; Gibbs, S.; Haque Hussain, S.; Mohd Mustapa, M.; Handfield-Jones, S.; Hughes, J.; Griffiths, M.; McDonagh, A.; Punjabi, S.; Buckley, D. British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br. J. Dermatol. 2014, 171, 696–712. [Google Scholar] [CrossRef] [PubMed]
- Thappa, D.M.; Chiramel, M.J. Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol. Online J. 2016, 7, 364. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.R.; Saikaly, S.K.; Schoch, J.J. Intralesional immunotherapy for pediatric warts: A review. Pediatric Dermatol. 2020, 37, 265–271. [Google Scholar] [CrossRef]
- Signore, R.J. Candida albicans intralesional injection immunotherapy of warts. Cutis 2002, 70, 185–192. [Google Scholar]
- Nofal, A.; Nofal, E.; Yosef, A.; Nofal, H. Treatment of recalcitrant warts with intralesional measles, mumps, and rubella vaccine: A promising approach. Int. J. Dermatol. 2015, 54, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.A.; Salem, S.A.M.; Fouad, D.A.; El-Fatah, A.A.A. Intralesional tuberculin (PPD) versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: A comparative clinical and immunological study. Dermatol. Ther. 2015, 28, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Boghdadi, G.; El Shahaway, A.A. Correlation between interferon-gamma production in vitro and clinical response to immunotherapy in recalcitrant genital warts. Egypt. J. Med Microbiol. 2017, 38, 1–7. [Google Scholar] [CrossRef]
- Nofal, A.; Marei, A.; Amer, A.; Amen, H. Significance of interferon gamma in the prediction of successful therapy of common warts by intralesional injection of Candida antigen. Int. J. Dermatol. 2017, 56, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.A.; Mansour, S.A.; Gerges, M.A.; Marei, A.M.; El-Hady, H. Human papilloma virus genotypes and induced protein-10 as predictors for the clinical response to Candida antigen immunotherapy of warts. Egypt J. Med. Microbiol. 2019, 28, 129–135. [Google Scholar]
- Keam, S.J.; Harper, D.M. Human papillomavirus types 16 and 18 vaccine (recombinant, AS04 adjuvanted adsorbed) [Cervarix™]. Drugs 2008, 68, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M. Currently approved prophylactic HPV vaccines. Expert Rev. Vaccines 2009, 8, 1663–1679. [Google Scholar] [CrossRef]
- Monie, A.; Hung, C.-F.; Roden, R.; Wu, T.C. Cervarix™: A vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biol. Targets Ther. 2008, 2, 107. [Google Scholar]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Junttila, I.S. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Nofal, A.; Marei, A.; Al-shimaa, M.I.; Nofal, E.; Nabil, M. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J. Am. Acad. Dermatol. 2020, 82, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Hammad, N.M.; Abdelhadi, A.A.; Fawzy, M.M.; Marei, A. Complement component 3c and tumor necrosis factor-α systemic assessment after Candida antigen immunotherapy in cutaneous warts. Braz. J. Microbiol. 2020, 51, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Daniel, B.S.; Murrell, D.F. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013, 149, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Bossart, S.; Imstepf, V.; Hunger, R.E.; Jafari, S. Nonavalent human papillomavirus vaccination as a treatment for skin warts in immunosuppressed adults: A case series. Acta Derm. Venereol. 2020, 100, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.A.G.; Donadi, E.A. Immune cellular response to HPV: Current concepts. Braz. J. Infect. Dis. 2004, 8, 1–9. [Google Scholar] [CrossRef]
- Hong, J.H.; Kim, M.K.; Lee, I.H.; Kim, T.J.; Kwak, S.H.; Song, S.H.; Lee, J.K. Association Between Serum Cytokine Profiles and Clearance or Persistence of High-Risk Human Papillomavirus Infection: A Prospective Study. Int. J. Gynecol. Cancer 2010, 20, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.D.; Johnson, S.M.; Helm, R.M.; Roberson, P.K. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: A single-blinded, randomized, and controlled trial. Arch. Dermatol. 2005, 141, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Deenadayalan, A.; Maddineni, P.; Raja, A. Comparison of whole blood and PBMC assays for T-cell functional analysis. BMC Res. Notes 2013, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Son, J.-H.; Kim, G.-W.; Kim, H.-S.; Ko, H.-C.; Kim, M.-B.; Lim, K.-M.; Kim, B.-S. Quadrivalent human papilloma virus vaccine for the treatment of multiple warts: A retrospective analysis of 30 patients. J. Dermatol. Treat. 2019, 30, 405–409. [Google Scholar] [CrossRef]
- Wideroff, L.; Schiffman, M.; Nonnenmacher, B.; Hubbert, N.; Kirnbauer, R.; Greer, C.; Lowy, D.; Lorincz, A.; Manos, M.; Glass, A. Evaluation of seroreactivity to human papillomavirus type 16 virus-like particles in an incident case-control study of cervical neoplasia. J. Infect. Dis. 1995, 172, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Ault, K.A. Human papillomavirus vaccines and the potential for cross-protection between related HPV types. Gynecol. Oncol. 2007, 107, S31–S33. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Huang, Y.S.; Tang, M.; Lv, X.P.; Li, T.Y.; Yin, Y.B. Distribution and clinical significance of human papillomavirus subtypes in Shenzhen city, People’s Republic of China. Int. J. Gynecol. Cancer 2008, 18, 295–299. [Google Scholar] [CrossRef]
- Cheng, Y.-P.; Chen, C.-W.; Sheen, Y.-S.; Tsai, T.-F. Genotype distribution of human papillomavirus in anogenital warts of male patients in Taiwan. Dermatol. Sin. 2012, 30, 85–89. [Google Scholar] [CrossRef]
- Herrin, D.M.; Coates, E.E.; Costner, P.J.; Kemp, T.J.; Nason, M.C.; Saharia, K.K.; Pan, Y.; Sarwar, U.N.; Holman, L.; Yamshchikov, G. Comparison of adaptive and innate immune responses induced by licensed vaccines for Human Papillomavirus. Hum. Vaccines Immunother. 2014, 10, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Tyring, S.K.; Stanley, M.A.; Tomai, M.A.; Miller, R.L.; Smith, M.H.; McDermott, D.J.; Slade, H.B. Enhancement of the innate and cellular immune response in patients with genital warts treated with topical imiquimod cream 5%. Antivir. Res. 1999, 43, 55–63. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F. AS04, an aluminum salt-and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [PubMed]
- Pasare, C.; Medzhitov, R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science 2003, 299, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.R.; Mitchell, T.C. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 2008, 65, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Gattoc, L.; Nair, N.; Ault, K. Human papillomavirus vaccination: Current indications and future directions. Obstet. Gynecol. Clin. 2013, 40, 177–197. [Google Scholar] [CrossRef] [PubMed][Green Version]



| Variable | Patients N = 40 | Controls N = 40 | Test of Significance | p-Value |
|---|---|---|---|---|
| Age (Year) | t-test | 0.14 | ||
| Mean ± SD | 32.8 ± 18.4 | 38.9 ± 18.3 | ||
| Gender | χ2 test | 0.5 | ||
| Female | 20 (50.0) | 17 (42.5) | ||
| Male | 20 (50.0) | 23 (57.5) | ||
| Wart site | N/A | |||
| Face | 7 (17.5) | |||
| Dorsum of hands | 14 (35.0) | |||
| Dorsum of foot | 2 (5.0) | |||
| Plantar | 10 (25.0) | |||
| Back and axilla | 1 (2.5) | |||
| Genital | 6 (15.0) | |||
| Recurrence | N/A | |||
| No | 34 (85.0) | |||
| Yes | 6 (15.0) | |||
| Recalcitrance | N/A | |||
| No | 17 (42.5) | |||
| Yes | 23 (57.5) | |||
| Response to IT | N/A | |||
| Responders | 28 (70.0) | |||
| Partial responders | 4 (10.0) | |||
| Non-responders | 8 (20.0) |
| Cytokine Response | Cutoff | AUC (95% CI) | p-Value | Sensitivity | Specificity | +PV | −PV | Accuracy |
|---|---|---|---|---|---|---|---|---|
| IFN-γ ↑ | ≥56.8% | 0.88 (0.751–1.00) | p < 0.001 | 92.9% | 75% | 89.7% | 81.8% | 87.5% |
| IL-4 ↓ | ≥16.6% | 0.688 (0.506–0.869) | p = 0.063 | 62.2% | 92.3% | 95.0% | 51.2% | 69.0% |
| Variable | Response (N = 40) | Test of Significance | p-Value | ||
|---|---|---|---|---|---|
| Responders n = 28 (%) | Partial Responders n = 4 (%) | Non-Responders n = 8 (%) | |||
| Age (Years) | One-way ANOVA | 0.29 | |||
| Mean ± SD | 29.9 ± 16.8 | 43.3 ± 20.3 | 37.7 ± 22.6 | ||
| Gender | Fisher exact | 0.08 | |||
| Female | 11 (39.3) | 4 (100.0) | 5 (62.5) | ||
| Male | 17 (60.7) | 0 (0.0) | 3 (37.5) | ||
| Wart Site | 0.016 | ||||
| Face | 5 (17.9) | 2 (50.0) | 0 (0.0) | ||
| Dorsum of hands | 6 (21.4) | 2 (50.0) | 6 (75.0) | ||
| Dorsum of foot | 2 (7.1) | 0 (0.0) | 0 (25.0) | ||
| Plantar | 8 (28.6) | 0 (0.0) | 2 (25.0) | ||
| Back and axilla | 1 (3.6) | 0 (0.0) | 0 (0.0) | ||
| Genital | 6 (21.4) | 0 (0.0) | 0 (0.0) | ||
| Recurrence | 0.62 | ||||
| Yes | 4 (14.3) | 0 (0.0) | 2 (25.0) | ||
| No | 24 (85.7) | 4 (100.0) | 6 (75.0) | ||
| Recalcitrance | 0.89 | ||||
| Yes | 15 (53.6) | 2 (50.0) | 5 (62.5) | ||
| No | 13 (46.4) | 2 (50.0) | 3 (37.5) | ||
| IL-4 reduction response a | Kruskal–Wallis | 0.14 | |||
| Median | 0.9 | 1.0 | 1.1 | ||
| Range | 0.4–4.8 | 0.7–8.0 | 8.0–11.4 | ||
| IFN-γ induction response a | 0.001 * | ||||
| Median | 3.5 | 1.8 | 1.1 | ||
| Range | 1.3–10.0 | 1.0–3.4 | 0.7–4.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, N.M.; Marei, A.; El-Didamony, G.; Mortada, Z.; Elradi, M.; Afifi, A.H.M.; Kadry, H.M. Predictors of the Therapeutic Response to Intralesional Bivalent HPV Vaccine in Wart Immunotherapy. Vaccines 2021, 9, 1280. https://doi.org/10.3390/vaccines9111280
Hammad NM, Marei A, El-Didamony G, Mortada Z, Elradi M, Afifi AHM, Kadry HM. Predictors of the Therapeutic Response to Intralesional Bivalent HPV Vaccine in Wart Immunotherapy. Vaccines. 2021; 9(11):1280. https://doi.org/10.3390/vaccines9111280
Chicago/Turabian StyleHammad, Noha M., Ayman Marei, Gamal El-Didamony, Zeinb Mortada, Mona Elradi, Amira Hamed Mohamed Afifi, and Heba M. Kadry. 2021. "Predictors of the Therapeutic Response to Intralesional Bivalent HPV Vaccine in Wart Immunotherapy" Vaccines 9, no. 11: 1280. https://doi.org/10.3390/vaccines9111280
APA StyleHammad, N. M., Marei, A., El-Didamony, G., Mortada, Z., Elradi, M., Afifi, A. H. M., & Kadry, H. M. (2021). Predictors of the Therapeutic Response to Intralesional Bivalent HPV Vaccine in Wart Immunotherapy. Vaccines, 9(11), 1280. https://doi.org/10.3390/vaccines9111280






