Development of Inactivated FAKHRAVAC® Vaccine against SARS-CoV-2 Virus: Preclinical Study in Animal Models
Abstract
:1. Introduction
2. Experimental Model
2.1. Animal Models
2.2. Isolation of Viral Strain
2.3. Viral Titration
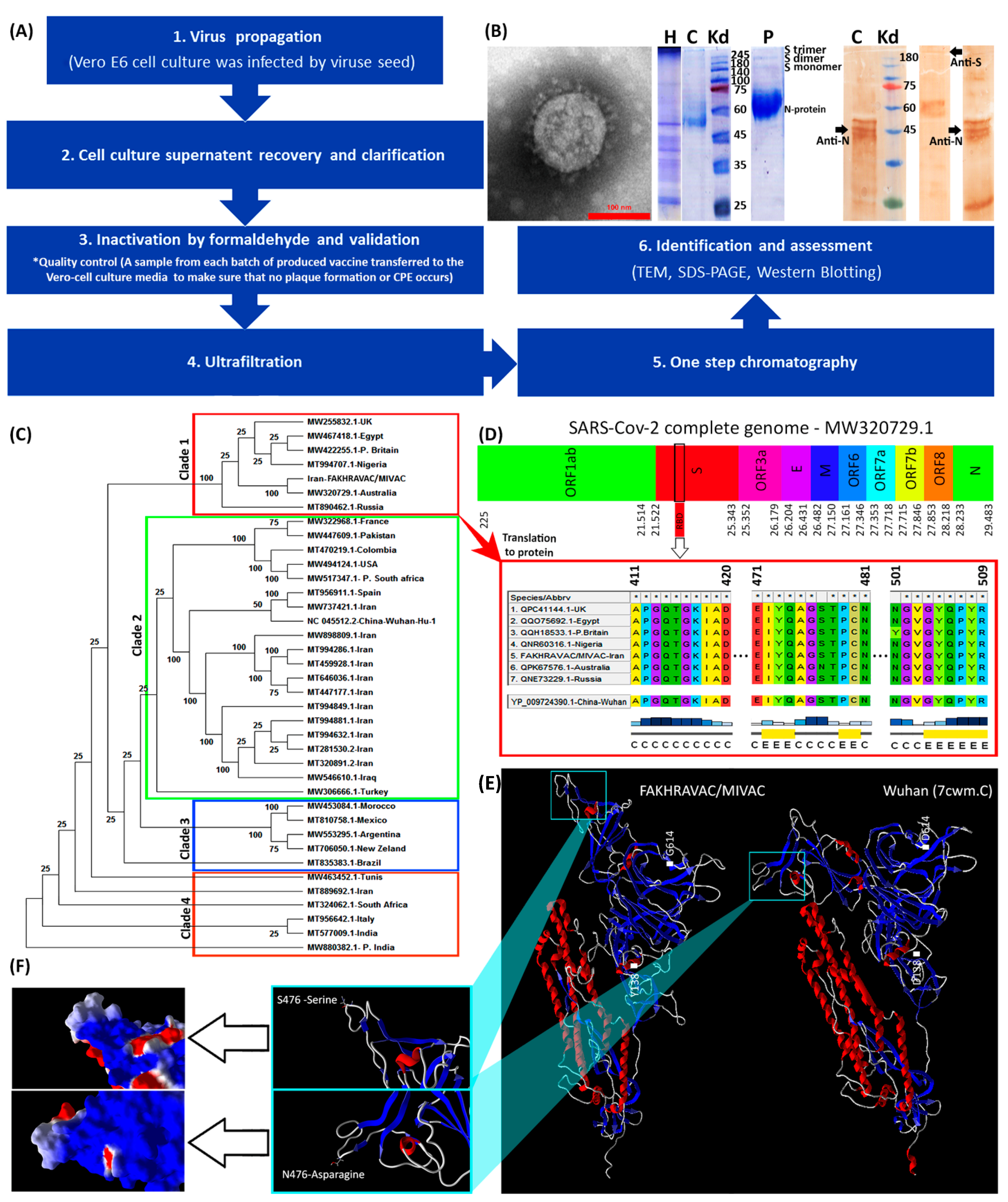
2.4. Vaccine Preparation and Identification
2.5. qRT-PCR
2.6. Humoral Immunization
2.7. Vaccine Immunogenicity Analysis and Protection in Animals
2.7.1. Neutralization Antibody (NAb) Assay
2.7.2. Challenge Assay in Rhesus Macaques
2.8. Safety Evaluation
2.9. Phylogenic Tree Analysis of SARS-CoV-2 Virus
2.10. Statistical Analysis
3. Results
3.1. Vaccine Design and Production
3.2. Immunogenicity in Mice, Guinea Pig, Rabbit and RMs
3.3. Safety
3.4. Protection in a Non-Human Primate Animal Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zellweger, R.M.; Wartel, T.A.; Marks, F.; Song, M.; Kim, J.H. Vaccination against SARS-CoV-2 and disease enhancement–knowns and unknowns. Expert Rev. Vaccines 2020, 19, 691–698. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.D.; Ella, R.; Kumar, S.; Patil, D.R.; Mohandas, S.; Shete, A.M.; Vadrevu, K.M.; Bhati, G.; Sapkal, G.; Kaushal, H.; et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat. Commun. 2021, 12, 1386. [Google Scholar] [CrossRef] [PubMed]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Hamilton, M.; Russo, R.; Thurston, R. Trimmed Spearman-Karber method for estimating median lethal concentrations in bioassays. Environ. Sci. Technol. 1978, 12, 417. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Barati, B.; Ebrahimi, F.; Nazarian, S. Production of Chicken Egg Yolk Antibody (IgY) Against Recombinant Cholera Toxin B Subunit and Evaluation of Its Prophylaxis Potency in Mice. Iran. J. Immunol. 2018, 15, 47–58. [Google Scholar]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85. [Google Scholar] [CrossRef]
- Safari, I.; InanlooRahatloo, K.; Elahi, E. Evolution of SARS-CoV-2 genome from December 2019 to late March 2020: Emerged haplotypes and informative Tag nucleotide variations. J. Med. Virol. 2021, 93, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, S.J.; Ahmad, K.R.; Jafar, A.; Iman, R.; Mahyat, J.; Ali, H.S. Designing and structure evaluation of multi-epitope vaccine against ETEC and EHEC, an in silico approach. Protein Pept. Lett. 2016, 23, 33–42. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Khodayari, K.; Alipour, M.; Rad, I.; Ramshini, H.; Abdolmaleki, P. Inhibition potential evaluation of two synthetic bis-indole compounds on amyloid fibrillation: A molecular simulation study. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt-Ferreira, G.; de Azevedo, W.F. Molegro virtual docker for docking. In Docking Screens for Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 149–167. [Google Scholar]
- Hatcher, E.L.; Sergey, A.Z.; Bao, Y.; Olga, B.; Eric, P.N.; Yuri, O.; Alejandro, A.S.; Rodney, J.B. Virus Variation Resource–improved response to emergent viral outbreaks. Nucleic Acids Res. 2017, 45, D482–D490. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for Valid Histopathologic Scoring in Research. Veter Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Blair, R.V.; Vaccari, M.; Doyle-Meyers, L.A.; Roy, C.J.; Russell-Lodrigue, K.; Fahlberg, M.; Monjure, C.J.; Beddingfield, B.; Plante, K.S.; Plante, J.A.; et al. Acute Respiratory Distress in Aged, SARS-CoV-2–Infected African Green Monkeys but Not Rhesus Macaques. Am. J. Pathol. 2021, 191, 274–282. [Google Scholar] [CrossRef]
- Planas, D.; Bruel, T.; Grzelak, L.; Guivel-Benhassine, F.; Staropoli, I.; Porrot, F.; Planchais, C.; Buchrieser, J.; Rajah, M.M.; Bishop, E.; et al. Sensitivity of infectious SARS-CoV-2 B. 1.1. 7 and B. 1.351 variants to neutralizing antibodies. Nat. Med. 2021, 27, 917–924. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. MedRxiv 2020. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Dingens, A.S.; Bloom, J.D. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Rep. Med. 2021, 2, 100255. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England. Tech. Brief. 2021, 12, 5. [Google Scholar]
- World Health Organization. COVID-19 Weekly Epidemiological Update; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi, S.; Naderi Saffar, K.; Ebrahimi, F.; Khatami, P.; Monazah, A.; Alizadeh, G.-A.; Ettehadi, H.-A.; Rad, I.; Nojehdehi, S.; Kehtari, M.; et al. Development of Inactivated FAKHRAVAC® Vaccine against SARS-CoV-2 Virus: Preclinical Study in Animal Models. Vaccines 2021, 9, 1271. https://doi.org/10.3390/vaccines9111271
Ghasemi S, Naderi Saffar K, Ebrahimi F, Khatami P, Monazah A, Alizadeh G-A, Ettehadi H-A, Rad I, Nojehdehi S, Kehtari M, et al. Development of Inactivated FAKHRAVAC® Vaccine against SARS-CoV-2 Virus: Preclinical Study in Animal Models. Vaccines. 2021; 9(11):1271. https://doi.org/10.3390/vaccines9111271
Chicago/Turabian StyleGhasemi, Soheil, Kosar Naderi Saffar, Firooz Ebrahimi, Pezhman Khatami, Arina Monazah, Ghorban-Ali Alizadeh, Hossein-Ali Ettehadi, Iman Rad, Shahrzad Nojehdehi, Mousa Kehtari, and et al. 2021. "Development of Inactivated FAKHRAVAC® Vaccine against SARS-CoV-2 Virus: Preclinical Study in Animal Models" Vaccines 9, no. 11: 1271. https://doi.org/10.3390/vaccines9111271
APA StyleGhasemi, S., Naderi Saffar, K., Ebrahimi, F., Khatami, P., Monazah, A., Alizadeh, G.-A., Ettehadi, H.-A., Rad, I., Nojehdehi, S., Kehtari, M., Kouhkan, F., Barjasteh, H., Moradi, S., Ghorbani, M.-H., Khodaeipour, A., Papizadeh, M., Najafi, R., Naghneh, E., Sadeghi, D., & Karimi Rahjerdi, A. (2021). Development of Inactivated FAKHRAVAC® Vaccine against SARS-CoV-2 Virus: Preclinical Study in Animal Models. Vaccines, 9(11), 1271. https://doi.org/10.3390/vaccines9111271





