Sweet Syndrome Following SARS-CoV2 Vaccination
Abstract
:1. Introduction
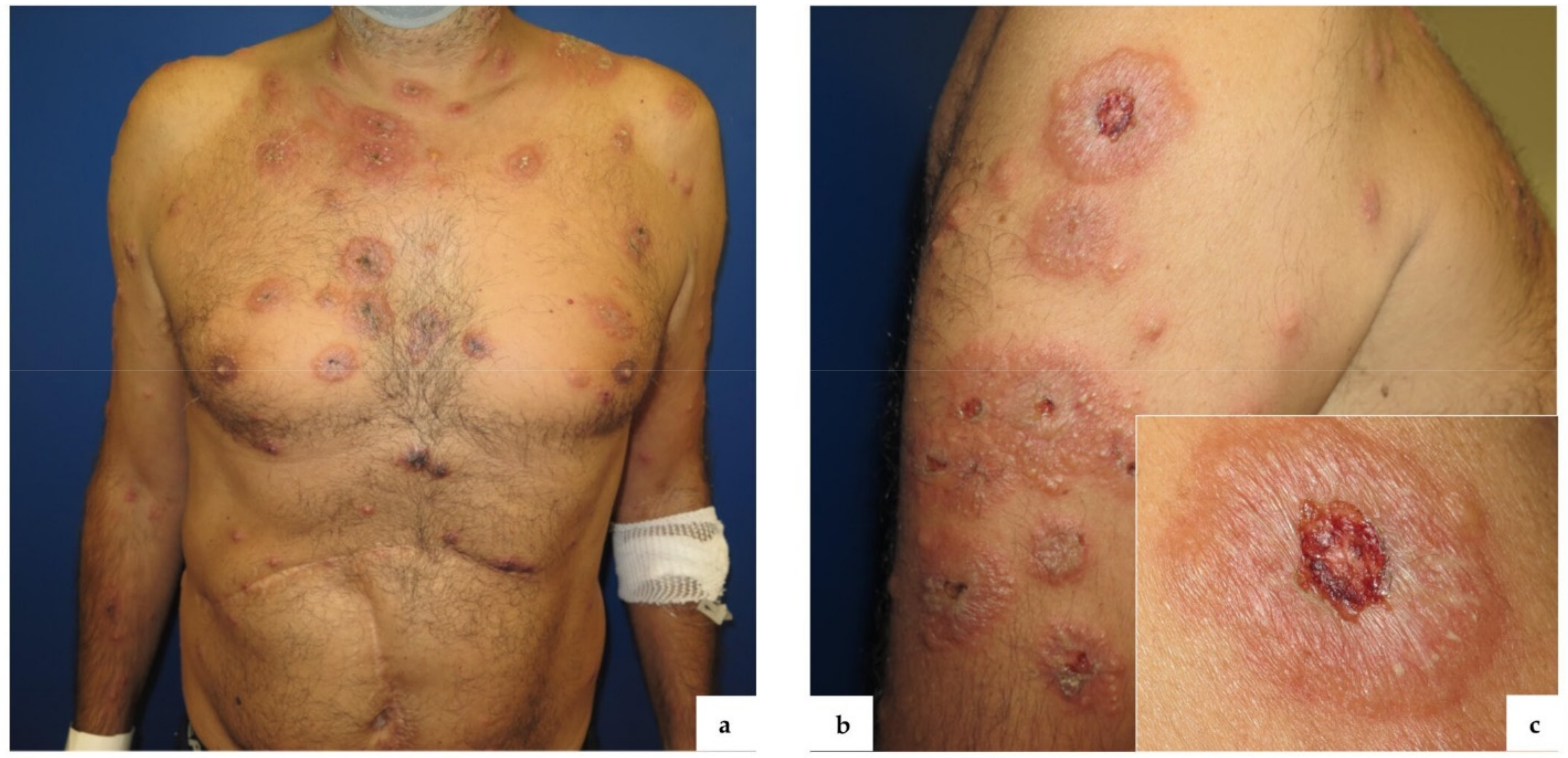
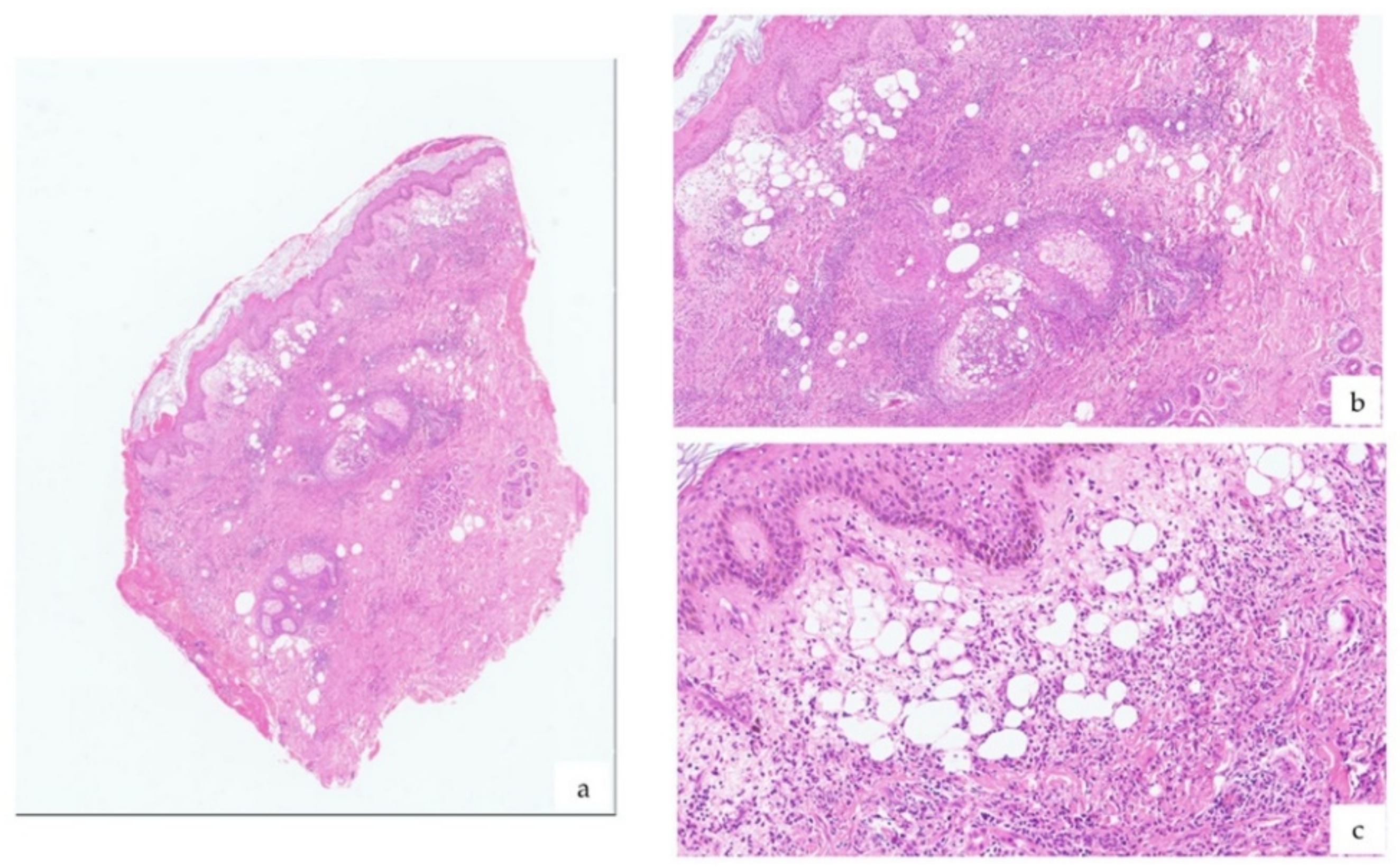
2. Case
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 16 October 2021).
- Pfizer. Pfizer and BioNTech Achieve First Authorization in the World for a Vaccine to Combat COVID-19 [Media Release]. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-achieve-first-authorization-world (accessed on 2 December 2020).
- European Medicine Agency. The European Commission Authorized the First Vaccine to Prevent COVID-19 in the EU, Following Evaluation by EMA. Available online: https://ec.europa.eu/health/documents/community-register/html/h1528.htm (accessed on 16 October 2021).
- US Food and Drug Administration. FDA Takes Key Action in Fight against COVID-19 by Issuing Emergency Use Authorization for First COVID-19 Vaccine [Media Release]. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 (accessed on 2 December 2020).
- Larson, V.; Seidenberg, R.; Caplan, A.; Brinster, N.K.; Meehan, S.A.; Kim, R.H. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J. Cutan. Pathol. 2021, 22, 10. [Google Scholar] [CrossRef]
- European Medicines Agency. Covid-19 mRNA Vaccine (Comirnaty): EU Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty (accessed on 16 October 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Torrealba-Acosta, G.; Martin, J.C.; Huttenbach, Y.; Garcia, C.R.; Sohail, M.R.; Agarwal, S.K.; Wasko, C.; Bershad, E.M.; Hirzallah, M.I. Acute encephalitis, myoclonus and Sweet syndrome after mRNA-1273 vaccine. BMJ Case Rep. 2021, 14, e243173. [Google Scholar] [CrossRef] [PubMed]
- Darrigade, A.S.; Théophile, H.; Sanchez-Pena, P.; Milpied, B.; Colbert, M.; Pedeboscq, S.; Pistone, T.; Jullié, M.L.; Seneschal, J. Sweet syndrome induced by SARS-CoV-2 Pfizer-BioNTech mRNA vaccine. Allergy 2021, 76, 3194–3196. [Google Scholar] [CrossRef] [PubMed]
- Capassoni, M.; Ketabchi, S.; Cassisa, A.; Caramelli, R.; Molinu, A.A.; Galluccio, F.; Guiducci, S. AstraZeneca (AZD1222) COVID-19 vaccine-associated adverse drug event: A case report. J. Med. Virol. 2021, 93, 5718–5720. [Google Scholar] [CrossRef] [PubMed]
- Rochet, N.M.; Chavan, R.N.; Cappel, M.A.; Wada, D.A.; Gibson, L.E. Sweet syndrome: Clinical presentation, associations, and response to treatment in 77 patients. J. Am. Acad. Dermatol. 2013, 69, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.S.; Ortega-Loayza, A.G. Insights into the Pathogenesis of Sweet’s Syndrome. Front. Immunol. 2019, 10, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrosa, A.F.; Morais, P.; Nogueira, A.; Pardal, J.; Azevedo, F. Sweet’s syndrome triggered by pneumococcal vaccination. Cutan. Ocul. Toxicol. 2013, 32, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Poljacki, M.; Vujanovic, L.; Duran, V. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) after influenza vaccination. J. Am. Acad. Dermatol. 2005, 5, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, O.; Piette, F.; Delaporte, E. Sweet’s syndrome after BCG vaccination. Acta Derm. Venereol. 2002, 82, 221. [Google Scholar] [CrossRef] [PubMed]
- Maddox, P.R.; Motley, R.J. Sweet’s syndrome: A severe complication of pneumococcal vaccination following emergency splenectomy. Br. J. Surg. 1990, 77, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Radeff, B.; Harms, M. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) following BCG vaccination. Acta Derm. Venereol. 1986, 66, 357–358. [Google Scholar] [PubMed]
- Marzano, A.V.; Fanoni, D.; Antiga, E.; Quaglino, P.; Caproni, M.; Crosti, C.; Meroni, P.L.; Cugno, M. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet’s syndrome. Clin. Exp. Immunol. 2014, 178, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Antiga, E.; Maglie, R.; Volpi, W.; Bianchi, B.; Berti, E.; Marzano, A.V.; Caproni, M. T helper type 1-related molecules as well as interleukin-15 are hyperexpressed in the skin lesions of patients with pyoderma gangrenosum. Clin. Exp. Immunol. 2017, 189, 383–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratzinger, G.; Burgdorf, W.; Zelger, B.G.; Zelger, B. Acute febrile neutrophilic dermatosis: A histopathologic study of 31 cases with review of literature. Am. J. Dermatopathol. 2007, 29, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Taşkın, B.; Vural, S.; Altuğ, E.; Demirkesen, C.; Kocatürk, E.; Çelebi, İ.; Ferhanoğlu, B.; Alper, S. Coronavirus 19 presenting with atypical Sweet’s syndrome. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e534–e535. [Google Scholar] [CrossRef] [PubMed]
- Berro, S.; Calas, A.; Sohier, P.; Darbord, D.; Dupin, N. Sweet’s Syndrome Three Weeks after a Severe COVID-19 Infection: A Case Report. Acta Derm. Venereol. 2021, 101, adv00486. [Google Scholar] [CrossRef] [PubMed]
| Age | Sex | Administered Vaccine | Symptoms Latency | Histological Confirmation | Associated Extracutaneous Manifestations | Other Investigations | |
|---|---|---|---|---|---|---|---|
| Capassoni et al. | 37 | F | ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca) | 96 h | YES | Arthritis; polymyositis | Immunofluorescence; ultrasounds; electromyography |
| Darrigade et al. | 45 | F | SARS-CoV-2 Pfizer-BioNTech mRNA vaccine (BNT162b2) | 24 h | YES | None | Patch tests; IDR 1 |
| Torrealba-Acosta et al. | 77 | M | Moderna mRNA-1273 vaccine | 24 h | YES | Acute encephalitis; myoclonus | CSF 2 analysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baffa, M.E.; Maglie, R.; Giovannozzi, N.; Montefusco, F.; Senatore, S.; Massi, D.; Antiga, E. Sweet Syndrome Following SARS-CoV2 Vaccination. Vaccines 2021, 9, 1212. https://doi.org/10.3390/vaccines9111212
Baffa ME, Maglie R, Giovannozzi N, Montefusco F, Senatore S, Massi D, Antiga E. Sweet Syndrome Following SARS-CoV2 Vaccination. Vaccines. 2021; 9(11):1212. https://doi.org/10.3390/vaccines9111212
Chicago/Turabian StyleBaffa, Maria Efenesia, Roberto Maglie, Neri Giovannozzi, Francesca Montefusco, Stefano Senatore, Daniela Massi, and Emiliano Antiga. 2021. "Sweet Syndrome Following SARS-CoV2 Vaccination" Vaccines 9, no. 11: 1212. https://doi.org/10.3390/vaccines9111212
APA StyleBaffa, M. E., Maglie, R., Giovannozzi, N., Montefusco, F., Senatore, S., Massi, D., & Antiga, E. (2021). Sweet Syndrome Following SARS-CoV2 Vaccination. Vaccines, 9(11), 1212. https://doi.org/10.3390/vaccines9111212






