Pharmaceutical Industry’s Engagement in the Global Equitable Distribution of COVID-19 Vaccines: Corporate Social Responsibility of EUL Vaccine Developers
Abstract
1. Introduction
2. Theory and Literature Review
2.1. Definition and Motivations of CSR
2.2. CSR in Pharmaceutical Companies
2.3. Measuring CSR
3. Materials and Methods
- 1.
- Research and Development
- a.
- Effectiveness: 1 point for over 90% effectiveness and consistent clinical trial results;
- b.
- Profits: 1 point for stating non-profit on vaccine sales.
- 2.
- Transparency and Accountability
- a.
- Clinical trials: 1 point for publicly sharing clinical trial protocols;
- b.
- Vaccine Contracts: 1 point for publishing opening over 20% of vaccine price data to public.
- 3.
- Product Delivery
- a.
- COVAX %: 1 point for providing more than 20% of vaccine supplies to COVAX;
- b.
- Distribution to Lower-Income Countries: 1 point for distributing more than 40% of vaccine supplies to countries other than HICs;
- c.
- Equitable pricing: 1 point for a positive correlation coefficient between GDP per capita and vaccine prices;
- d.
- Intellectual Property Strategy: 1 point for agreeing to waive IP rights during the COVID-19 pandemic;
- e.
- Manufacturing agreements: 1 point for actively participating in manufacturing agreements with various countries, including lower-income countries; more than 15% of manufacturing agreements (including CDMO and Technology Transfer) are made with countries other than high-income countries.
4. Results
4.1. Research and Development
4.1.1. Effectiveness, Storage, and Manufacturing Capacity
4.1.2. Profit Generation
| Pfizer/BioNTech | AstraZeneca–Oxford | Janssen | Moderna | Sinopharm | Sinovac | |
|---|---|---|---|---|---|---|
| Funding 1 (APA included) (million USD) | 18,549 | 4967 | 5928 | 8337 | 145 | 515 |
| Funding 2 (APA excluded) (million USD) | 800 | 115 | 1028 | 955 | 145 | 515 |
| Revenues from vaccine sales in 2021 (Q1–Q2 this year, billion USD) | 11.3 [48,55] | 1.2 [56] | 0.3 [57,58] | 5.9 [59,60] | - 3 | - |
| Announcements | “marginal profit” [51] | no profit [54] | no profit [52] | “will not charge too high a price” [53] | - | - |
4.2. Transparency and Accountability
4.2.1. Clinical Trials
4.2.2. Vaccine Contracts
4.3. Product Delivery
4.3.1. Percentage of Vaccines in COVAX Vaccine Supply Agreements
4.3.2. Distribution to Lower-Income Countries
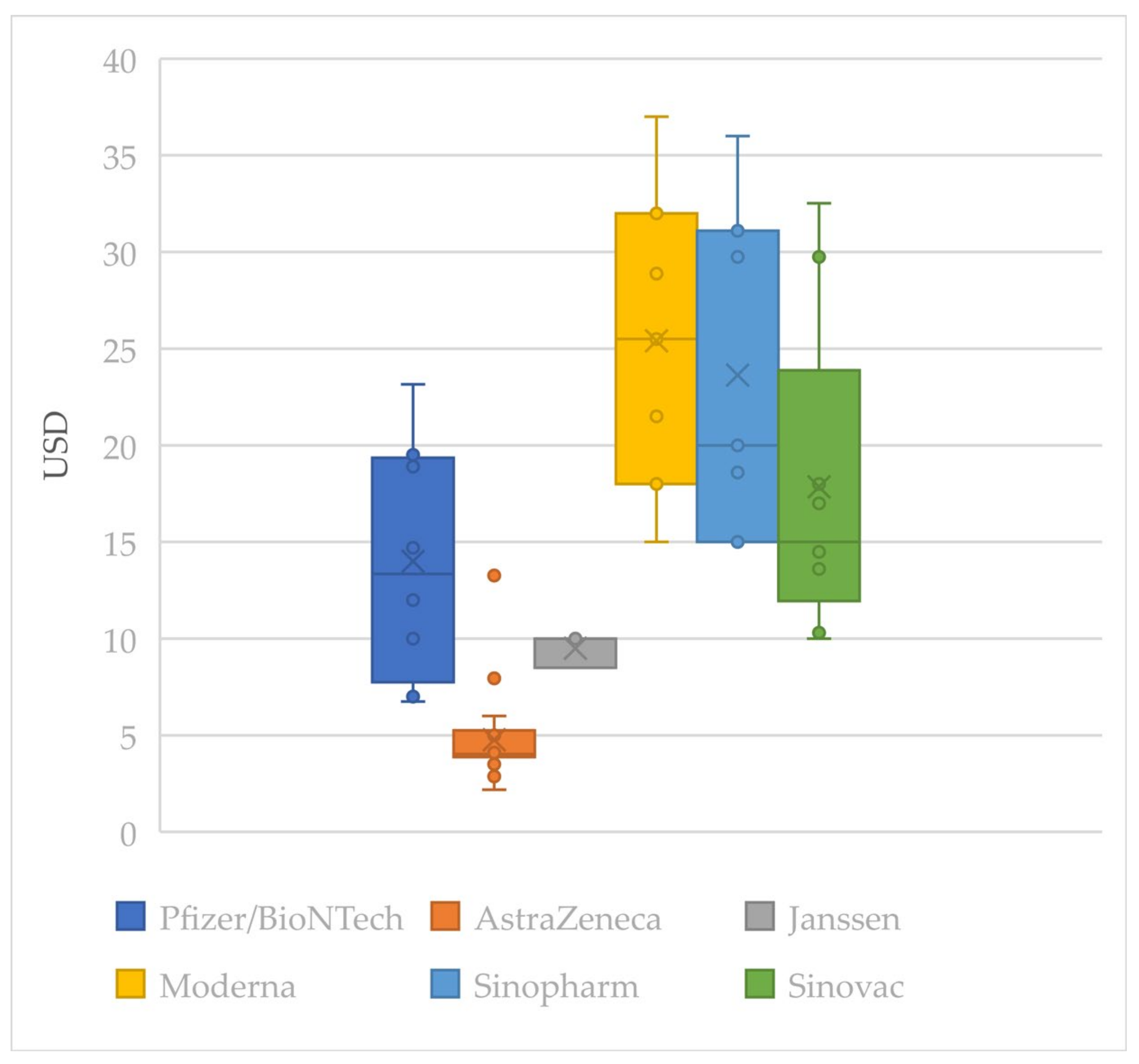
4.3.3. Equitable Pricing
4.3.4. Intellectual Property Strategy
4.3.5. Manufacturing Agreements
4.4. Scoring of the EUL-Listed Vaccine Developers
5. Discussion
5.1. Findings
5.1.1. Research and Development
5.1.2. Transparency and Accountability
5.1.3. Product Delivery
5.1.4. Assessment of Vaccine Developer’s CSR and Suggestions
5.2. Contributions and Implications
5.3. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S. The Coronavirus Is Here Forever. This Is How We Live with It. Available online: https://www.theatlantic.com/science/archive/2021/08/how-we-live-coronavirus-forever/619783/ (accessed on 12 September 2021).
- WHO Team. COVAX Facility Explainer; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Berkley, S. COVAX Explained. Available online: https://www.gavi.org/vaccineswork/covax-explained (accessed on 9 July 2021).
- WHO. Interim Statement on COVID-19 Vaccine Booster Doses. Available online: https://www.who.int/news/item/10-08-2021-interim-statement-on-covid-19-vaccine-booster-doses (accessed on 12 September 2021).
- RAND Corporation. COVID-19 and the Cost of Vaccine Nationalism. Available online: https://www.gavi.org/vaccineswork/covid-19-and-cost-vaccine-nationalism?gclid=Cj0KCQjwiqWHBhD2ARIsAPCDzan_4BAWlUDRTxtW5m27jtWRCiFxv3QOI8-XF3gbYof4ZcuztfO1qb0aAow6EALw_wcB (accessed on 10 July 2021).
- Rhodes, N.; Wright, N.; Rusu, V.; Bakhtari, H.; Cushing, J.; Kohler, J. For Whose Benefit? Transparency in the Development and Procurement of COVID-19 Vaccines; Transparency International: London, UK, 2021. [Google Scholar]
- UNICEF. COVID-19 Vaccine Market Dashboard; UNICEF: New York, NY, USA, 2021. [Google Scholar]
- Shrotri, M.; Swinnen, T.; Kampmann, B.; Parker, E.P.K. An interactive website tracking COVID-19 vaccine development. Lancet Glob. Health 2021, 9, e590–e592. [Google Scholar] [CrossRef]
- International Organization for Standardization. Social Responsibility—Discovering ISO 26000; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Carroll, A. Corporate social responsibility: Evolution of a definitional construct. Bus. Soc. 1999, 38, 268–295. [Google Scholar] [CrossRef]
- Matten, D.; Moon, J. “Implicit” and “Explicit” CSR: A Conceptual Framework for a Comparative Understanding of Corporate Social Responsibility. Acad. Manag. Rev. 2008, 33, 1–46. [Google Scholar] [CrossRef]
- Mahmud, A.; Ding, D.; Kiani, A.; Hasan, M. Corporate Social Responsibility Programs and Community Perceptions of Societal Progress in Bangladesh: A Multimethod Approach. SAGE Open 2020, 10, 2158244020924046. [Google Scholar] [CrossRef]
- Resnik, D.B. Scientific misconduct and research integrity. In Encylcopedia of Global Bioethics; ten Have, H., Ed.; Springer International Publishing: Geneva, Switzerland, 2014; pp. 2606–2615. [Google Scholar]
- Cuesta-Valiño, P.; Rodríguez, P.G.; Núñez-Barriopedro, E. The impact of corporate social responsibility on customer loyalty in hypermarkets: A new socially responsible strategy. Corp. Soc. Responsib. Environ. Manag. 2019, 26, 761–769. [Google Scholar] [CrossRef]
- Lins, K.V.; Servaes, H.; Tamayo, A. Social Capital, Trust, and Firm Performance: The Value of Corporate Social Responsibility during the Financial Crisis. J. Financ. 2017, 72, 1785–1824. [Google Scholar] [CrossRef]
- Brulhart, F.; Gherra, S.; Quélin, B. Do Stakeholder Orientation and Environmental Proactivity Impact Firm Profitability? J. Bus. Ethics 2019, 158, 25–46. [Google Scholar] [CrossRef]
- Deng, X.; Long, X.; Schuler, D.A.; Luo, H.; Zhao, X. External corporate social responsibility and labor productivity: A S-curve relationship and the moderating role of internal CSR and government subsidy. Corp. Soc. Responsib. Environ. Manag. 2020, 27, 393–408. [Google Scholar] [CrossRef]
- Kapelus, P. Mining, Corporate Social Responsibility and the “Community”: The Case of Rio Tinto, Richards Bay Minerals and the Mbonambi. J. Bus. Ethics 2002, 39, 275–296. [Google Scholar] [CrossRef]
- Idemudia, U. Oil Extraction and Poverty Reduction in the Niger Delta: A Critical Examination of Partnership Initiatives. J. Bus. Ethics 2009, 90, 91–116. [Google Scholar] [CrossRef]
- Kochhar, S.K. Putting community first: Mainstreaming CSR for community-building in India and China. Asian J. Commun. 2014, 24, 421–440. [Google Scholar] [CrossRef]
- Skouloudis, A.; Avlonitis, G.J.; Malesios, C.; Evangelinos, K. Priorities and perceptions of corporate social responsibility. Manag. Decis. 2015, 53, 375–401. [Google Scholar] [CrossRef]
- Carroll, A.B. The pyramid of corporate social responsibility: Toward the moral management of organizational stakeholders. Bus. Horiz. 1991, 34, 39–48. [Google Scholar] [CrossRef]
- George, R.D. Moral responsibility and the corporation. Philos. Exch. 1981, 12, 3. [Google Scholar]
- García-Sánchez, I.-M.; García-Sánchez, A. Corporate Social Responsibility during COVID-19 Pandemic. J. Open Innov. Technol. Mark. Complex. 2020, 6, 126. [Google Scholar] [CrossRef]
- Brammer, S.; Branicki, L.; Linnenluecke, M.K. COVID-19, Societalization, and the Future of Business in Society. Acad. Manag. Perspect. 2020, 34, 493–507. [Google Scholar] [CrossRef]
- Verma, S.; Gustafsson, A. Investigating the emerging COVID-19 research trends in the field of business and management: A bibliometric analysis approach. J. Bus. Res. 2020, 118, 253–261. [Google Scholar] [CrossRef]
- Haessler, P. Strategic Decisions between Short-Term Profit and Sustainability. Adm. Sci. 2020, 10, 63. [Google Scholar] [CrossRef]
- Hurst, D.J. Restoring a reputation: Invoking the UNESCO Universal Declaration on Bioethics and Human Rights to bear on pharmaceutical pricing. Med. Health Care Philos. 2017, 20, 105–117. [Google Scholar] [CrossRef]
- Bangalee, V.; Suleman, F. Access considerations for a COVID-19 vaccine for South Africa. S. Afr. Fam. Pract. 2020, 62, 5152. [Google Scholar] [CrossRef]
- Macklin, R. Double Standards in Medical Research in Developing Countries; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Moon, S.; Jambert, E.; Childs, M.; von Schoen-Angerer, T. A win-win solution?: A critical analysis of tiered pricing to improve access to medicines in developing countries. Glob. Health 2011, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Droppert, H.; Bennett, S. Corporate social responsibility in global health: An exploratory study of multinational pharmaceutical firms. Glob. Health 2015, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Esposito, M.; Desmoulins-Lebeault, F. Should Pharmaceutical Companies Engage in Corporate Social Responsibility? J. Manag. Dev. 2017, 36, 58. [Google Scholar] [CrossRef]
- Kanwar, A.; Rahim, M. Social Responsibilities of the Global Pharmaceutical Companies: Towards an Ethical Health Care Paradigm. SSRN Electron. J. 2019, 26. [Google Scholar] [CrossRef]
- Bluestone, K.; Heaton, A.; Lewis, C. Beyond Philanthropy: The Pharmaceutical Industry, Corporate Social Responsibility and the Developing World; Oxfam/Save the Children/VSO: London, UK, 2002. [Google Scholar]
- 2015 Methodology for the 2017 Access to Vaccines Index; Access to Medicine Foundation: Amsterdam, The Netherlands, 1 December 2015.
- Smith, N. Corporate Social Responsibility: Not Whether, But How. Calif. Manag. Rev. 2003, 45, 52–76. [Google Scholar] [CrossRef]
- Gruskin, S.; Raad, Z. Are drug companies living up to their human rights responsibilities? Moving toward assessment. PLoS Med. 2010, 7, e1000310. [Google Scholar] [CrossRef]
- Dow Jones Sustainability World Index; S&P Dow Jones Indices LLC.: New York, NY, USA, 2021.
- Senz, K. What Does an ESG Score Really Say about a Company? Harvard Business School: Boston, MA, USA, 2021. [Google Scholar]
- MSCI. ESG Ratings Corporate Search Tool; MSCI: New York, NY, USA, 2021. [Google Scholar]
- Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process; WHO: Geneva, Switzerland, 19 August 2021.
- Tan, Y. Covid: What do we know about China’s coronavirus vaccines? BBC News, 14 January 2021. [Google Scholar]
- Edwards, D.; Diallo, M.; Gülser, S.; Kong, A.; Romero, C.; Warren, M. The Methodology for the 2021 Access to Medicine Index; Access to Medicine Foundation: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Carstens, A. The Role of Transparency and Accountability for Economic Development in Resource-rich Countries. In Proceedings of the Regional Workshop on Transparency and Accountability in Resource Management in CEMAC Countries, Malabo, Equatorial Guinea, 27 January 2005. [Google Scholar]
- Ruchir Agarwal, G.G. 9781513577609/2617-6750; International Monetary Fund: Washington, DC, USA, 19 May 2021. [Google Scholar]
- Centre, G.H. COVID-19 Vaccines R&D Investments; Graduate Institute of International and Development Studies: Geneva, Switzerland, 2021. [Google Scholar]
- Pfizer. Pfizer Reports Second-Quarter 2021 Results; Pfizer: New York, NY, USA, 4 July 2021. [Google Scholar]
- Sinopharm. 2021 Interim Report; Sinopharm: Beijing, China, 2021. [Google Scholar]
- Parton, J. Covid Shot Makers to Share in up to $190 Billion Sales Bonaza. Bloomberg, 26 May 2021. [Google Scholar]
- Shapiro, E. Pfizer CEO Albert Bourla Raises Expectations That the Pharmaceutical Giant Can Deliver a COVID-19 Vaccine by Fall. Time, 9 July 2020. [Google Scholar]
- Dunn, A. The CEO of the buzzy biotech that’s working on a potential coronavirus vaccine just pledged he won’t set a high price for the shot. Insider, 5 March 2020. [Google Scholar]
- Douoguih, M. Testimony of Macaya Douoguih, M.D., M.P.H; Submitted to the Oversight & Investigation Subcommittee of the U.S. House of Representatives Energy & Commerce Committee; 2020. Available online: https://energycommerce.house.gov/sites/democrats.energycommerce.house.gov/files/documents/Testimony%20-%20Pangalos%2020200721_0.pdf (accessed on 10 October 2021).
- Pangalos, M. Pathway to a Vaccine: Efforts to Develop a Safe, Effective and Accessible COVID-19 Vaccine; Statement of Sir Menelas Pangalos, Ph.D., Executive Vice President, Biopharmaceutical Research & Development AstraZeneca before the Subcommittee on Oversight and Investigations, Committee on Energy and Commerce, U.S. House of Representatives; 2020. Available online: https://energycommerce.house.gov/sites/democrats.energycommerce.house.gov/files/documents/Testimony%20-%20Pangalos%2020200721_0.pdf (accessed on 10 October 2021).
- Pfizer. Pfizer Reports Strong First-Quarter 2021 Results; Pfizer: New York, NY, USA, 2021. [Google Scholar]
- AstraZeneca. H1 2021 Results; AstraZeneca: Cambridge, UK, 2021. [Google Scholar]
- Johnson&Johnson. Johnson & Johnson Reports Q2 2021 Results; Johnson&Johnson: New Brunswick, NJ, USA, 2021. [Google Scholar]
- Johnson&Johnson. Johnson&Johnson Reports 2021 First-Quarter Results; Johnson&Johnson: New Brunswick, NJ, USA, 2021. [Google Scholar]
- Moderna. 10-Q Quarterly Report (Q1); Moderna: Cambridge, MA, USA, 2021. [Google Scholar]
- Moderna. 10-Q Quarterly Report (Q2); Moderna: Cambridge, MA, USA, 2021. [Google Scholar]
- Roberts, M. Oxford/AstraZeneca Covid vaccine ‘dose error’ explained. BBC News Online, 27 November 2020. [Google Scholar]
- The World Bank. World Bank Country and Lending Groups; The World Bank: Washington, DC, USA, 2021. [Google Scholar]
- Gavi. Gavi Signs Agreements with Sinopharm and Sinovac for Immediate Supply to COVAX; Gavi: Geneva, Switzerland, 2021. [Google Scholar]
- Low-income countries have received just 0.2 per cent of all COVID-19 shots given. UN News, 9 April 2021.
- Council for Trade-Related Aspects of Intellectual Property Rights. Waiver from Certain Provisions of the TRIPS Agreement for the Prevention, Containment and Treatment of COVID-19—Responses to Questions. In Proceedings of the Communication from the Plurinational State of Bolivia, Eswatini, India, Kenya, Mozambique, Mongolia, Pakistan, South Africa, The Bolivarian Repulbic of Venezuela and Zimbabwe. Available online: https://docs.wto.org/dol2fe/Pages/SS/directdoc.aspx?filename=q:/IP/C/W672.pdf&Open=True (accessed on 15 January 2021).
- Kapalan, T.; Stolberg, S.G.; Robbins, R. Taking ‘Extraordinary Measures’, Biden Backs Suspending Patents on Vaccines. The New York Times, 5 May 2021. [Google Scholar]
- Oxford University Innovation. Expedited Access for COVID-19 Related IP. Available online: https://innovation.ox.ac.uk/technologies-available/technology-licensing/expedited-access-covid-19-related-ip/ (accessed on 15 July 2021).
- Loftus, P. Moderna Vows to Not Enforce Covid-19 Vaccine Patents during Pandemic. The Wall Street Journal, 8 October 2020. [Google Scholar]
- McCarthy, S. China backs IP waiver for coronavirus vaccines. South China Morning Post, 17 May 2021. [Google Scholar]
- Bourla, A. An Open Letter from Pfizer Chairman and CEO Albert Bourla. Available online: https://www.pfizer.com/news/hot-topics/an_open_letter_from_pfizer_chairman_and_ceo_albert_bourla (accessed on 15 July 2021).
- Eakin, B. J&J’s Chief Patent Atty Says COVID IP Waiver Won’t Work. Law360, 22 April 2021. [Google Scholar]
- Sagonowsky, E. Where do COVID-19 vaccine players stand on pricing? So far, it’s no profit, slight profit or undecided. Fiercepharma, 21 July 2020. [Google Scholar]
- WHO. COVAX Announces New Agreement, Plans for First Deliveries; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Hickey, K.J. The PREP Act and COVID-19: Limiting Liability for Medical Countermeasures; Congressional Research Service: Washington, DC, USA, 23 September 2021.
- Eccleston-Turner, M.; Upton, H. International Collaboration to Ensure Equitable Access to Vaccines for COVID-19: The ACT-Accelerator and the COVAX Facility. Milbank Q 2021, 99, 426–449. [Google Scholar] [CrossRef]
- Dreisbach, T. ‘Bad Optics’ Or Something More? Moderna Executives’ Stock Sales Raise Concerns. NPR, 4 September 2020. [Google Scholar]
- Dreisbach, T. Pfizer CEO Sold Millions in Stock after Coronavirus Vaccine News, Raising Questions. NPR, 11 November 2020. [Google Scholar]
- Amin, L. Making the Case for Open Contracting in Healthcare Procurement; Transparency International: London, UK, 2017. [Google Scholar]
- Coles, L.C. Operational Productivity and Performance in English NHS Acute Hospitals: Unwarranted Variations; Crown: London, UK, 2016. [Google Scholar]
- McIntosh, T. Transparency Becomes a Casualty for Contracts to Buy Covid-19 Vaccines. Available online: https://eyeonglobaltransparency.net/2021/01/29/transparency-becomes-a-casualty-for-contracts-to-buy-covid-19-vaccines/ (accessed on 1 October 2021).
- Mancini, D.P. Vaccine contracts shrouded in secrecy despite massive public funding. Financial Times, 23 November 2020. [Google Scholar]
- Armstrong, D.; Randall, T. Tracking the COVID-19 Vaccine Rollout Around the Globe. Bloomberg, 4 December 2020. [Google Scholar]
- The Race for Global COVID-19 Vaccine Equity; Duke Global Health Innovation Center: Durham, NC, USA, 2021.
- Guzman, J.; Hafner, T.; Maiga, L.A.; Giedion, U. COVID-19 vaccines pricing policy options for low-income and middle-income countries. BMJ Glob. Health 2021, 6, e005347. [Google Scholar] [CrossRef]
- A Patent Waiver on COVID Vaccines Is Right and Fair. Available online: https://www.nature.com/articles/d41586-021-01242-1 (accessed on 10 October 2021).
- McNeil, D.G., Jr. Oxfam Joins Campaign to Cut Drug Prices for Poor Nations. The New York Times, 13 February 2001. [Google Scholar]
- Médecins Sans Frontières. Untangling the Antiretroviral Price Reductions; MSF: Geneva, Switzerland, 2014. [Google Scholar]
- Elfarouk, O.; Wong, K.Y.; Wong, W.P. Multi-objective optimization for multi-echelon, multi-product, stochastic sustainable closed-loop supply chain. J. Ind. Prod. Eng. 2021, 1–19. [Google Scholar] [CrossRef]
- Taylor, P. AbbVie won’t enforce patents for COVID-19 drug candidate Kaletra. Pharmaphorum, 25 March 2020. [Google Scholar]
- Merck Sustainability Report 2020. Available online: https://www.merckgroup.com/en/cr-report/2020/products/health-for-all/innovation-sharing.html (accessed on 15 July 2021).
- Fidler, D.P. Vaccine nationalism’s politics. Science 2020, 369, 749. [Google Scholar] [CrossRef] [PubMed]
| Pfizer/BioNTech | AstraZeneca–Oxford | Janssen | Moderna | Sinopharm | Sinovac | |
|---|---|---|---|---|---|---|
| Effectiveness [43] 1 | 95% | 62–90% | 66% | 95% | 79–86% | 50–92% |
| Storage 2 | Ultra-cold (−60 to −80 °C) | Refrigeration (2~8 °C) | Refrigeration (2~8 °C) | Refrigeration (2 °C to 8 °C) for up to 30 days or frozen (−15 °C to −25 °C) for long-term storage | Refrigeration (2~8 °C) | Refrigeration (2~8 °C) |
| Manufacturing capacity in 2021 2 | 3 billion doses per year | 3 billion doses | 1 billion doses per year | Up to 1 billion doses | Up to 1 billion doses Beijing/Sinopharm combined with Wuhan/Sinopharm vaccine | Up to 2 billion doses per year |
| Company | Clinical Trial Protocol Shared | Clinical Trial Results Shared (Peer Reviewed) 1 | Published Contracts | |
|---|---|---|---|---|
| Number of Published Contracts 3 (%) | Number of Released Vaccine Prices (%) 4 | |||
| Pfizer/BioNTech | Yes | Phase I/II/III: 7 (3) Phase I/II: 3 (-) | 3 (7.1) | 8 (10.7) |
| AstraZeneca–Oxford | Yes | Phase I/II: 7 (3) Phase II/III: 4 (1) Phase III: 6 (-) | 5 (7.9) | 22 (31.4) |
| Janssen | Yes | Phase I/IIa: 2 (2) Phase III: 1 (-) | 1 (10.0) | 3 (16.7) |
| Moderna | Yes | Phase I: 3 (3) Phase III: 3 (1) | 1 (9.1) | 7 (24.1) |
| Sinopharm | No | Phase I/II: 2 (-) Phase III: 3 (-) | - | 7 (14.9) |
| Sinovac | Yes | Phase I/II: 3 (-) Phase III: 6 (-) | - | 9 (22.5) |
| Overall 2 |
|
|
| |
| Pfizer/BioNTech | AstraZeneca–Oxford | Janssen | Moderna | Sinopharm | Sinovac | Total | ||
|---|---|---|---|---|---|---|---|---|
| Total Secured Doses (Millions) | 4040 | 3434 | 923 | 1859 | 418 | 645 | 11,319 | |
| Number of doses by type of agreement (millions) | multilateral (%) | 1544 (38.2) | 1020 (29.7) | 657 (71.1) | 960 (51.6) | 60 (14.3) | 50 (7.7) | 4291 (37.9) |
| bilateral 1 (%) | 2496 (61.8) | 2414 (70.3) | 266 (28.9) | 899 (48.4) | 358 (85.6) | 595 (92.2) | 7029 (62.1) | |
| Number of doses by country income level (millions) 2 | COVAX 3 (%) | 540 5 (13.4) | 720 (21.0) | 200 (21.7) | 500 (26.9) | 60 (14.3) | 50 (7.7) | 2070 (18.3) |
| HIC (%) | 2701 (66.9) | 1025 (29.8) | 417 (45.1) | 1297 (69.7) | 11 (2.5) | 169 (26.2) | 5619 (49.6) | |
| UMIC (%) | 619 (15.3) | 455 (13.3) | 63 (6.8) | 37 (2.0) | 80 (19.2) | 198 (30.7) | 1453 (12.8) | |
| LMIC (%) | 179 (4.4) | 1216 (35.4) | 243 (26.4) | 25 (1.3) | 256 (61.3) | 227 (35.2) | 2147 (19.0) | |
| LIC (%) | 0 | 18 (0.5) | 0 | 0 | 11 (2.7) | 0.3 (0.1) | 11 (0.1) | |
| Total number of agreements | 75 | 70 | 18 | 29 | 47 | 40 | 279 | |
| Number of agreements by type of agreement 4 | multilateral (%) | 4 | 3 | 4 | 4 | 1 | 1 | 17 |
| bilateral (%) | 71 | 67 | 14 | 25 | 46 | 39 | 262 | |
| Number of agreements by country income level | COVAX | 2 | 2 | 1 | 1 | 1 | 1 | 8 |
| HIC | 33 (30 + 3) * | 20 (19 + 1) | 8 (6 + 2) | 20 | 5 | 5 | 7 | |
| UMIC | 24 | 19 | 4 | 5 | 19 | 19 | 90 | |
| LMIC | 16 | 28 | 5 (4 + 1) | 3 | 20 | 14 | 81 | |
| LIC | 0 | 1 | 0 | 0 | 2 | 1 | 4 | |
| (USD). | Pfizer/BioNTech | AstraZeneca–Oxford | Janssen | Moderna | Sinopharm | Sinovac | Average |
|---|---|---|---|---|---|---|---|
| Average | 14 | 5 | 9 | 25 | 24 | 18 | 13 |
| COVAX | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Minimum | 7 | 2 | 8 | 15 | 15 | 10 | N/A |
| HIC Average | 19 | 4 | 9 | 25 | 36 | N/A | 16 |
| UMIC Average | 11 | 4 | N/A2 | 25 | 24 | 22 | 15 |
| LMIC Average | 7 | 5 | 10 | N/A | 17 | 15 | 9 |
| Maximum | 23 | 13 | 10 | 37 | 36 | 32 | N/A |
| Coefficient | 0.82 | −0.26 | −0.02 | −0.13 | 0.78 | 0.67 | 0.22 |
| # 1 | 8 | 22 | 3 | 7 | 7 | 9 | N/A |
| Contract Development and Manufacturing Organization (CDMO) | Technology Transfer | |||||
|---|---|---|---|---|---|---|
| Company | HIC | UMIC, LMIC | Total | HIC | UMIC, LMIC | Total |
| Pfizer/BioNTech | 16 | 1 | 17 | 1 | 3 | 4 |
| AstraZeneca–Oxford | 16 | - | 16 | 2 | 7 | 9 |
| Janssen | 7 | 1 | 8 | - | 1 | 1 |
| Moderna | 10 | 1 | 11 | - | - | - |
| Sinopharm | - | - | - | 1 | 4 | 5 |
| Sinovac | - | - | - | - | 7 | 7 |
| Category | Indicators | Good | Need Improvements |
|---|---|---|---|
| Research and Development (4.1) | Effectiveness (4.1.1) |
|
|
| Storage 1 (4.1.1) |
|
| |
| Manufacturing capacity 2 (4.1.1) |
|
| |
| Funding (4.1.2) |
|
| |
| Profits (4.1.2) |
|
| |
| Transparency and Accountability (4.2) | Clinical trials (4.2.1) |
|
|
| Vaccine Contracts (4.2.2) |
| ||
| Product Delivery (4.3) | COVAX % (4.3.1) |
|
|
| Distribution to Lower Income Countries (4.3.2) |
| ||
| Equitable Pricing (4.3.3) |
|
| |
| Intellectual Property Strategy (4.3.4) |
|
| |
| Manufacturing Agreements (4.3.5) |
|
|
| Category | Indicators | Pfizer/BioNTech | AstraZeneca–Oxford | Janssen | Moderna | Sinopharm | Sinovac |
|---|---|---|---|---|---|---|---|
| Research and Development | Effectiveness | 1 | 1 | ||||
| Profits | 1 | 1 | |||||
| Transparency and Accountability | Clinical trials | 1 | 1 | 1 | 1 | 1 | |
| Vaccine Contracts | 1 | 1 | 1 | ||||
| Product Delivery | COVAX % | 1 | 1 | 1 | |||
| Distribution to Lower Income Countries | 1 | 1 | 1 | 1 | |||
| Equitable Pricing | 1 | 1 | 1 | ||||
| Intellectual Property Strategy | 1 | 1 | 1 | 1 | |||
| Manufacturing Agreements | 1 | 1 | 1 | 1 | |||
| Total | 4 | 6 | 5 | 4 | 5 | 6 | |
| Ranking | 3 | 1 | 2 | 3 | 2 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, M.; Huang, Y.; Duan, Y.; Liu, F.; Jin, Y.; Zheng, Z. Pharmaceutical Industry’s Engagement in the Global Equitable Distribution of COVID-19 Vaccines: Corporate Social Responsibility of EUL Vaccine Developers. Vaccines 2021, 9, 1183. https://doi.org/10.3390/vaccines9101183
Sung M, Huang Y, Duan Y, Liu F, Jin Y, Zheng Z. Pharmaceutical Industry’s Engagement in the Global Equitable Distribution of COVID-19 Vaccines: Corporate Social Responsibility of EUL Vaccine Developers. Vaccines. 2021; 9(10):1183. https://doi.org/10.3390/vaccines9101183
Chicago/Turabian StyleSung, Meekang, Yangmu Huang, Yuqi Duan, Fangjing Liu, Yinzi Jin, and Zhijie Zheng. 2021. "Pharmaceutical Industry’s Engagement in the Global Equitable Distribution of COVID-19 Vaccines: Corporate Social Responsibility of EUL Vaccine Developers" Vaccines 9, no. 10: 1183. https://doi.org/10.3390/vaccines9101183
APA StyleSung, M., Huang, Y., Duan, Y., Liu, F., Jin, Y., & Zheng, Z. (2021). Pharmaceutical Industry’s Engagement in the Global Equitable Distribution of COVID-19 Vaccines: Corporate Social Responsibility of EUL Vaccine Developers. Vaccines, 9(10), 1183. https://doi.org/10.3390/vaccines9101183








