Resistance Mechanisms Influencing Oncolytic Virotherapy, a Systematic Analysis
Abstract
1. Introduction
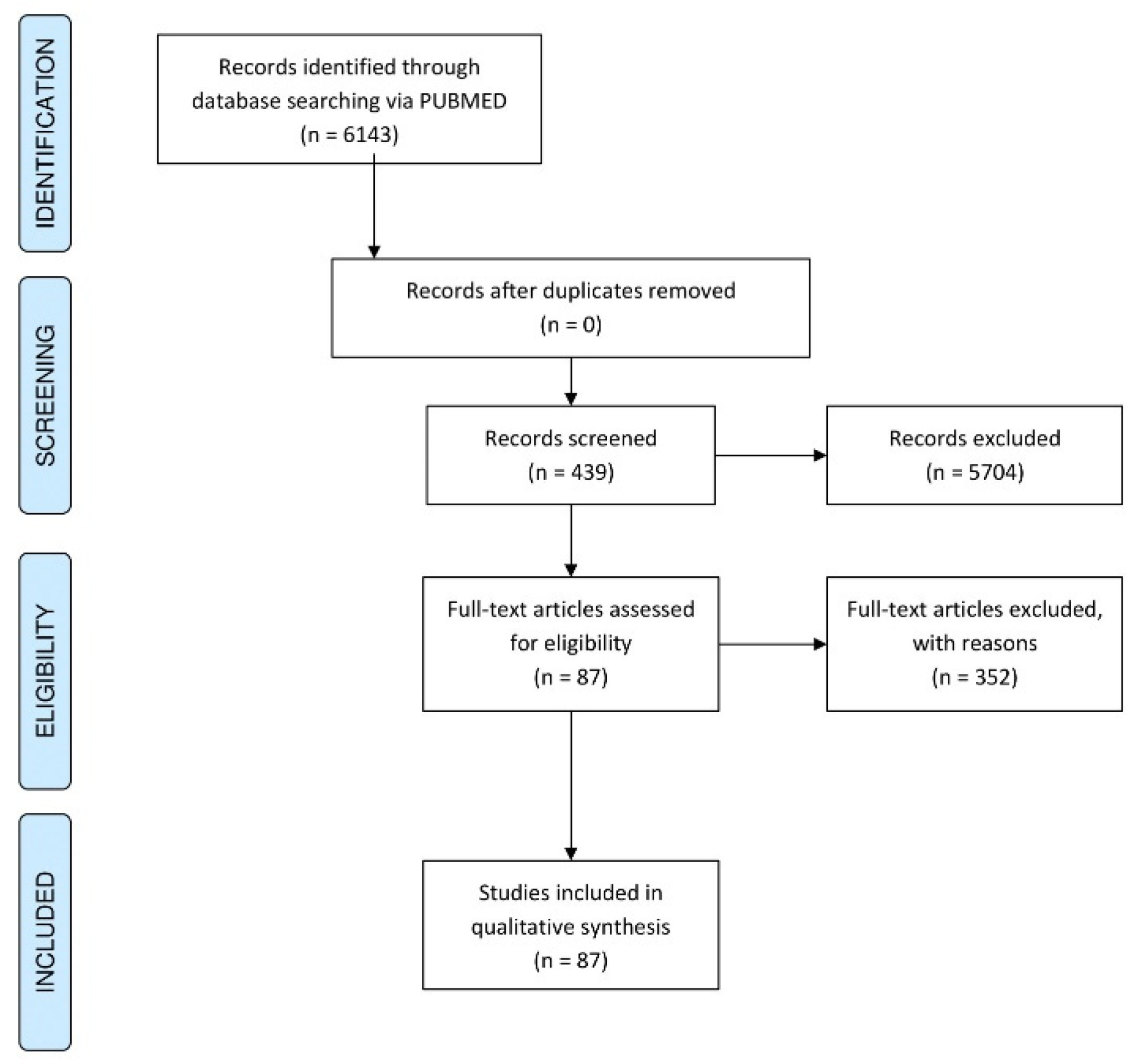
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aktipis, C.A.; Kwan, V.S.Y.; Johnson, K.A.; Neuberg, S.L.; Maley, C.C. Overlooking Evolution: A Systematic Analysis of Cancer Relapse and Therapeutic Resistance Research. PLoS ONE 2011, 6, e26100. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat. Rev. Drug. Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Twumasi-Boateng, K.; Pettigrew, J.L.; Kwok, Y.Y.E.; Bell, J.C.; Nelson, B.H. Oncolytic Viruses as Engineering Platforms for Combination Immunotherapy. Nat. Rev. Cancer 2018, 18, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Buijs, P.R.A.; Verhagen, J.H.E.; van Eijck, C.H.J.; van den Hoogen, B.G. Oncolytic Viruses: From Bench to Bedside with a Focus on Safety. Hum. Vaccin Immunother. 2015, 11, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Možina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. In Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18 September 2017; ACM: Cagliari, Italy, 2017; pp. 1–5. [Google Scholar]
- Arwert, E.N.; Milford, E.L.; Rullan, A.; Derzsi, S.; Hooper, S.; Kato, T.; Mansfield, D.; Melcher, A.; Harrington, K.J.; Sahai, E. STING and IRF3 in Stromal Fibroblasts Enable Sensing of Genomic Stress in Cancer Cells to Undermine Oncolytic Viral Therapy. Nat. Cell Biol. 2020, 22, 758–766. [Google Scholar] [CrossRef]
- Van Asten, S.D.; Raaben, M.; Nota, B.; Spaapen, R.M. Secretome Screening Reveals Fibroblast Growth Factors as Novel Inhibitors of Viral Replication. J. Virol. 2018, 92, e00260-18. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.G.; Valdes, Y.R.; Sivapragasam, M.; Barrett, J.W.; Bell, J.C.; Stojdl, D.; DiMattia, G.E.; Shepherd, T.G. Spatial and Temporal Epithelial Ovarian Cancer Cell Heterogeneity Impacts Maraba Virus Oncolytic Potential. BMC Cancer 2017, 17, 594. [Google Scholar] [CrossRef] [PubMed]
- Yumul, R.; Richter, M.; Lu, Z.-Z.; Saydaminova, K.; Wang, H.; Wang, C.-H.K.; Carter, D.; Lieber, A. Epithelial Junction Opener Improves Oncolytic Adenovirus Therapy in Mouse Tumor Models. Hum. Gene Ther. 2016, 27, 325–337. [Google Scholar] [CrossRef]
- Tseng, J.-C.; Granot, T.; DiGiacomo, V.; Levin, B.; Meruelo, D. Enhanced Specific Delivery and Targeting of Oncolytic Sindbis Viral Vectors by Modulating Vascular Leakiness in Tumor. Cancer Gene Ther. 2010, 17, 244–255. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Suksanpaisan, L.; Steele, M.B.; Russell, S.J.; Peng, K.-W. Induction of Antiviral Genes by the Tumor Microenvironment Confers Resistance to Virotherapy. Sci. Rep. 2013, 3, 2375. [Google Scholar] [CrossRef]
- Janelle, V.; Brassard, F.; Lapierre, P.; Lamarre, A.; Poliquin, L. Mutations in the Glycoprotein of Vesicular Stomatitis Virus Affect Cytopathogenicity: Potential for Oncolytic Virotherapy. J. Virol. 2011, 85, 6513–6520. [Google Scholar] [CrossRef]
- Fu, X.; Tao, L.; Rivera, A.; Zhang, X. Rapamycin Enhances the Activity of Oncolytic Herpes Simplex Virus against Tumor Cells That Are Resistant to Virus Replication. Int. J. Cancer 2011, 129, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Paglino, J.C.; van den Pol, A.N. Vesicular Stomatitis Virus Has Extensive Oncolytic Activity against Human Sarcomas: Rare Resistance Is Overcome by Blocking Interferon Pathways. J. Virol. 2011, 85, 9346–9358. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-J.; Haddad, D.; Adusumilli, P.; Kim, T.; Stiles, B.; Hezel, M.; Socci, N.D.; Gönen, M.; Fong, Y. Molecular Network Pathways and Functional Analysis of Tumor Signatures Associated with Development of Resistance to Viral Gene Therapy. Cancer Gene Ther. 2012, 19, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; Berchtold, S.; Lampe, J.; Malek, N.P.; Bitzer, M.; Lauer, U.M. Primary Resistance Phenomena to Oncolytic Measles Vaccine Viruses. Int. J. Oncol. 2013, 43, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Le Bœuf, F.; Batenchuk, C.; Vähä-Koskela, M.; Breton, S.; Roy, D.; Lemay, C.; Cox, J.; Abdelbary, H.; Falls, T.; Waghray, G.; et al. Model-Based Rational Design of an Oncolytic Virus with Improved Therapeutic Potential. Nat. Commun. 2013, 4, 1974. [Google Scholar] [CrossRef] [PubMed]
- Voros, A.; Kormos, B.; Valyi-Nagy, T.; Valyi-Nagy, K. Increased Resistance of Breast, Prostate, and Embryonic Carcinoma Cells against Herpes Simplex Virus in Three-Dimensional Cultures. ISRN Oncol. 2013, 2013, 104913. [Google Scholar] [CrossRef] [PubMed]
- Paglino, J.C.; Andres, W.; van den Pol, A.N. Autonomous Parvoviruses Neither Stimulate nor Are Inhibited by the Type I Interferon Response in Human Normal or Cancer Cells. J. Virol. 2014, 88, 4932–4942. [Google Scholar] [CrossRef]
- Cronin, M.; Le Boeuf, F.; Murphy, C.; Roy, D.G.; Falls, T.; Bell, J.C.; Tangney, M. Bacterial-Mediated Knockdown of Tumor Resistance to an Oncolytic Virus Enhances Therapy. Mol. Ther. 2014, 22, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Su, B.-H.; Shieh, G.-S.; Tseng, Y.-L.; Shiau, A.-L.; Wu, C.-L. Etoposide Enhances Antitumor Efficacy of MDR1-Driven Oncolytic Adenovirus through Autoupregulation of the MDR1 Promoter Activity. Oncotarget 2015, 6, 38308–38326. [Google Scholar] [CrossRef]
- Vähä-Koskela, M.; Tähtinen, S.; Grönberg-Vähä-Koskela, S.; Taipale, K.; Saha, D.; Merisalo-Soikkeli, M.; Ahonen, M.; Rouvinen-Lagerström, N.; Hirvinen, M.; Veckman, V.; et al. Overcoming Tumor Resistance by Heterologous Adeno-Poxvirus Combination Therapy. Mol. Ther. Oncolytics 2015, 1, 14006. [Google Scholar] [CrossRef]
- Hou, W.; Sampath, P.; Rojas, J.J.; Thorne, S.H. Oncolytic Virus-Mediated Targeting of PGE2 in the Tumor Alters the Immune Status and Sensitizes Established and Resistant Tumors to Immunotherapy. Cancer Cell 2016, 30, 108–119. [Google Scholar] [CrossRef]
- Kleinlützum, D.; Hanauer, J.D.S.; Muik, A.; Hanschmann, K.-M.; Kays, S.-K.; Ayala-Breton, C.; Peng, K.-W.; Mühlebach, M.D.; Abel, T.; Buchholz, C.J. Enhancing the Oncolytic Activity of CD133-Targeted Measles Virus: Receptor Extension or Chimerism with Vesicular Stomatitis Virus Are Most Effective. Front. Oncol. 2017, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Ou, P.; Rousso, C.; Bergeron, A.; Krishnan, R.; Pikor, L.; Chen, A.; Keller, B.A.; Ilkow, C.; Bell, J.C.; et al. Dimethyl Fumarate Potentiates Oncolytic Virotherapy through NF-ΚB Inhibition. Sci. Transl. Med. 2018, 10, eaao1613. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Ricca, J.M.; Sadekova, S.; Oseledchyk, A.; Yu, Y.; Blumenschein, W.M.; Wong, J.; Gigoux, M.; Merghoub, T.; Wolchok, J.D. PD-L1 in Tumor Microenvironment Mediates Resistance to Oncolytic Immunotherapy. J. Clin. Investig. 2018, 128, 1413–1428. [Google Scholar] [CrossRef]
- Kurokawa, C.; Iankov, I.D.; Anderson, S.K.; Aderca, I.; Leontovich, A.A.; Maurer, M.J.; Oberg, A.L.; Schroeder, M.A.; Giannini, C.; Greiner, S.M.; et al. Constitutive Interferon Pathway Activation in Tumors as an Efficacy Determinant Following Oncolytic Virotherapy. J. Nat. Cancer Inst. 2018, 110, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liang, J.; Huang, C.; Li, K.; Xing, F.; Zhu, W.; Lin, Z.; Xu, W.; Wu, G.; Zhang, J.; et al. DNA-PK Inhibition Synergizes with Oncolytic Virus M1 by Inhibiting Antiviral Response and Potentiating DNA Damage. Nat. Commun. 2018, 9, 4342. [Google Scholar] [CrossRef]
- Nakatake, M.; Kurosaki, H.; Kuwano, N.; Horita, K.; Ito, M.; Kono, H.; Okamura, T.; Hasegawa, K.; Yasutomi, Y.; Nakamura, T. Partial Deletion of Glycoprotein B5R Enhances Vaccinia Virus Neutralization Escape While Preserving Oncolytic Function. Mol. Ther. Oncolytics 2019, 14, 159–171. [Google Scholar] [CrossRef]
- Berchtold, S.; Beil, J.; Raff, C.; Smirnow, I.; Schell, M.; D’Alvise, J.; Gross, S.; Lauer, U.M. Assessing and Overcoming Resistance Phenomena against a Genetically Modified Vaccinia Virus in Selected Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 7618. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kojima, T.; Kagawa, S.; Uno, F.; Hashimoto, Y.; Kyo, S.; Mizuguchi, H.; Tanaka, N.; Kawamura, H.; Ichimaru, D.; et al. A Novel Translational Approach for Human Malignant Pleural Mesothelioma: Heparanase-Assisted Dual Virotherapy. Oncogene 2010, 29, 1145–1154. [Google Scholar] [CrossRef]
- Felt, S.A.; Droby, G.N.; Grdzelishvili, V.Z. Ruxolitinib and Polycation Combination Treatment Overcomes Multiple Mechanisms of Resistance of Pancreatic Cancer Cells to Oncolytic Vesicular Stomatitis Virus. J. Virol. 2017, 91, e00461-17. [Google Scholar] [CrossRef] [PubMed]
- Tuzmen, C.; Cairns, T.M.; Atanasiu, D.; Lou, H.; Saw, W.T.; Hall, B.L.; Cohen, J.B.; Cohen, G.H.; Glorioso, J.C. Point Mutations in Retargeted GD Eliminate the Sensitivity of EGFR/EGFRvIII-Targeted HSV to Key Neutralizing Antibodies. Mol. Ther. Methods Clin. Dev. 2020, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; O’Leary, M.P.; Lu, J.; Kim, S.-I.; Fong, Y.; Chen, N.G. Endogenous Akt Activity Promotes Virus Entry and Predicts Efficacy of Novel Chimeric Orthopoxvirus in Triple-Negative Breast Cancer. Mol. Ther. Oncolytics 2018, 9, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhu, W.; Huang, X.; You, L.; Han, X.; Yang, C.; Qian, W. PI3K Inhibitor LY294002 Inhibits Activation of the Akt/MTOR Pathway Induced by an Oncolytic Adenovirus Expressing TRAIL and Sensitizes Multiple Myeloma Cells to the Oncolytic Virus. Oncol. Rep. 2014, 31, 1581–1588. [Google Scholar] [CrossRef]
- Lucas, T.; Benihoud, K.; Vigant, F.; Schmidt, C.Q.; Wortmann, A.; Bachem, M.G.; Simmet, T.; Kochanek, S. Hexon Modification to Improve the Activity of Oncolytic Adenovirus Vectors against Neoplastic and Stromal Cells in Pancreatic Cancer. PLoS ONE 2015, 10, e0117254. [Google Scholar] [CrossRef]
- Shulak, L.; Beljanski, V.; Chiang, C.; Dutta, S.M.; Van Grevenynghe, J.; Belgnaoui, S.M.; Nguyên, T.L.-A.; Di Lenardo, T.; Semmes, O.J.; Lin, R.; et al. Histone Deacetylase Inhibitors Potentiate Vesicular Stomatitis Virus Oncolysis in Prostate Cancer Cells by Modulating NF-ΚB-Dependent Autophagy. J. Virol. 2014, 88, 2927–2940. [Google Scholar] [CrossRef]
- Bieler, A.; Mantwill, K.; Dravits, T.; Bernshausen, A.; Glockzin, G.; Köhler-Vargas, N.; Lage, H.; Gansbacher, B.; Holm, P.S. Novel Three-Pronged Strategy to Enhance Cancer Cell Killing in Glioblastoma Cell Lines: Histone Deacetylase Inhibitor, Chemotherapy, and Oncolytic Adenovirus Dl520. Hum. Gene Ther. 2006, 17, 55–70. [Google Scholar] [CrossRef]
- Trus, I.; Berube, N.; Jiang, P.; Rak, J.; Gerdts, V.; Karniychuk, U. Zika Virus with Increased CpG Dinucleotide Frequencies Shows Oncolytic Activity in Glioblastoma Stem Cells. Viruses 2020, 12, 579. [Google Scholar] [CrossRef]
- Huff, A.L.; Wongthida, P.; Kottke, T.; Thompson, J.M.; Driscoll, C.B.; Schuelke, M.; Shim, K.G.; Harris, R.S.; Molan, A.; Pulido, J.S.; et al. APOBEC3 Mediates Resistance to Oncolytic Viral Therapy. Mol. Ther. Oncolytics 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Evgin, L.; Huff, A.L.; Kottke, T.; Thompson, J.; Molan, A.M.; Driscoll, C.B.; Schuelke, M.; Shim, K.G.; Wongthida, P.; Ilett, E.J.; et al. Suboptimal T-Cell Therapy Drives a Tumor Cell Mutator Phenotype That Promotes Escape from First-Line Treatment. Cancer Immunol. Res. 2019, 7, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Toribio, R.; Díaz-López, I.; Berlanga, J.J.; Molina-Jiménez, F.; Majano, P.; Ventoso, I. Naturally Occurring and Engineered Alphaviruses Sensitive to Double-Stranded-RNA-Activated Protein Kinase Show Restricted Translation in Mammalian Cells, Increased Sensitivity to Interferon, and Marked Oncotropism. J. Virol. 2020, 94, e01630-19. [Google Scholar] [CrossRef]
- Lv, C.; Su, Q.; Liang, Y.; Hu, J.; Yuan, S. Oncolytic Vaccine Virus Harbouring the IL-24 Gene Suppresses the Growth of Lung Cancer by Inducing Apoptosis. Biochem. Biophys. Res. Commun. 2016, 476, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; You, L.-S.; Mao, L.-P.; Jin, S.-H.; Chen, X.-H.; Qian, W.-B. Combing Oncolytic Adenovirus Expressing Beclin-1 with Chemotherapy Agent Doxorubicin Synergistically Enhances Cytotoxicity in Human CML Cells in Vitro. Acta Pharm. Sin. 2018, 39, 251–260. [Google Scholar] [CrossRef]
- Colunga, A.; Bollino, D.; Schech, A.; Aurelian, L. Calpain-Dependent Clearance of the Autophagy Protein P62/SQSTM1 Is a Contributor to ΔPK Oncolytic Activity in Melanoma. Gene Ther. 2014, 21, 371–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, K.; Li, Y.; Zhu, Q.; Xu, J.; Wang, Y.; Deng, W.; Liu, Q.; Zhang, G.; Meng, S. Pharmacological Modulation of Autophagy Enhances Newcastle Disease Virus-Mediated Oncolysis in Drug-Resistant Lung Cancer Cells. BMC Cancer 2014, 14, 551. [Google Scholar] [CrossRef]
- Hastie, E.; Cataldi, M.; Steuerwald, N.; Grdzelishvili, V.Z. An Unexpected Inhibition of Antiviral Signaling by Virus-Encoded Tumor Suppressor P53 in Pancreatic Cancer Cells. Virology 2015, 483, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Sunamura, M.; Hamada, H.; Motoi, F.; Oonuma, M.; Abe, H.; Saitoh, Y.; Hoshida, T.; Ottomo, S.; Omura, N.; Matsuno, S. Oncolytic Virotherapy as a Novel Strategy for Pancreatic Cancer. Pancreas 2004, 28, 326–329. [Google Scholar] [CrossRef]
- Cataldi, M.; Shah, N.R.; Felt, S.A.; Grdzelishvili, V.Z. Breaking Resistance of Pancreatic Cancer Cells to an Attenuated Vesicular Stomatitis Virus through a Novel Activity of IKK Inhibitor TPCA-1. Virology 2015, 485, 340–354. [Google Scholar] [CrossRef]
- Hoang, H.-D.; Graber, T.E.; Jia, J.-J.; Vaidya, N.; Gilchrist, V.H.; Xiang, X.; Li, W.; Cowan, K.N.; Gkogkas, C.G.; Jaramillo, M.; et al. Induction of an Alternative MRNA 5’ Leader Enhances Translation of the Ciliopathy Gene Inpp5e and Resistance to Oncolytic Virus Infection. Cell Rep. 2019, 29, 4010–4023.e5. [Google Scholar] [CrossRef]
- Gholami, S.; Chen, C.-H.; Gao, S.; Lou, E.; Fujisawa, S.; Carson, J.; Nnoli, J.E.; Chou, T.-C.; Bromberg, J.; Fong, Y. Role of MAPK in Oncolytic Herpes Viral Therapy in Triple-Negative Breast Cancer. Cancer Gene Ther. 2014, 21, 283–289. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Aspromonte, S.; Zloza, A.; Rabkin, S.D.; Kaufman, H.L. MEK Inhibition Enhances Oncolytic Virus Immunotherapy through Increased Tumor Cell Killing and T Cell Activation. Sci. Transl. Med. 2018, 10, 417. [Google Scholar] [CrossRef]
- Li, W.; Turaga, R.C.; Li, X.; Sharma, M.; Enadi, Z.; Dunham Tompkins, S.N.; Hardy, K.C.; Mishra, F.; Tsao, J.; Liu, Z.-R.; et al. Overexpression of Smac by an Armed Vesicular Stomatitis Virus Overcomes Tumor Resistance. Mol. Ther. Oncolytics 2019, 14, 188–195. [Google Scholar] [CrossRef]
- Dobson, C.C.; Naing, T.; Beug, S.T.; Faye, M.D.; Chabot, J.; St-Jean, M.; Walker, D.E.; LaCasse, E.C.; Stojdl, D.F.; Korneluk, R.G.; et al. Oncolytic Virus Synergizes with Smac Mimetic Compounds to Induce Rhabdomyosarcoma Cell Death in a Syngeneic Murine Model. Oncotarget 2017, 8, 3495–3508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muscolini, M.; Castiello, L.; Palermo, E.; Zevini, A.; Ferrari, M.; Olagnier, D.; Hiscott, J. SIRT1 Modulates the Sensitivity of Prostate Cancer Cells to Vesicular Stomatitis Virus Oncolysis. J. Virol. 2019, 93, e00626-19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhuo, B.; Yin, Y.; Han, T.; Li, S.; Li, Z.; Wang, J. Anti-Cancer Effect of Oncolytic Adenovirus-Armed ShRNA Targeting MYCN Gene on Doxorubicin-Resistant Neuroblastoma Cells. Biochem. Biophys. Res. Commun. 2017, 491, 134–139. [Google Scholar] [CrossRef]
- Li, I.; Nabet, B.Y. Exosomes in the Tumor Microenvironment as Mediators of Cancer Therapy Resistance. Mol. Cancer 2019, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Urbiola, C.; Santer, F.R.; Petersson, M.; van der Pluijm, G.; Horninger, W.; Erlmann, P.; Wollmann, G.; Kimpel, J.; Culig, Z.; von Laer, D. Oncolytic Activity of the Rhabdovirus VSV-GP against Prostate Cancer. Int. J. Cancer 2018, 143, 1786–1796. [Google Scholar] [CrossRef]
- Allagui, F.; Achard, C.; Panterne, C.; Combredet, C.; Labarrière, N.; Dréno, B.; Elgaaied, A.B.; Pouliquen, D.; Tangy, F.; Fonteneau, J.-F.; et al. Modulation of the Type I Interferon Response Defines the Sensitivity of Human Melanoma Cells to Oncolytic Measles Virus. Curr. Gene Ther. 2017, 16, 419–428. [Google Scholar] [CrossRef]
- Tarasova, I.A.; Tereshkova, A.V.; Lobas, A.A.; Solovyeva, E.M.; Sidorenko, A.S.; Gorshkov, V.; Kjeldsen, F.; Bubis, J.A.; Ivanov, M.V.; Ilina, I.Y.; et al. Comparative Proteomics as a Tool for Identifying Specific Alterations within Interferon Response Pathways in Human Glioblastoma Multiforme Cells. Oncotarget 2018, 9, 1785–1802. [Google Scholar] [CrossRef]
- Kaowinn, S.; Cho, I.-R.; Moon, J.; Jun, S.W.; Kim, C.S.; Kang, H.Y.; Kim, M.; Koh, S.S.; Chung, Y.-H. Pancreatic Adenocarcinoma Upregulated Factor (PAUF) Confers Resistance to Pancreatic Cancer Cells against Oncolytic Parvovirus H-1 Infection through IFNA Receptor-Mediated Signaling. Biochem. Biophys. Res. Commun. 2015, 459, 313–318. [Google Scholar] [CrossRef]
- Westcott, M.M.; Liu, J.; Rajani, K.; D’Agostino, R.J.; Lyles, D.S.; Porosnicu, M. Interferon Beta and Interferon Alpha 2a Differentially Protect Head and Neck Cancer Cells from Vesicular Stomatitis Virus-Induced Oncolysis. J. Virol. 2015, 89, 7944–7954. [Google Scholar] [CrossRef]
- Vähä-Koskela, M.J.V.; Le Boeuf, F.; Lemay, C.; De Silva, N.; Diallo, J.-S.; Cox, J.; Becker, M.; Choi, Y.; Ananth, A.; Sellers, C.; et al. Resistance to Two Heterologous Neurotropic Oncolytic Viruses, Semliki Forest Virus and Vaccinia Virus, in Experimental Glioma. J. Virol. 2013, 87, 2363–2366. [Google Scholar] [CrossRef]
- Moerdyk-Schauwecker, M.; Shah, N.R.; Murphy, A.M.; Hastie, E.; Mukherjee, P.; Grdzelishvili, V.Z. Resistance of Pancreatic Cancer Cells to Oncolytic Vesicular Stomatitis Virus: Role of Type I Interferon Signaling. Virology 2013, 436, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Liikanen, I.; Monsurrò, V.; Ahtiainen, L.; Raki, M.; Hakkarainen, T.; Diaconu, I.; Escutenaire, S.; Hemminki, O.; Dias, J.D.; Cerullo, V.; et al. Induction of Interferon Pathways Mediates in Vivo Resistance to Oncolytic Adenovirus. Mol. Ther. 2011, 19, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Saloura, V.; Wang, L.-C.S.; Fridlender, Z.G.; Sun, J.; Cheng, G.; Kapoor, V.; Sterman, D.H.; Harty, R.N.; Okumura, A.; Barber, G.N.; et al. Evaluation of an Attenuated Vesicular Stomatitis Virus Vector Expressing Interferon-Beta for Use in Malignant Pleural Mesothelioma: Heterogeneity in Interferon Responsiveness Defines Potential Efficacy. Hum. Gene Ther. 2010, 21, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Dash, A.; Jacobson, B.A.; Ji, Y.; Baumann, D.; Ismail, K.; Kratzke, R.A. JAK/STAT Inhibition with Ruxolitinib Enhances Oncolytic Virotherapy in Non-Small Cell Lung Cancer Models. Cancer Gene Ther. 2019, 26, 411–418. [Google Scholar] [CrossRef]
- Udayakumar, T.S.; Betancourt, D.M.; Ahmad, A.; Tao, W.; Totiger, T.M.; Patel, M.; Marples, B.; Barber, G.; Pollack, A. Radiation Attenuates Prostate Tumor Antiviral Responses to Vesicular Stomatitis Virus Containing IFNβ, Resulting in Pronounced Antitumor Systemic Immune Responses. Mol. Cancer Res. 2020, 18, 1232–1243. [Google Scholar] [CrossRef]
- Dold, C.; Rodriguez Urbiola, C.; Wollmann, G.; Egerer, L.; Muik, A.; Bellmann, L.; Fiegl, H.; Marth, C.; Kimpel, J.; von Laer, D. Application of Interferon Modulators to Overcome Partial Resistance of Human Ovarian Cancers to VSV-GP Oncolytic Viral Therapy. Mol. Ther. Oncolytics 2016, 3, 16021. [Google Scholar] [CrossRef]
- Diallo, J.-S.; Le Boeuf, F.; Lai, F.; Cox, J.; Vaha-Koskela, M.; Abdelbary, H.; MacTavish, H.; Waite, K.; Falls, T.; Wang, J.; et al. A High-Throughput Pharmacoviral Approach Identifies Novel Oncolytic Virus Sensitizers. Mol. Ther. 2010, 18, 1123–1129. [Google Scholar] [CrossRef]
- Lypova, N.; Lanceta, L.; Gibson, A.; Vega, S.; Garza-Morales, R.; McMasters, K.M.; Chesney, J.; Gomez-Gutierrez, J.G.; Imbert-Fernandez, Y. Targeting Palbociclib-Resistant Estrogen Receptor-Positive Breast Cancer Cells via Oncolytic Virotherapy. Cancers 2019, 11, 684. [Google Scholar] [CrossRef]
- Sivanandam, V.; LaRocca, C.J.; Chen, N.G.; Fong, Y.; Warner, S.G. Oncolytic Viruses and Immune Checkpoint Inhibition: The Best of Both Worlds. Mol. Ther. Oncolytics 2019, 13, 93–106. [Google Scholar] [CrossRef]
- Melero, I.; Quetglas, J.I.; Reboredo, M.; Dubrot, J.; Rodriguez-Madoz, J.R.; Mancheño, U.; Casales, E.; Riezu-Boj, J.I.; Ruiz-Guillen, M.; Ochoa, M.C.; et al. Strict Requirement for Vector-Induced Type I Interferon in Efficacious Antitumor Responses to Virally Encoded IL12. Cancer Res. 2015, 75, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.K.; Daffis, S.; Brien, J.D.; Gainey, M.D.; Yokoyama, W.M.; Sheehan, K.C.F.; Murphy, K.M.; Schreiber, R.D.; Diamond, M.S. A Temporal Role of Type I Interferon Signaling in CD8+ T Cell Maturation during Acute West Nile Virus Infection. PLoS Pathog. 2011, 7, e1002407. [Google Scholar] [CrossRef]
- Biswas, M.; Johnson, J.B.; Kumar, S.R.P.; Parks, G.D.; Elankumarana, S. Incorporation of Host Complement Regulatory Proteins into Newcastle Disease Virus Enhances Complement Evasion. J. Virol. 2012, 86, 12708–12716. [Google Scholar] [CrossRef] [PubMed]
- Zemp, F.J.; McKenzie, B.A.; Lun, X.; Reilly, K.M.; McFadden, G.; Yong, V.W.; Forsyth, P.A. Cellular Factors Promoting Resistance to Effective Treatment of Glioma with Oncolytic Myxoma Virus. Cancer Res. 2014, 74, 7260–7273. [Google Scholar] [CrossRef]
- Carey, B.L.; Ahmed, M.; Puckett, S.; Lyles, D.S. Early Steps of the Virus Replication Cycle Are Inhibited in Prostate Cancer Cells Resistant to Oncolytic Vesicular Stomatitis Virus. J. Virol. 2008, 82, 12104–12115. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Seshadri, M.; Komorowski, M.P.; Abrams, S.I.; Kozbor, D. Targeting CXCL12/CXCR4 Signaling with Oncolytic Virotherapy Disrupts Tumor Vasculature and Inhibits Breast Cancer Metastases. Proc. Natl. Acad. Sci. USA 2013, 110, E1291–E1300. [Google Scholar] [CrossRef]
- Reinblatt, M.; Pin, R.H.; Federoff, H.J.; Fong, Y. Utilizing Tumor Hypoxia to Enhance Oncolytic Viral Therapy in Colorectal Metastases. Ann. Surg. 2004, 239, 892–899. [Google Scholar] [CrossRef]
- Valyi-Nagy, K.; Dosa, S.; Kovacs, S.K.; Bacsa, S.; Voros, A.; Shukla, D.; Folberg, R.; Valyi-Nagy, T. Identification of Virus Resistant Tumor Cell Subpopulations in Three-Dimensional Uveal Melanoma Cultures. Cancer Gene Ther. 2010, 17, 223–234. [Google Scholar] [CrossRef]
- Sun, C.W.; Willmon, C.; Wu, L.-C.; Knopick, P.; Thoerner, J.; Vile, R.; Townes, T.M.; Terman, D.S. Sickle Cells Abolish Melanoma Tumorigenesis in Hemoglobin SS Knockin Mice and Augment the Tumoricidal Effect of Oncolytic Virus In Vivo. Front. Oncol. 2016, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Ilkow, C.S.; Marguerie, M.; Batenchuk, C.; Mayer, J.; Ben Neriah, D.; Cousineau, S.; Falls, T.; Jennings, V.A.; Boileau, M.; Bellamy, D.; et al. Reciprocal Cellular Cross-Talk within the Tumor Microenvironment Promotes Oncolytic Virus Activity. Nat. Med. 2015, 21, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Ilett, E.J.; Bárcena, M.; Errington-Mais, F.; Griffin, S.; Harrington, K.J.; Pandha, H.S.; Coffey, M.; Selby, P.J.; Limpens, R.W.A.L.; Mommaas, M.; et al. Internalization of Oncolytic Reovirus by Human Dendritic Cell Carriers Protects the Virus from Neutralization. Clin. Cancer Res. 2011, 17, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Jennings, V.A.; Ilett, E.J.; Scott, K.J.; West, E.J.; Vile, R.; Pandha, H.; Harrington, K.; Young, A.; Hall, G.D.; Coffey, M.; et al. Lymphokine-activated Killer and Dendritic Cell Carriage Enhances Oncolytic Reovirus Therapy for Ovarian Cancer by Overcoming Antibody Neutralization in Ascites. Int. J. Cancer 2014, 134, 1091–1101. [Google Scholar] [CrossRef]
- Eisenstein, S.; Coakley, B.A.; Briley-Saebo, K.; Ma, G.; Chen, H.; Meseck, M.; Ward, S.; Divino, C.; Woo, S.; Chen, S.-H.; et al. Myeloid-Derived Suppressor Cells as a Vehicle for Tumor-Specific Oncolytic Viral Therapy. Cancer Res. 2013, 73, 5003–5015. [Google Scholar] [CrossRef]
- Guillerme, J.-B.; Boisgerault, N.; Roulois, D.; Ménager, J.; Combredet, C.; Tangy, F.; Fonteneau, J.-F.; Gregoire, M. Measles Virus Vaccine–Infected Tumor Cells Induce Tumor Antigen Cross-Presentation by Human Plasmacytoid Dendritic Cells. Clin. Cancer Res. 2013, 19, 1147–1158. [Google Scholar] [CrossRef]
- Kilinc, M.O.; Ehrig, K.; Pessian, M.; Minev, B.R.; Szalay, A.A. Colonization of Xenograft Tumors by Oncolytic Vaccinia Virus (VACV) Results in Enhanced Tumor Killing Due to the Involvement of Myeloid Cells. J. Transl. Med. 2016, 14, 340. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, D.K.; Chammas, R.; Daemen, T. Resistance Mechanisms Influencing Oncolytic Virotherapy, a Systematic Analysis. Vaccines 2021, 9, 1166. https://doi.org/10.3390/vaccines9101166
Bhatt DK, Chammas R, Daemen T. Resistance Mechanisms Influencing Oncolytic Virotherapy, a Systematic Analysis. Vaccines. 2021; 9(10):1166. https://doi.org/10.3390/vaccines9101166
Chicago/Turabian StyleBhatt, Darshak K., Roger Chammas, and Toos Daemen. 2021. "Resistance Mechanisms Influencing Oncolytic Virotherapy, a Systematic Analysis" Vaccines 9, no. 10: 1166. https://doi.org/10.3390/vaccines9101166
APA StyleBhatt, D. K., Chammas, R., & Daemen, T. (2021). Resistance Mechanisms Influencing Oncolytic Virotherapy, a Systematic Analysis. Vaccines, 9(10), 1166. https://doi.org/10.3390/vaccines9101166






