Pentavalent Disabled Infectious Single Animal (DISA)/DIVA Vaccine Provides Protection in Sheep and Cattle against Different Serotypes of Bluetongue Virus
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Rescue of DISA/DIVA Vaccines
3.2. Animal Trials
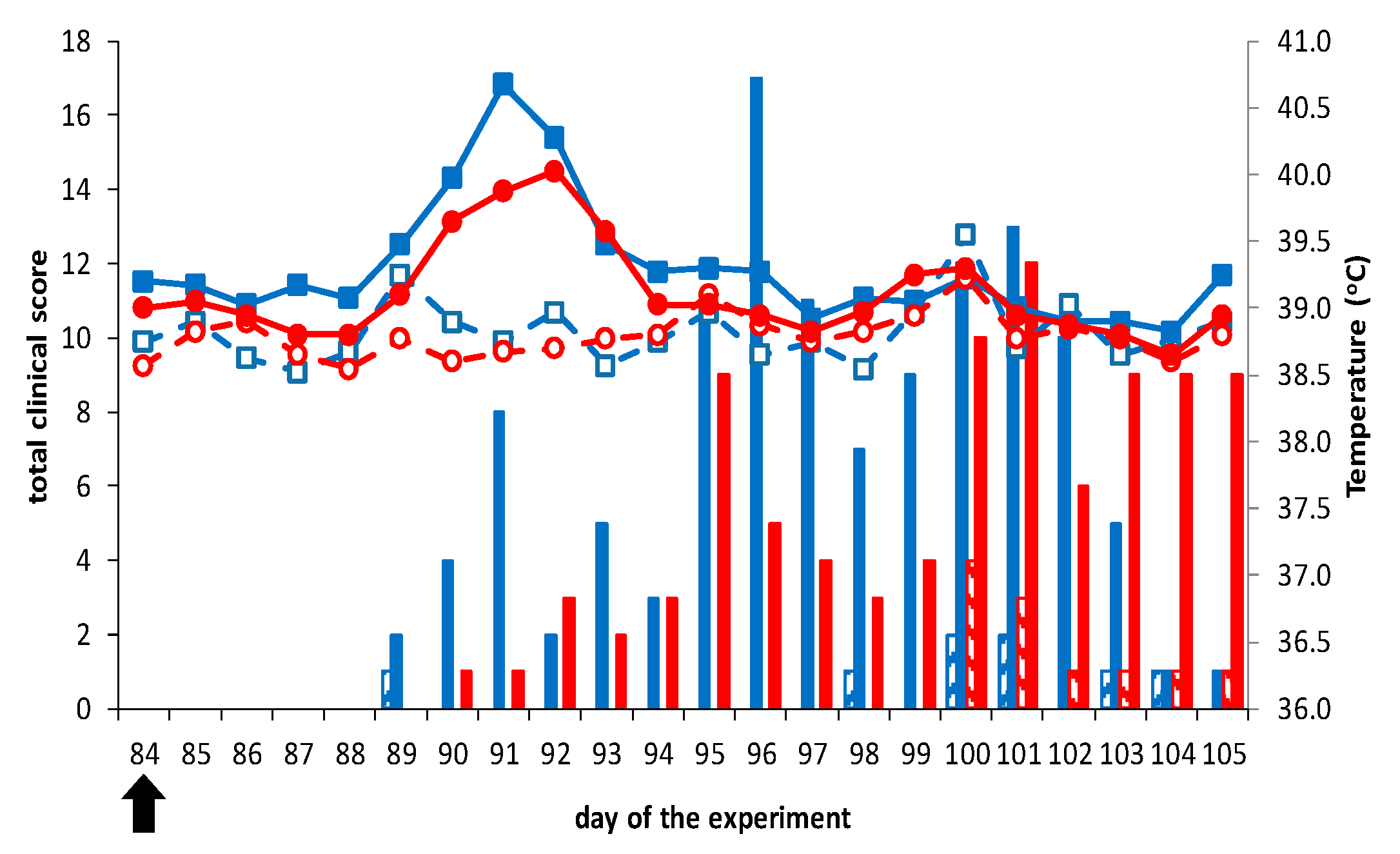
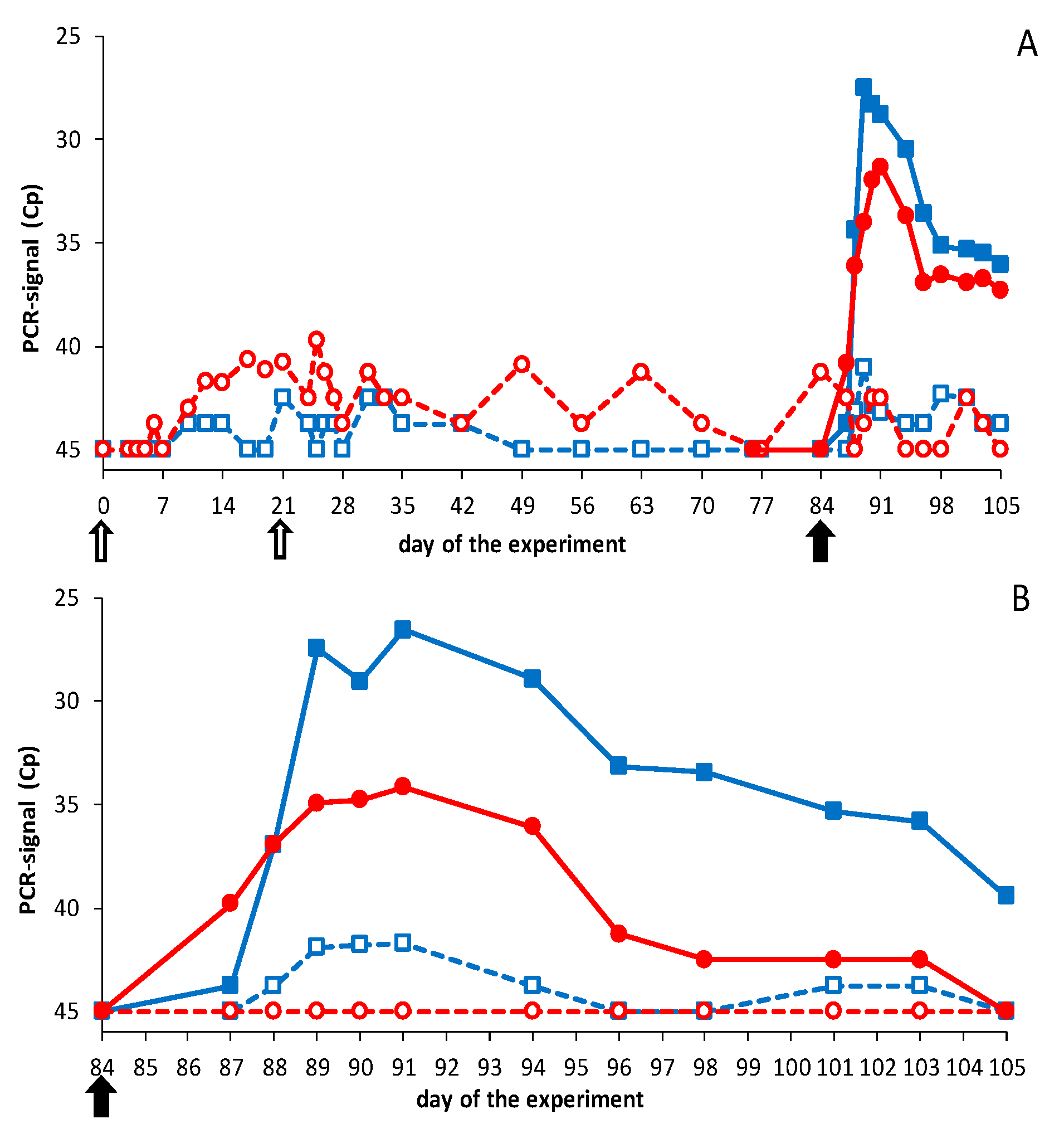
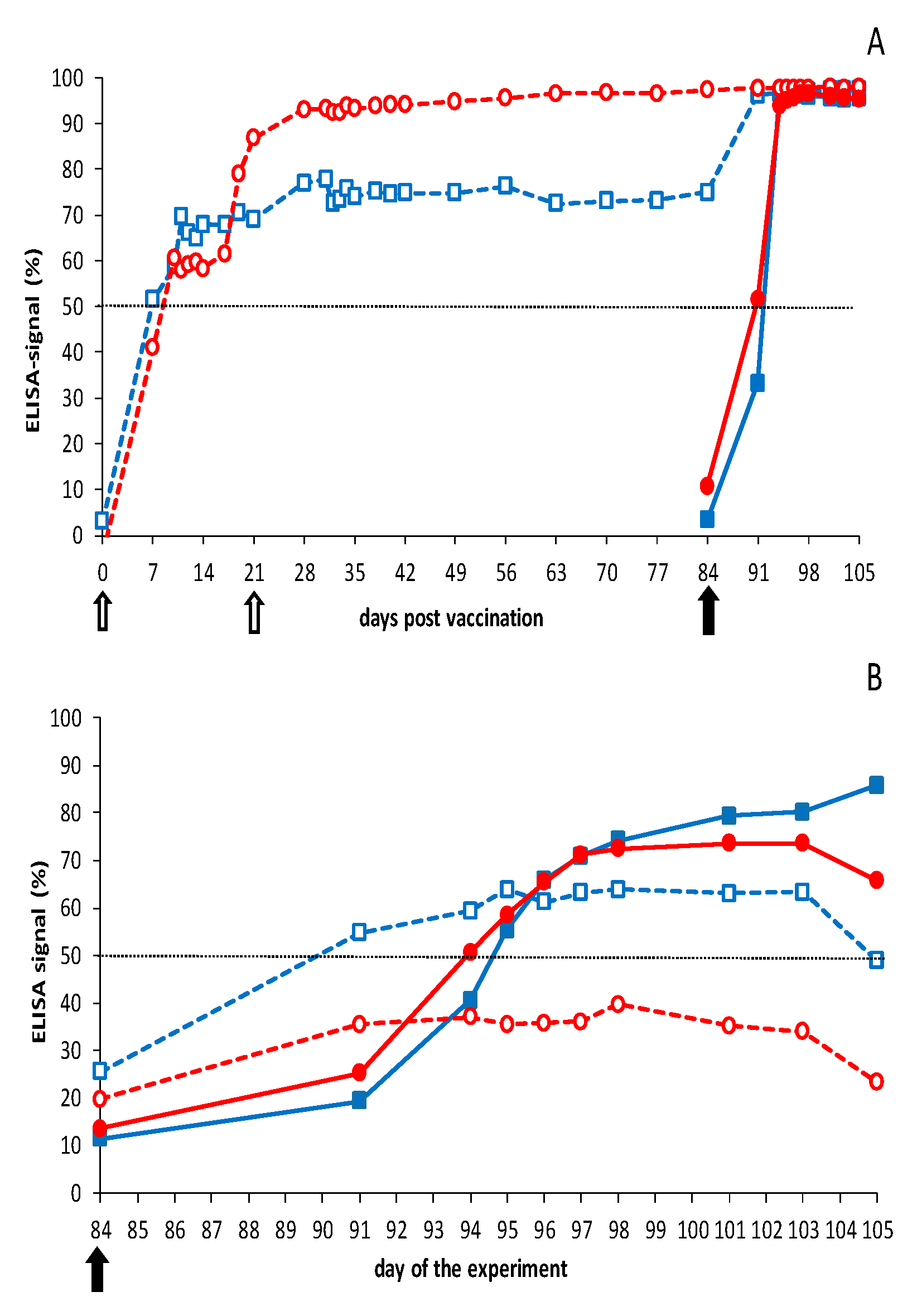
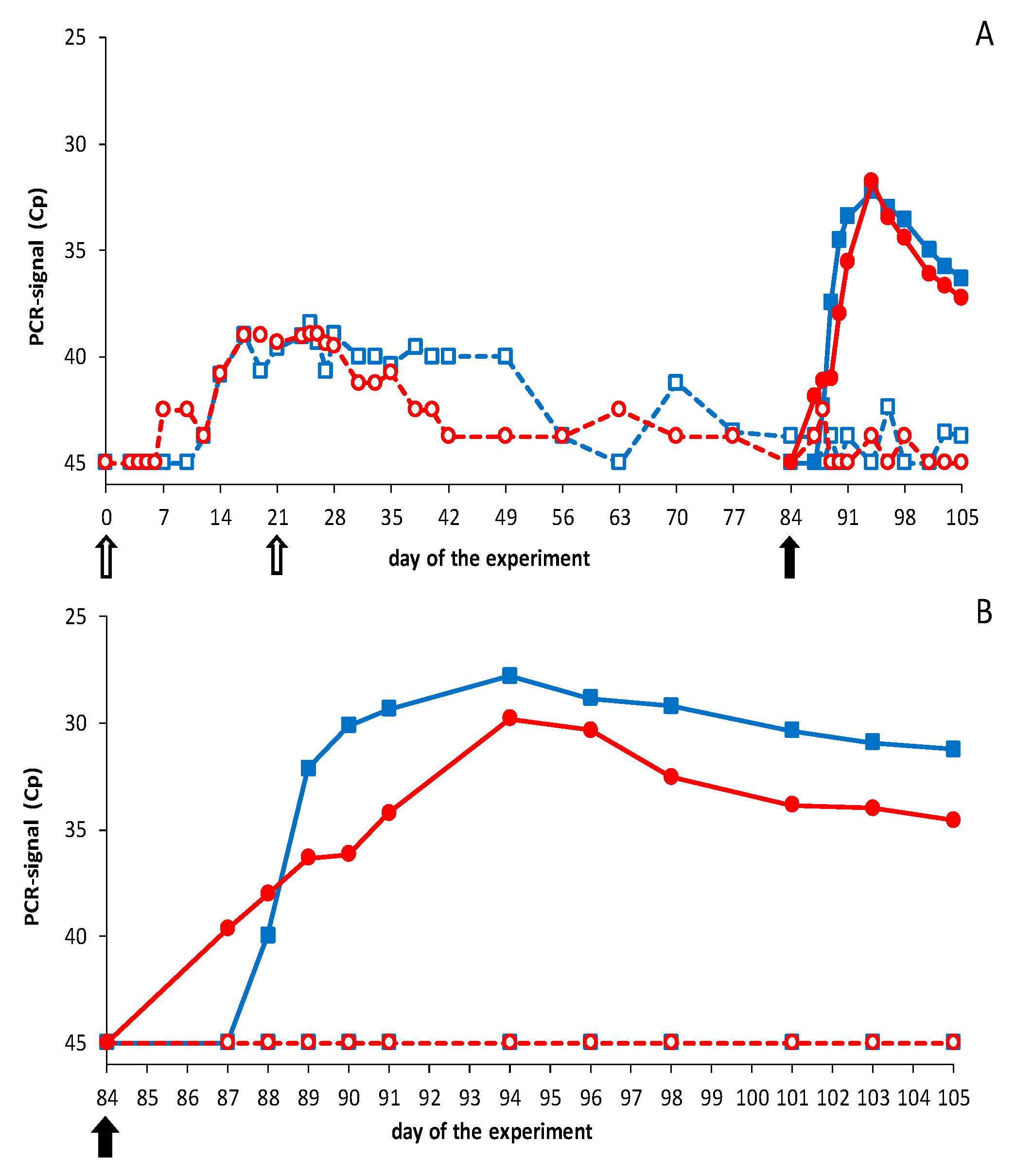
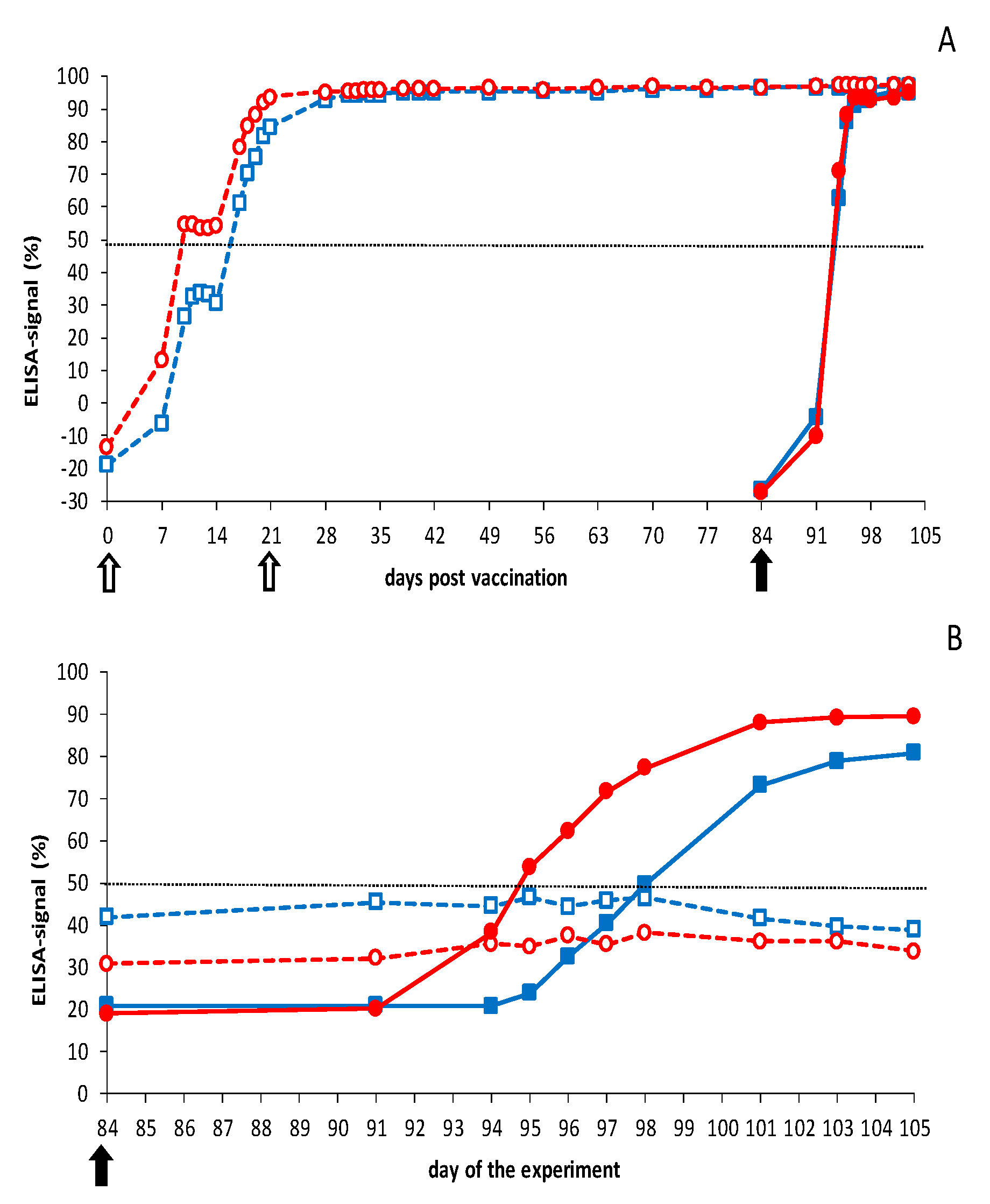
3.2.1. Vaccination-Challenge Experiment in Sheep
3.2.2. Vaccination-Challenge Experiment in Cattle
3.3. Neutralizing Abs by Pentavalent DISA12348 Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- OIE. Terrestrial Animal Health Code; OIE: Paris, France, 2019. [Google Scholar]
- MacLachlan, N.J.; Crafford, J.E.; Vernau, W.; Gardner, I.A.; Goddard, A.; Guthrie, A.J.; Venter, E.H. Experimental reproduction of severe bluetongue in sheep. Vet. Pathol. 2008, 45, 310–315. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Drew, C.P.; Darpel, K.E.; Worwa, G. The pathology and pathogenesis of bluetongue. J. Comp. Pathol. 2009, 141, 1–16. [Google Scholar] [CrossRef]
- Tago, D.; Hammitt, J.K.; Thomas, A.; Raboisson, D. Cost assessment of the movement restriction policy in France during the 2006 bluetongue virus episode (BTV-8). Prev. Vet. Med. 2014, 117, 577–589. [Google Scholar] [CrossRef]
- Velthuis, A.G.; Saatkamp, H.W.; Mourits, M.C.; de Koeijer, A.A.; Elbers, A.R. Financial consequences of the Dutch bluetongue serotype 8 epidemics of 2006 and 2007. Prev. Vet. Med. 2010, 93, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Attoui, H.; Mertens, P.; Becnel, J.; Belaganahalli, S.; Bergoin, M.; Brussaard, C.P.; Chappel, J.D.; Ciarlet, M.; del Vas, M. Virus taxonomy. In Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Oxford, UK, 2011; Volume 9, p. 1338. [Google Scholar]
- Belhouchet, M.; Mohd Jaafar, F.; Firth, A.E.; Grimes, J.M.; Mertens, P.P.; Attoui, H. Detection of a fourth orbivirus non-structural protein. PLoS ONE 2011, 6, e25697. [Google Scholar] [CrossRef]
- Ratinier, M.; Caporale, M.; Golder, M.; Franzoni, G.; Allan, K.; Nunes, S.F.; Armezzani, A.; Bayoumy, A.; Rixon, F.; Shaw, A.; et al. Identification and characterization of a novel non-structural protein of bluetongue virus. PLoS Pathog. 2011, 7, e1002477. [Google Scholar] [CrossRef]
- Stewart, M.; Hardy, A.; Barry, G.; Pinto, R.M.; Caporale, M.; Melzi, E.; Hughes, J.; Taggart, A.; Janowicz, A.; Varela, M.; et al. Characterization of a second open reading frame in genome segment 10 of bluetongue virus. J. Gen. Virol. 2015, 96, 3280–3293. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. Inhibition of the IFN Response by Bluetongue Virus: The Story So Far. Front. Microbiol. 2021, 12, 692069. [Google Scholar] [CrossRef]
- Labadie, T.; Sullivan, E.; Roy, P. Multiple Routes of Bluetongue Virus Egress. Microorganisms 2020, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Huismans, H.; Erasmus, B.J. Identification of the serotype-specific and group-specific antigens of bluetongue virus. Onderstepoort J. Vet. Res. 1981, 48, 51–58. [Google Scholar] [PubMed]
- Erasmus, B.J. Virus Infections of Ruminants; Elsevier Science Publishers: New York, NY, USA, 1990; Volume 3. [Google Scholar]
- Du Toit, R.A. The Transmission of Blue-Tongue andn Horse Sickness by Culicoides. Onderstepoort J. Vet. Sci. Anim. Ind. 1944, 19, 7–16. [Google Scholar]
- Gibbs, E.P.; Greiner, E.C. The epidemiology of bluetongue. Comp. Immunol. Microbiol. Infect. Dis. 1994, 17, 207–220. [Google Scholar] [CrossRef]
- Gould, A.R. The complete nucleotide sequence of bluetongue virus serotype 1 RNA3 and a comparison with other geographic serotypes from Australia, South Africa and the United States of America, and with other orbivirus isolates. Virus Res. 1987, 7, 169–183. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Ross-Smith, N.; Batten, C.A.; Shaw, A.E.; Anthony, S.J.; Samuel, A.R.; Darpel, K.E.; Veronesi, E.; Oura, C.A.; et al. Sequence analysis of bluetongue virus serotype 8 from the Netherlands 2006 and comparison to other European strains. Virology 2008, 377, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, L.I.; Gould, A.R.; Wilson, W.C.; Thompson, L.; Mertens, P.P.; Wade-Evans, A.M. Complete nucleotide sequence of RNA segment 3 of bluetongue virus serotype 2 (Ona-A). Phylogenetic analyses reveal the probable origin and relationship with other orbiviruses. Virus Res. 1995, 35, 247–261. [Google Scholar] [CrossRef]
- Breard, E.; Schulz, C.; Sailleau, C.; Bernelin-Cottet, C.; Viarouge, C.; Vitour, D.; Guillaume, B.; Caignard, G.; Gorlier, A.; Attoui, H.; et al. Bluetongue virus serotype 27: Experimental infection of goats, sheep and cattle with three BTV-27 variants reveal atypical characteristics and likely direct contact transmission BTV-27 between goats. Transbound. Emerg. Dis. 2018, 65, e251–e263. [Google Scholar] [CrossRef] [PubMed]
- Batten, C.A.; Henstock, M.R.; Steedman, H.M.; Waddington, S.; Edwards, L.; Oura, C.A. Bluetongue virus serotype 26: Infection kinetics, pathogenesis and possible contact transmission in goats. Vet. Microbiol. 2013, 162, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Chaignat, V.; Schwermer, H.; Casati, S.; Planzer, J.; Worwa, G.; Vanzetti, T.; Batten, C.; Hofmann, M.; Thür, B. Occurrence and spatial distribution of Toggenburg Orbivirus in Switzerland. Small Rumin. Res. 2010, 93, 157–164. [Google Scholar] [CrossRef]
- Wright, I.M. Serological and Genetic Characterisation of Putative New Serotypes of Bluetongue Virus and Epizootic Haemorrhagic Disease Virus Isolated from an Alpaca. Ph.D. Dissertation, North-West University, Potchefstroom, South Africa, 2014. [Google Scholar]
- Savini, G.; Puggioni, G.; Meloni, G.; Marcacci, M.; Di Domenico, M.; Rocchigiani, A.M.; Spedicato, M.; Oggiano, A.; Manunta, D.; Teodori, L.; et al. Novel putative Bluetongue virus in healthy goats from Sardinia, Italy. Infect. Genet. Evol. 2017, 51, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.C.; Huang, L.P.; Xu, Q.Y.; Wang, H.X.; Xue, X.M.; Lu, P.; Li, W.J.; Liu, W.; Bu, Z.G.; Wu, D.L. Emergence of a Novel Bluetongue Virus Serotype, China 2014. Transbound. Emerg. Dis. 2016, 63, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Bumbarov, V.; Golender, N.; Jenckel, M.; Wernike, K.; Beer, M.; Khinich, E.; Zalesky, O.; Erster, O. Characterization of bluetongue virus serotype 28. Transbound. Emerg. Dis. 2020, 67, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Sant, S.; Mangone, I.; Goria, M.; Dondo, A.; Zoppi, S.; van Gennip, R.G.P.; Radaelli, M.C.; Camma, C.; van Rijn, P.A.; et al. One after the other: A novel Bluetongue virus strain related to Toggenburg virus detected in the Piedmont region (North-western Italy), extends the panel of novel atypical BTV strains. Transbound. Emerg. Dis. 2018, 65, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Sharav, T.; Tseren-Ochir, E.O.; Beer, M.; Hoffmann, B. Putative Novel Serotypes ‘33’ and ‘35’ in Clinically Healthy Small Ruminants in Mongolia Expand the Group of Atypical BTV. Viruses 2020, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Vogtlin, A.; Hussy, D.; Jandt, T.; Gobet, H.; Hilbe, M.; Burgener, C.; Schweizer, L.; Hafliger-Speiser, S.; Beer, M.; et al. Putative Novel Atypical BTV Serotype ‘36′ Identified in Small Ruminants in Switzerland. Viruses 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- OIE Bluetongue Restricted Zones as of July 2021. Available online: https://ec.europa.eu/food/system/files/2021-07/ad_control-measures_bt_restrictedzones-map.pdf (accessed on 7 July 2021).
- Feenstra, F.; van Rijn, P.A. Current and next-generation bluetongue vaccines: Requirements, strategies, and prospects for different field situations. Crit. Rev. Microbiol. 2017, 43, 142–155. [Google Scholar] [CrossRef]
- Van Oirschot, J.T. Diva vaccines that reduce virus transmission. J. Biotechnol. 1999, 73, 195–205. [Google Scholar] [CrossRef]
- Savini, G.; MacLachlan, N.J.; Sanchez-Vizcaino, J.M.; Zientara, S. Vaccines against bluetongue in Europe. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 101–120. [Google Scholar] [CrossRef]
- Savini, G.; Hamers, C.; Conte, A.; Migliaccio, P.; Bonfini, B.; Teodori, L.; Di Ventura, M.; Hudelet, P.; Schumacher, C.; Caporale, V. Assessment of efficacy of a bivalent BTV-2 and BTV-4 inactivated vaccine by vaccination and challenge in cattle. Vet. Microbiol. 2009, 133, 1–8. [Google Scholar] [CrossRef]
- Dungu, B.; Gerdes, T.; Smit, T. The use of vaccination in the control of bluetongue in southern Africa. Vet. Ital. 2004, 40, 616–622. [Google Scholar]
- Savini, G.; Cannas, A.; Casaccia, C.; Di Gialleonardo, L.; Leone, A.; Patta, C.; Nicolussi, P. Risk factors associated with the occurrence of undesired effects in sheep and goats after field vaccination with modified-live vaccine against bluetongue virus serotypes 2, 4 and 16. Vet. Microbiol. 2010, 146, 44–50. [Google Scholar] [CrossRef]
- Batten, C.A.; Maan, S.; Shaw, A.E.; Maan, N.S.; Mertens, P.P. A European field strain of bluetongue virus derived from two parental vaccine strains by genome segment reassortment. Virus Res. 2008, 137, 56–63. [Google Scholar] [CrossRef]
- Van Rijn, P.A. Prospects of Next-Generation Vaccines for Bluetongue. Front. Vet. Sci. 2019, 6, 407. [Google Scholar] [CrossRef] [PubMed]
- van Gennip, R.G.; van de Water, S.G.; Maris-Veldhuis, M.; van Rijn, P.A. Bluetongue viruses based on modified-live vaccine serotype 6 with exchanged outer shell proteins confer full protection in sheep against virulent BTV8. PLoS ONE 2012, 7, e44619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Celma, C.C.; Boyce, M.; van Rijn, P.A.; Eschbaumer, M.; Wernike, K.; Hoffmann, B.; Beer, M.; Haegeman, A.; De Clercq, K.; Roy, P. Rapid generation of replication-deficient monovalent and multivalent vaccines for bluetongue virus: Protection against virulent virus challenge in cattle and sheep. J. Virol. 2013, 87, 9856–9864. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.F.; Hamers, C.; Ratinier, M.; Shaw, A.; Brunet, S.; Hudelet, P.; Palmarini, M. A synthetic biology approach for a vaccine platform against known and newly emerging serotypes of bluetongue virus. J. Virol. 2014, 88, 12222–12232. [Google Scholar] [CrossRef]
- Feenstra, F.; Pap, J.S.; van Rijn, P.A. Application of Bluetongue Disabled Infectious Single Animal (DISA) vaccine for different serotypes by VP2 exchange or incorporation of chimeric VP2. Vaccine 2015, 33, 812–818. [Google Scholar] [CrossRef]
- Feenstra, F.; van Gennip, R.G.; Maris-Veldhuis, M.; Verheij, E.; van Rijn, P.A. Bluetongue virus without NS3/NS3a expression is not virulent and protects against virulent bluetongue virus challenge. J. Gen. Virol. 2014, 95 Pt 9, 2019–2029. [Google Scholar] [CrossRef]
- Feenstra, F.; Drolet, B.S.; Boonstra, J.; van Rijn, P.A. Non-structural protein NS3/NS3a is required for propagation of bluetongue virus in Culicoides sonorensis. Parasites Vectors 2015, 8, 476. [Google Scholar] [CrossRef]
- Feenstra, F.; Maris-Veldhuis, M.; Daus, F.J.; Tacken, M.G.; Moormann, R.J.; van Gennip, R.G.; van Rijn, P.A. VP2-serotyped live-attenuated bluetongue virus without NS3/NS3a expression provides serotype-specific protection and enables DIVA. Vaccine 2014, 32, 7108–7114. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2019. [Google Scholar]
- Van Gennip, R.G.P.; Drolet, B.S.; Rozo Lopez, P.; Roost, A.J.C.; Boonstra, J.; van Rijn, P.A. Vector competence is strongly affected by a small deletion or point mutations in bluetongue virus. Parasites Vectors 2019, 12, 470. [Google Scholar] [CrossRef]
- Van Rijn, P.A.; Maris-Veldhuis, M.A.; van Gennip, R.G.P. The Bluetongue Disabled Infectious Single Animal (DISA) Vaccine Platform Based on Deletion NS3/NS3a Protein Is Safe and Protective in Cattle and Enables DIVA. Viruses 2021, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tanaka, H.; Yamada, T.; Yamamoto, N. Persistent infection of BHK21/WI-2 cells with rubella virus and characterization of rubella variants. Arch. Virol. 1977, 54, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Monaco, F.; Conte, A.; Migliaccio, P.; Casaccia, C.; Salucci, S.; Di Ventura, M. Virological and serological response of sheep following field vaccination with bivalent modified-live vaccine against bluetongue virus serotypes 2 and 9. Vet. Ital. 2004, 40, 631–634. [Google Scholar] [PubMed]
- Van Rijn, P.A.; Geurts, Y.; van der Spek, A.N.; Veldman, D.; van Gennip, R.G. Bluetongue virus serotype 6 in Europe in 2008-Emergence and disappearance of an unexpected non-virulent BTV. Vet. Microbiol. 2012, 158, 23–32. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; van Rijn, P.A.; van Gennip, R.G.; Sanders, A.; Wright, I.M.; Batten, C.; Hoffmann, B.; Eschbaumer, M.; Oura, C.A.; et al. Full genome characterisation of bluetongue virus serotype 6 from the Netherlands 2008 and comparison to other field and vaccine strains. PLoS ONE 2010, 5, e10323. [Google Scholar] [CrossRef]
- Van Rijn, P.A.; van de Water, S.G.; Feenstra, F.; van Gennip, R.G. Requirements and comparative analysis of reverse genetics for bluetongue virus (BTV) and African horse sickness virus (AHSV). Virol. J. 2016, 13, 119. [Google Scholar] [CrossRef]
- Van Gennip, R.G.; van de Water, S.G.; Potgieter, C.A.; Wright, I.M.; Veldman, D.; van Rijn, P.A. Rescue of recent virulent and avirulent field strains of bluetongue virus by reverse genetics. PLoS ONE 2012, 7, e30540. [Google Scholar] [CrossRef]
- Tacken, M.G.; Daus, F.J.; Feenstra, F.; van Gennip, R.G.; van Rijn, P.A. Development of a competitive ELISA for NS3 antibodies as DIVA test accompanying the novel Disabled Infectious Single Animal (DISA) vaccine for Bluetongue. Vaccine 2015, 33, 5539–5545. [Google Scholar] [CrossRef]
- Haig, D.A.; McKercher, D.G.; Alexander, D.J. The cytopathic action of Bluetongue virus on tissue cultures and its application to the detection of antibodies in the serum of sheep. Onderstepoort J. Vet. Res. 1956, 27, 171–177. [Google Scholar]
- Celma, C.C.; Stewart, M.; Wernike, K.; Eschbaumer, M.; Gonzalez-Molleda, L.; Breard, E.; Schulz, C.; Hoffmann, B.; Haegeman, A.; De Clercq, K.; et al. Replication-Deficient Particles: New Insights into the Next Generation of Bluetongue Virus Vaccines. J. Virol. 2017, 91, e27795442. [Google Scholar] [CrossRef]
- Merial Merial’s Bluetongue Vaccine—Information for Veterinary Professionals and Farmers. Available online: https://www.boehringer-ingelheim.com/animal-health/livestock-products/btv-pur (accessed on 22 June 2021).
- Van Rijn, P.A.; Daus, F.J.; Maris-Veldhuis, M.A.; Feenstra, F.; van Gennip, R.G. Bluetongue Disabled Infectious Single Animal (DISA) vaccine: Studies on the optimal route and dose in sheep. Vaccine 2017, 35, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, P.A.; van de Water, S.G.; van Gennip, H.G. Bluetongue virus with mutated genome segment 10 to differentiate infected from vaccinated animals: A genetic DIVA approach. Vaccine 2013, 31, 5005–5008. [Google Scholar] [CrossRef] [PubMed]
| DISA Vaccine | Outer Shell | NCBI Accession nr. | |||
|---|---|---|---|---|---|
| VP2 | VP5 | Seg-2[VP2] | Seg-6[VP5] | Rescue | |
| 1 | 1 | 1 | FJ969720 | FJ969723 | 7.3 |
| 2 | 2IT | 2RSA | JN255863 | AJ586696 | + |
| 2 | 2USA | 2USA | JQ822249 | JQ822253 | 7.1 |
| 3 | 3RSA | 3RSA | MG255540 | AJ586697 | 5.8 |
| 4 | 4 | 4 | AJ585125 | AJ586699 | 6.8 |
| 6 | 6 | 6 | FJ183375 | FJ183379 | + |
| 7 | 7 | 6 | AJ585128 | FJ183379 | - |
| 8 | 8 | 8 | GQ506452 | GQ506456 | 6.9 |
| 9 | 9 | 9 | AJ585130 | AJ586708 | - |
| 9 | 9 | 1 | AJ585130 | FJ969723 | + |
| 14 | 14 | 6 | AJ585135 | FJ183379 | + |
| 16 | 16 | 16 | FJ969720 | FJ969723 | - |
| 16 | 16 | 6 | AJ585137 | FJ183379 | - |
| 16 | 16 | 1 | FJ969720 | FJ969723 | - |
| 1/16 | 1(16) | 1 | FJ969720/AJ585137 | FJ969723 | + |
| 17 | 17 | 17 | AJ585138 | AJ586720 | + |
| 22 | 22 | 6 | AJ585143 | FJ183379 | - |
| 25 | 25 | 25 | EU839840 | EU839842 | + |
| 29 | 29 | 29 | KP196604 | KP196608 | - |
| 29 | 29 | 6 | KP196604 | FJ183379 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Rijn, P.A.; Maris-Veldhuis, M.A.; Spedicato, M.; Savini, G.; van Gennip, R.G.P. Pentavalent Disabled Infectious Single Animal (DISA)/DIVA Vaccine Provides Protection in Sheep and Cattle against Different Serotypes of Bluetongue Virus. Vaccines 2021, 9, 1150. https://doi.org/10.3390/vaccines9101150
van Rijn PA, Maris-Veldhuis MA, Spedicato M, Savini G, van Gennip RGP. Pentavalent Disabled Infectious Single Animal (DISA)/DIVA Vaccine Provides Protection in Sheep and Cattle against Different Serotypes of Bluetongue Virus. Vaccines. 2021; 9(10):1150. https://doi.org/10.3390/vaccines9101150
Chicago/Turabian Stylevan Rijn, Piet A., Mieke A. Maris-Veldhuis, Massimo Spedicato, Giovanni Savini, and René G. P. van Gennip. 2021. "Pentavalent Disabled Infectious Single Animal (DISA)/DIVA Vaccine Provides Protection in Sheep and Cattle against Different Serotypes of Bluetongue Virus" Vaccines 9, no. 10: 1150. https://doi.org/10.3390/vaccines9101150
APA Stylevan Rijn, P. A., Maris-Veldhuis, M. A., Spedicato, M., Savini, G., & van Gennip, R. G. P. (2021). Pentavalent Disabled Infectious Single Animal (DISA)/DIVA Vaccine Provides Protection in Sheep and Cattle against Different Serotypes of Bluetongue Virus. Vaccines, 9(10), 1150. https://doi.org/10.3390/vaccines9101150







