Immunological Response to COVID-19 Vaccination in Ovarian Cancer Patients Receiving PARP Inhibitors
Abstract
:1. Introduction
2. Material and Methods
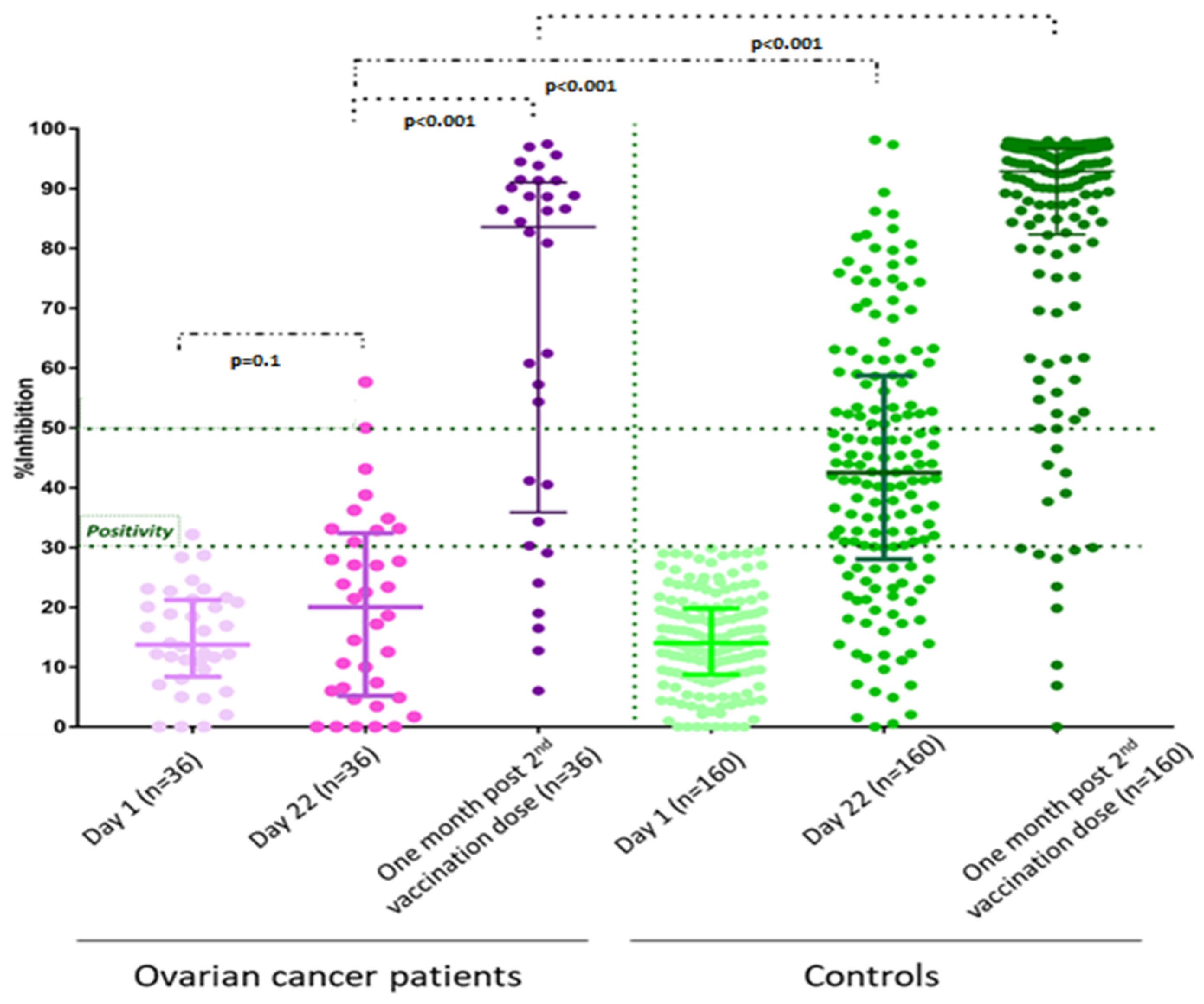
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Ferguson, J.M.; Kurian, A.; Bondy, M.; Patel, M.I. The Impact of COVID-19 on Patients with Cancer: A National Study of Patient Experiences. Am. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chai, P.; Yu, J.; Fan, X. Effects of cancer on patients with COVID-19: A systematic review and meta-analysis of 63,019 participants. Cancer Biol. Med. 2021, 18, 298–307. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Yi, M.; Dong, B.; Qin, S.; Chu, Q.; Wu, K.; Luo, S. Advances and perspectives of PARP inhibitors. Exp. Hematol. Oncol. 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, J.; Lord, C.J.; Serra, V.; Tutt, A.; Balmaña, J.; Castroviejo-Bermejo, M.; Cruz, C.; Oaknin, A.; Kaye, S.B.; de Bono, J.S. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1437–1447. [Google Scholar] [CrossRef] [Green Version]
- Yélamos, J.; Moreno-Lama, L.; Jimeno, J.; Ali, S.O. Immunomodulatory Roles of PARP-1 and PARP-2: Impact on PARP-Centered Cancer Therapies. Cancers 2020, 12, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.-D.; Liacos, C.-I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Mitra, M.; Basu, M. A Study on Challenges to Health Care Delivery Faced by Cancer Patients in India During the COVID-19 Pandemic. J. Prim. Care Community Health 2020, 11, 2150132720942705. [Google Scholar] [CrossRef] [PubMed]
- Ayubi, E.; Bashirian, S.; Khazaei, S. Depression and Anxiety Among Patients with Cancer During COVID-19 Pandemic: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2021, 52, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.; Bányai, K.; Thaventhiran, J.; Le Quesne, J.; Helyes, Z.; Bai, P. Repositioning PARP inhibitors for SARS-CoV-2 infection (COVID-19); a new multi-pronged therapy for acute respiratory distress syndrome? Br. J. Pharmacol. 2020, 177, 3635. [Google Scholar] [CrossRef] [PubMed]
- Capoluongo, E. PARP-inhibitors in a non-oncological indication as COVID-19: Are we aware about its potential role as anti-thrombotic drugs? The discussion is open. Biomed. Pharmacother. 2020, 130, 110536. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Konstantinopoulos, P.A. PARP inhibition and immune modulation: Scientific rationale and perspectives for the treatment of gynecologic cancers. Ther. Adv. Med. Oncol. 2020, 12, 1758835920944116. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; Del Molino Del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet. Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Terpos, E.; Zagouri, F.; Liontos, M.; Sklirou, A.D.; Koutsoukos, K.; Markellos, C.; Briasoulis, A.; Papanagnou, E.-D.; Trougakos, I.P.; Dimopoulos, M.-A. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J. Hematol. Oncol. 2020, 14, 86. [Google Scholar] [CrossRef]
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet. Oncol. 2021, 22, 581–583. [Google Scholar] [CrossRef]
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.-M.; et al. SARS-CoV-2 Variants and Vaccines. N. Engl. J. Med. 2021, 385, 179–186. [Google Scholar] [CrossRef] [PubMed]
| # | SEX | AGE | BMI | TYPE OF CANCER | TYPE OF THERAPY | MONTHS ON TREATMENT | COMORBIDITIES | VACCINE | ADVERSE EVENTS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 82 | 28.1 | Ovarian cancer | Niraparib | 2 | Hypertension, Dyslipidemia | BNT162b2 | None |
| 2 | F | 77 | 34.3 | Ovarian cancer | Olaparib | 13 | Breast Cancer, Dyslipidemia, Osteopenia, Diabetes | BNT162b2 | None |
| 3 | F | 60 | 30.4 | Ovarian cancer | Olaparib | 6 | None | AZD12222 | Pain at the site of injection, Fatigue |
| 4 | F | 75 | 34.5 | Ovarian cancer | Olaparib | 16 | Hypertension, Dyslipidemia, Chronic Obstructive Pulmonary Disease | mRNA-1273 | Fever |
| 5 | F | 60 | 26.2 | Ovarian cancer | Olaparib | 13 | Pulmonary Embolism | AZD12222 | None |
| 6 | F | 47 | 34.2 | Ovarian cancer | Niraparib | 2 | None | BNT162b2 | None |
| 7 | F | 51 | 22.8 | Ovarian cancer | Olaparib | 14 | Hyperthyroidism | BNT162b2 | Pain at the site of injection |
| 8 | F | 42 | 24.5 | Ovarian cancer | Olaparib | 1 | None | BNT162b2 | Pain at the site of injection |
| 9 | F | 75 | 28.1 | Ovarian cancer | Olaparib | 2 | Dyslipidemia, Osteoporosis | mRNA-1273 | None |
| 10 | F | 57 | 23.4 | Ovarian cancer | Olaparib | 11 | Hypertension, Dyslipidemia, Hypothyroidism | BNT162b2 | Fever, Pain at the site of injection |
| 11 | F | 68 | 26 | Ovarian cancer | Olaparib | 13 | Arthritis | BNT162b2 | None |
| 12 | F | 56 | 23.6 | Ovarian cancer | Niraparib | 4 | Hypothyroidism | BNT162b2 | Pain at the site of Injection, Numbness of upper extremity |
| 13 | F | 64 | 23.7 | Ovarian cancer | Niraparib | 1 | Hypertension | AZD12222 | None |
| 14 | F | 68 | 21.8 | Ovarian cancer | Niraparib | 4 | Dyslipidemia, Hypothyroidism, Depression | BNT162b2 | None |
| 15 | F | 57 | 44.9 | Ovarian cancer | Niraparib | 8 | Hypothyroidism | BNT162b2 | None |
| 16 | F | 74 | 25.6 | Ovarian cancer | Niraparib | 2 | Rheumatoid Arthritis, Asthma, Atrial Fibrillation | BNT162b2 | Fever, Leg pain |
| 17 | F | 70 | 32.7 | Ovarian cancer | Olaparib | 1 | Glaucoma, Osteoporosis, Hypertension | BNT162b2 | None |
| 18 | F | 74 | 20.8 | Ovarian cancer | Olaparib | 17 | Hypothyroidism | BNT162b2 | None |
| 19 | F | 71 | 21.6 | Ovarian cancer | Niraparib | 2 | Dyspipidemia, Glaucoma | BNT162b2 | None |
| 20 | F | 79 | 21.4 | Ovarian cancer | Niraparib | 10 | Dyspipidemia, Osteoporosis, Irritable Bowel, Gastritis, Deep Vein Thrombosis | BNT162b2 | None |
| 21 | F | 74 | 26 | Ovarian cancer | Niraparib | 4 | Gastritis, Aneurysm, Hypertension | BNT162b2 | None |
| 22 | F | 67 | 23.4 | Ovarian cancer | Olaparib | 5 | None | BNT162b2 | None |
| 23 | F | 73 | 29.3 | Ovarian cancer | Olaparib | 16 | None | BNT162b2 | Pain at the site of injection |
| 24 | F | 44 | 27.7 | Ovarian cancer | Olaparib | 1 | None | BNT162b2 | Fatigue |
| 25 | F | 70 | 22.5 | Ovarian cancer | Olaparib | 3 | Hypothyroidism | BNT162b2 | Fatigue |
| 26 | F | 53 | 32 | Ovarian cancer | Olaparib | 1 | Dyslipidemia, Hypothyroidism, Depression | BNT162b2 | Pain at the site of injection |
| 27 | F | 51 | 24 | Ovarian cancer | Olaparib | 4 | Hypothyroidism | BNT162b2 | None |
| 28 | F | 48 | 26.2 | Ovarian cancer | Olaparib | 10 | None | BNT162b2 | None |
| 29 | F | 62 | 25 | Ovarian cancer | Niraparib | 1 | Dyslipidemia, Hypothyroidism | mRNA-1273 | None |
| 30 | F | 46 | 28.1 | Ovarian cancer | Rucaparib | 15 | None | BNT162b2 | Headache |
| 31 | F | 47 | 19.5 | Ovarian cancer | Niraparib | 7 | None | BNT162b2 | None |
| 32 | F | 64 | 21.8 | Ovarian cancer | Niraparib | 3 | None | BNT162b2 | None |
| 33 | F | 52 | 17.5 | Ovarian cancer | Niraparib | 1 | Hypothyroidism | BNT162b2 | Fever |
| 34 | F | 70 | 27.4 | Ovarian cancer | Olaparib | 3 | Dyslipidemia | BNT162b2 | Pain at the site of injection |
| 35 | F | 67 | 27.9 | Ovarian cancer | Olaparib | 17 | Hypothyroidism | BNT162b2 | Fever |
| 36 | F | 50 | 21 | Ovarian cancer | Niraparib | 13 | None | BNT162b2 | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liontos, M.; Terpos, E.; Markellos, C.; Zagouri, F.; Briasoulis, A.; Katsiana, I.; Skafida, E.; Fiste, O.; Kunadis, E.; Andrikopoulou, A.; et al. Immunological Response to COVID-19 Vaccination in Ovarian Cancer Patients Receiving PARP Inhibitors. Vaccines 2021, 9, 1148. https://doi.org/10.3390/vaccines9101148
Liontos M, Terpos E, Markellos C, Zagouri F, Briasoulis A, Katsiana I, Skafida E, Fiste O, Kunadis E, Andrikopoulou A, et al. Immunological Response to COVID-19 Vaccination in Ovarian Cancer Patients Receiving PARP Inhibitors. Vaccines. 2021; 9(10):1148. https://doi.org/10.3390/vaccines9101148
Chicago/Turabian StyleLiontos, Michalis, Evangelos Terpos, Christos Markellos, Flora Zagouri, Alexandros Briasoulis, Ioanna Katsiana, Efthymia Skafida, Oraianthi Fiste, Elena Kunadis, Angeliki Andrikopoulou, and et al. 2021. "Immunological Response to COVID-19 Vaccination in Ovarian Cancer Patients Receiving PARP Inhibitors" Vaccines 9, no. 10: 1148. https://doi.org/10.3390/vaccines9101148
APA StyleLiontos, M., Terpos, E., Markellos, C., Zagouri, F., Briasoulis, A., Katsiana, I., Skafida, E., Fiste, O., Kunadis, E., Andrikopoulou, A., Kaparelou, M., Koutsoukos, K., Gavriatopoulou, M., Kastritis, E., Trougakos, I. P., & Dimopoulos, M.-A. (2021). Immunological Response to COVID-19 Vaccination in Ovarian Cancer Patients Receiving PARP Inhibitors. Vaccines, 9(10), 1148. https://doi.org/10.3390/vaccines9101148









