Pulmonary Infection Caused by Mycobacterium malmoense in a Chronic HIV-Infected Individual Participating in a Therapeutic Vaccine Trial
Abstract
1. Introduction
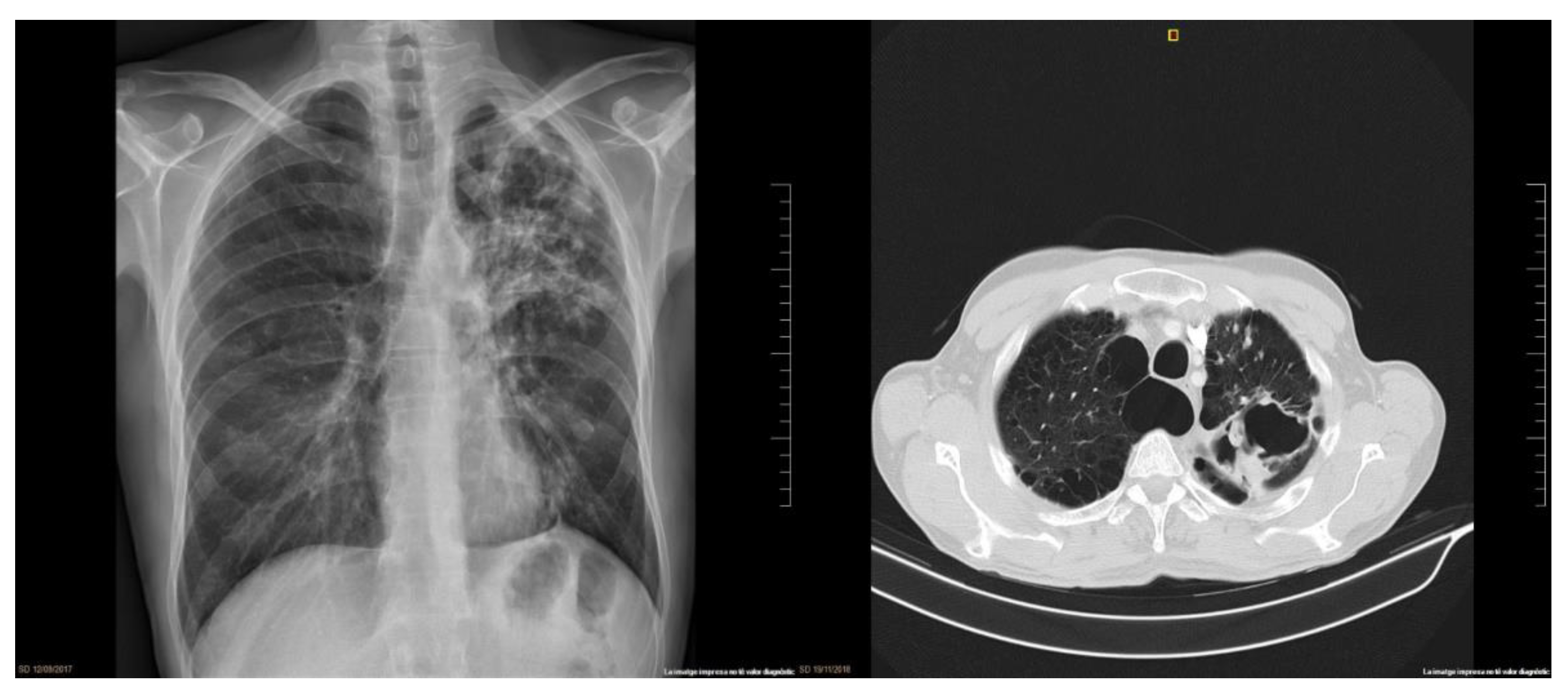
2. Clinical Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, P.A.; Tsukamura, M. Infections with Mycobacterium malmoense in England and Wales. Tubercle 1979, 60, 71–76. [Google Scholar] [CrossRef]
- Schroder, K.H.; Muller, U. Mycobacterium malmoense in deutschland. Zent. Bakteriol. Mikrobiol. Hyg.-Abt. 1 Orig. A 1982, 253, 279–283. [Google Scholar] [CrossRef]
- Pigem, R.; Cairó, M.; Martínez-Lacasa, X.; Irigoyen, D.; Miró, J.M.; Acevedo, J.; Fernández, J.; Alsina-Gibert, M. Disseminated infection with cutaneous involvement caused by Mycobacterium malmoense in an immunocompromised patient. J. Am. Acad. Dermatol. 2013, 69, e192–e193. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.; Lucero, C.; Gatell, J.M.; Gallart, T.; Plana, M.; García, F. New challenges in therapeutic vaccines against HIV infection. Expert Rev. Vaccines 2017, 16, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Hoefsloot, W.; Boeree, M.J.; Van Ingen, J.; Bendien, S.; Magis, C.; De Lange, W.; Dekhuijzen, P.N.R.; Van Soolingen, D. The rising incidence and clinical relevance of Mycobacterium malmoense: A review of the literature. Int. J. Tuberc. Lung Dis. 2008, 12, 987–993. [Google Scholar]
- Thomsen, V.; Andersen, Å.B.; Miörner, H. Incidence and clinical significance of non-tuberculous mycobacteria isolated from clinical specimens during a 2-y nationwide survey. Scand. J. Infect. Dis. 2002, 34, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F.; Leitch, A.; Loebinger, M.R.; Milburn, H.J.; Nightingale, M.; et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72 (Suppl. 2), ii1–ii64. [Google Scholar] [CrossRef]
- Jenkins, P.A.; Campbell, I.A.; Banks, J.; Gelder, C.M.; Prescott, R.J.; Smith, A.P. Clarithromycin vs. ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax 2008, 63, 627–634. [Google Scholar] [CrossRef]
- Jenkins, P.A.; Banks, J.; Campbell, I.A.; Prescott, R.J.; Smith, A.P. First randomised trial of treatments for pulmonary disease caused by M avium intracellulare, M malmoense, and M xenopi in HIV negative patients: Rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol. Thorax 2001, 56, 167–172. [Google Scholar] [CrossRef]
- Podzamczer, D.; Tiraboschi, J.M.; Saumoy, M.; Benetucci, J.A.; Cahn, P.; Corti, M.; Frola, C. Profilaxis y tratamiento de las infecciones más frecuentes en pacientes adultos con infección por el VIH. In Guía Práctica del Sida; Editorial Antares: Barcelona, Spain, 2019; pp. 283–332. [Google Scholar]
- García, F.; Climent, N.; Guardo, A.C.; Gil, C.; León, A.; Autran, B.; Lifson, J.D.; Martínez-Picado, J.; Dalmau, J.; Clotet, B.; et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci. Transl. Med. 2013, 5, 166ra2. [Google Scholar] [CrossRef]
- Cintolo, J.A.; Datta, J.; Mathew, S.J.; Czerniecki, B.J. Dendritic cell-based vaccines: Barriers and opportunities. Futur. Oncol. 2012. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Visconti, R. Type I IFNs and regulation of TH I responses: Enigmas both resolved and emerge. Nat. Immunol. 2000, 1, 17–19. [Google Scholar] [CrossRef]
- Giosuè, S.; Casarini, M.; Alemanno, L.; Galluccio, G.; Mattia, P.; Pedicelli, G.; Rebek, L.; Bisetti, A.; Ameglio, F. Effects of aerosolized interferon-α in patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 1998, 158, 1156–1162. [Google Scholar] [CrossRef]
- Giosuè, S.; Casarini, M.; Ameglio, F.; Zangrilli, P.; Palla, M.; Altieri, A.M.; Bisetti, A. Aerosolized interferon-alpha treatment in patients with multi-drug-resistant pulmonary tuberculosis. Eur. Cytokine Netw. 2000, 11, 99–104. [Google Scholar]
- Telesca, C.; Angelico, M.; Piccolo, P.; Nosotti, L.; Morrone, A.; Longhi, C.; Carbone, M.; Baiocchi, L. Interferon-alpha treatment of hepatitis D induces tuberculosis exacerbation in an immigrant. J. Infect. 2007, 54, e223–e226. [Google Scholar] [CrossRef] [PubMed]
- Sabbatani, S.; Manfredi, R.; Marinacci, G.; Pavoni, M.; Cristoni, L.; Chiodo, F. Reactivation of severe, acute pulmonary tuberculosis during treatment with pegylated interferon-alpha and ribavirin for chronic HCV hepatitis. Scand. J. Infect. Dis. 2006, 38, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Mattner, J.; Schleicher, U. The role of type I interferons in non-viral infections. Immunol. Rev. 2004, 202, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Daou, S.; Ambrosioni, J.; Merkler, D.; Calmy, A. Intracranial hypertension following highly active antiretroviral therapy interruption in an HIV-infected woman: Case report and review of the literature. AIDS 2013, 27, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Garlin, A.B.; Sax, P.E. Retroviral Rebound Syndrome with Fatal Outcome after Discontinuation of Antiretroviral Therapy. Clin. Infect. Dis. 2005, 41, e83–e85. [Google Scholar] [CrossRef][Green Version]
- Ruiz, L.; Martinez-Picado, J.; Romeu, J.; Paredes, R.; Zayat, M.K.; Marfil, S.; Negredo, E.; Sirera, G.; Tural, C.; Clotet, B. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. AIDS 2000, 14, 397–403. [Google Scholar] [CrossRef]
- Ananworanich, J.; Gayet-Ageron, A.; Le Braz, M.; Prasithsirikul, W.; Chetchotisakd, P.; Kiertiburanakul, S.; Munsakul, W.; Raksakulkarn, P.; Tansuphasawasdikul, S.; Sirivichayakul, S.; et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: Results of the Staccato randomised trial. Lancet 2006, 368, 459–465. [Google Scholar] [CrossRef]
| 2017 | 2019 | 2021 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weeks | 0–4 | 16 | 22 | 26 | 39 | 41 | 56 | 80 | 117 (January) | 125 (March) |
| ANTIMYCOBACTERIAL TREATMENT (MCBT) | LVX 750 mg AZM 500 mg RIF 600 mg ETB 1200 mg | LVX 750 mg AZM 500 mg RIF 600 mg | AZM 500 mg ETB 1200 mg PRTN 1000 mg | AZM 500 mg ETB 1200 mg PRTN 750 mg | AZM 500 mg ETB 1200 mg MFX 400 mg LZD 600 mg | AZM 500 mg ETB 1200 mg MFX 400 mg | AZM 500 mg ETB 1200 mg MFX 400 mg RFB 300 mg AMK 1grm IV | AZM 500 mg ETB 1200 mg MFX 400 mg RFB 300 mg | ||
| INTERVENTION | Week 4 → Resume ART ABC 600 mg 3TC 300 mg DTG 100 mg | Worsened dizziness → Stop ETB | Following antibiogram → Stop LVX and RIF Restart ETB Start PRTN Reduce DTG | Reduce PRTN Ophtalmology OK | Following antibiogram → Stop PRTN Start MFX and LZD | Restart MFX | Start RFB AMK TX 18 days Endoscopy BAL no malignancies | After 14m STOP MCBT Continue ART | Left apical lobectomy | Restart MCBT STOP in JAN 2021 Total duration 112w |
| MICROBIOLOGY (−) negative (+) positive | QUAN (−) Sputum (+) Gene-xpert (−) Culture (+) Maldi-Tof Complete genome sequencing | Sputum (−) Culture (+) | Sputum (−) Culture (−) | Sputum (−) Culture (−) | Sputum (−) Culture (−) | Sputum (+) Culture (−) BAL: Aspergillus (−) Respiratory virus (−) P. Carinni (−) Smear (−) Culture (−) | Sputum (−) Culture (−) | Sputum (−) Culture (−) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, J.S.; Bodro, M.; Torres, B.; García, F.; Martínez, J.A.; Leal, L. Pulmonary Infection Caused by Mycobacterium malmoense in a Chronic HIV-Infected Individual Participating in a Therapeutic Vaccine Trial. Vaccines 2021, 9, 1103. https://doi.org/10.3390/vaccines9101103
Marques JS, Bodro M, Torres B, García F, Martínez JA, Leal L. Pulmonary Infection Caused by Mycobacterium malmoense in a Chronic HIV-Infected Individual Participating in a Therapeutic Vaccine Trial. Vaccines. 2021; 9(10):1103. https://doi.org/10.3390/vaccines9101103
Chicago/Turabian StyleMarques, Joana Silva, Marta Bodro, Berta Torres, Felipe García, José Antonio Martínez, and Lorna Leal. 2021. "Pulmonary Infection Caused by Mycobacterium malmoense in a Chronic HIV-Infected Individual Participating in a Therapeutic Vaccine Trial" Vaccines 9, no. 10: 1103. https://doi.org/10.3390/vaccines9101103
APA StyleMarques, J. S., Bodro, M., Torres, B., García, F., Martínez, J. A., & Leal, L. (2021). Pulmonary Infection Caused by Mycobacterium malmoense in a Chronic HIV-Infected Individual Participating in a Therapeutic Vaccine Trial. Vaccines, 9(10), 1103. https://doi.org/10.3390/vaccines9101103






