A Retrospective Cross-Sectional Study Assessing Self-Reported Adverse Events following Immunization (AEFI) of the COVID-19 Vaccine in Bangladesh
Abstract
:1. Background
2. Method
2.1. Design and Sample Selection
2.2. Questionnaire on Adverse Events following COVID-19 Vaccine Immunization
2.3. Duration of the Study
2.4. Statistical Analysis
3. Results
3.1. Demographic Analysis
3.2. Respondents Who Took Preventive Measurements
3.3. Pre-Existing Diseases of the Respondents Prior to the COVID-19 Vaccination
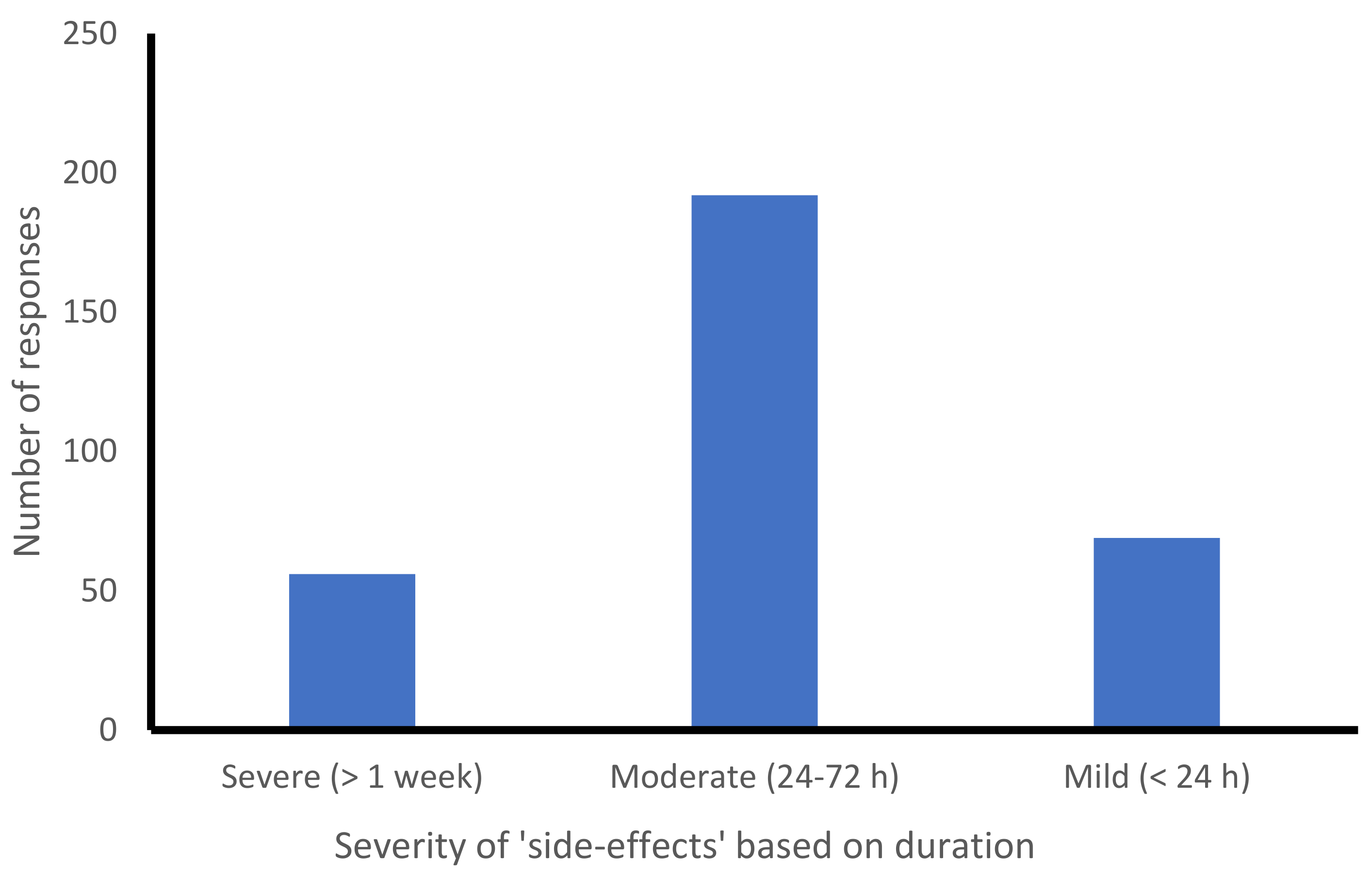
3.4. COVID-19 Vaccination Side-Effects and Their Severity
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padron-Regalado, E. Vaccines for SARS-CoV-2: Lessons from Other Coronavirus Strains. Infect. Dis. Ther. 2020, 9, 255–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahriar, S.; Koly, F.J. A Cross-Sectional Study on Bangladeshi Students Regarding Physiological Challenges of Online Education. Pharm. Educ. 2021, 21, 267–275. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Nopp, S.; Moik, F.; Jilma, B.; Pabinger, I.; Ay, C. Risk of Venous Thromboembolism in Patients with COVID-19: A Systematic Review and Meta-Analysis. Res. Pract. Thromb. Haemost. 2020, 4, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, S.; Rana, M.; Hossain, M.; Karim, A.; Mredula, T.N.; Nourin, N.; Uddin, S.; Amran, M. COVID-19: Epidemiology, Pathology, Diagnosis, Treatment, and Impact. Curr. Pharm. Des. 2021, 27, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.; Kluge, S.; Klamroth, R.; Oldenburg, J. Coagulopathy in COVID-19 and Its Implication for Safe and Efficacious Thromboprophylaxis. Hämostaseologie 2020, 40, 264–269. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 11 July 2021).
- Khan, Y.H.; Mallhi, T.H.; Alotaibi, N.H.; Alzarea, A.I.; Alanazi, A.S.; Tanveer, N.; Hashmi, F.K. Threat of COVID-19 Vaccine Hesitancy in Pakistan: The Need for Measures to Neutralize Misleading Narratives. Am. J. Trop. Med. Hyg. 2020, 103, 603–604. [Google Scholar] [CrossRef]
- Dodd, R.H.; Pickles, K.; Nickel, B.; Cvejic, E.; Ayre, J.; Batcup, C.; Bonner, C.; Copp, T.; Cornell, S.; Dakin, T.; et al. Concerns and Motivations about COVID-19 Vaccination. Lancet Infect. Dis. 2021, 21, 161–163. [Google Scholar] [CrossRef]
- Wu, Q.; Dudley, M.Z.; Chen, X.; Bai, X.; Dong, K.; Zhuang, T.; Salmon, D.; Yu, H. Evaluation of the Safety Profile of COVID-19 Vaccines: A Rapid Review. BMC Med. 2021, 19, 173. [Google Scholar] [CrossRef]
- Cohen, J. South Africa Suspends Use of AstraZeneca’s COVID-19 Vaccine after It Fails to Clearly Stop Virus Variant. Science 2021. [Google Scholar] [CrossRef]
- Albert, E.; Aurigemma, G.; Saucedo, J.; Gerson, D.S. Myocarditis Following COVID-19 Vaccination. Radiol. Case Rep. 2021, 16, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: Israel sees new infections plummet following vaccinations. BMJ 2021, 372, n338. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Bergmann, K.-C.; Brehler, R.; Pfützner, W.; Zuberbier, T.; Hartmann, K.; Jakob, T.; Novak, N.; Ring, J.; Merk, H.; et al. Practical Handling of Allergic Reactions to COVID-19 Vaccines. Allergo J. Int. 2021, 30, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, C. What Do We Know about China’s Covid-19 Vaccines? BMJ 2021, 373, n912. [Google Scholar] [CrossRef]
- COVID-19: Vaccine Surveillance Strategy. Available online: https://www.gov.uk/government/publications/covid-19-vaccine-surveillance-strategy (accessed on 11 July 2021).
- Lee, G.M.; Romero, J.R.; Bell, B.P. Postapproval Vaccine Safety Surveillance for COVID-19 Vaccines in the US. JAMA 2020, 324, 1937–1938. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahriar, S.; Koly, F.J.; Kabir, S.; Choudhury, A.A.; Chowdhury, J.A.; Tahsin, M.R.; Amran, M.S. A Cross-Sectional Pilot Study on COVID-19 Disease Pattern, Recovery Status and Effect of Co-Morbidities in Bangladesh. Afr. J. Pharm. Pharmacol. 2021, 15, 84–91. [Google Scholar]
- Abedin, M.; Islam, M.A.; Rahman, F.N.; Reza, H.M.; Hossain, M.Z.; Hossain, M.A.; Arefin, A.; Hossain, A. Willingness to Vaccinate against COVID-19 among Bangladeshi Adults: Understanding the Strategies to Optimize Vaccination Coverage. PLoS ONE 2021, 16, e0250495. [Google Scholar] [CrossRef]
- Mallapaty, S. China’s COVID Vaccines Are Going Global—But Questions Remain. Nature 2021, 593, 178–179. [Google Scholar] [CrossRef]
- Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus. (accessed on 18 August 2021).
- CDC. Cases, Data, and Surveillance. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/index.html (accessed on 18 August 2021).
- Islam, M.S.; Sujan, M.S.H.; Tasnim, R.; Mohona, R.A.; Ferdous, M.Z.; Kamruzzaman, S.; Toma, T.Y.; Sakib, M.N.; Pinky, K.N.; Islam, M.R.; et al. Problematic Smartphone and Social Media Use among Bangladeshi College and University Students amid COVID-19: The Role of Psychological Well-Being and Pandemic Related Factors. Front. Psychiatry 2021, 12, 647386. [Google Scholar] [CrossRef]
- Tavakol, M.; Dennick, R. Making Sense of Cronbach’s Alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef]
- Borriello, A.; Master, D.; Pellegrini, A.; Rose, J.M. Preferences for a COVID-19 Vaccine in Australia. Vaccine 2021, 39, 473–479. [Google Scholar] [CrossRef]
- Dasgupta, N.; Lazard, A.; Brownstein, J.S. Covid-19 Vaccine Apps Should Deliver More to Patients. Lancet Digit. Health 2021, 3, e278–e279. [Google Scholar] [CrossRef]
- Intent to Get a COVID-19 Vaccine Rises to 60% as Confidence in Research and Development Process Increases. Available online: https://www.pewresearch.org/science/2020/12/03/intent-to-get-a-covid-19-vaccine-rises-to-60-as-confidence-in-research-and-development-process-increases/ (accessed on 18 August 2021).
- Wang, J.; Jing, R.; Lai, X.; Zhang, H.; Lyu, Y.; Knoll, M.D.; Fang, H. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 2020, 8, 482. [Google Scholar] [CrossRef]
- Goss, A.L.; Samudralwar, R.D.; Das, R.R.; Nath, A. ANA Investigates: Neurological Complications of COVID-19 Vaccines. Ann. Neurol. 2021, 89, 856–857. [Google Scholar] [CrossRef]
- Kadali, R.A.; Janagama, R.; Peruru, S.; Malayala, S.V. Side Effects of BNT162b2 MRNA COVID-19 Vaccine: A Randomized, Cross-Sectional Study with Detailed Self-Reported Symptoms from Healthcare Workers. Int. J. Infect. Dis. 2021, 106, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ 2021, 372, n149. [Google Scholar] [CrossRef]
- Oldenburg, J.; Klamroth, R.; Langer, F.; Albisetti, M.; von Auer, C.; Ay, C.; Korte, W.; Scharf, R.E.; Pötzsch, B.; Greinacher, A. Diagnosis and Management of Vaccine-Related Thrombosis Following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hämostaseologie 2021, 41, 184–189. [Google Scholar] [PubMed]
- Wolf, M.E.; Luz, B.; Niehaus, L.; Bhogal, P.; Bäzner, H.; Henkes, H. Thrombocytopenia and Intracranial Venous Sinus Thrombosis after “COVID-19 Vaccine AstraZeneca” Exposure. J. Clin. Med. 2021, 10, 1599. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis after Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef]
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethylene Glycol (PEG) Is a Cause of Anaphylaxis to the Pfizer/BioNTech MRNA COVID-19 Vaccine. Clin. Exp. Allergy 2021, 51, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, C. Covid-19: How the UK Vaccine Rollout Delivered Success, so Far. BMJ 2021, 372, n421. [Google Scholar] [CrossRef] [PubMed]

| Variables | Outcome | Frequency | Percentage (%) |
|---|---|---|---|
| Gender | Male | 372 | 59.6 |
| Female | 251 | 40.4 | |
| Age | 18–30 years | 100 | 16.1 |
| 30–60 years | 446 | 71.4 | |
| >60 years | 77 | 12.5 | |
| Area of residence | Rural | 182 | 29.37 |
| Urban | 429 | 69.02 | |
| Foreign | 12 | 2.08 | |
| Educational qualification | Primary | 28 | 4.6 |
| Secondary | 70 | 11.3 | |
| Higher secondary | 53 | 8.6 | |
| Undergraduate | 60 | 9.6 | |
| Graduate | 233 | 37.3 | |
| Postgraduate | 179 | 28.6 |
| Disease Condition | Frequency | Percentage (%) |
|---|---|---|
| Allergy | 5 | 0.79 |
| COVID-19 infection | 53 | 8.5 |
| Asthma | 15 | 2.39 |
| Diabetes | 84 | 13.41 |
| Hypertension | 107 | 17.09 |
| Heart problem | 23 | 3.67 |
| Hyperlipidaemia | 12 | 1.91 |
| Hypothyroidism | 4 | 0.63 |
| Back and joint pain | 6 | 0.95 |
| Stomach upset | 5 | 0.79 |
| Kidney disease | 4 | 0.63 |
| Disease-free state | 379 | 60.54 |
| Side-Effects after the First or/and Second Dose of the COVID-19 Vaccine | Number of Symptoms | Percentage Reported (%) |
|---|---|---|
| Sore arm, pain and swelling at the site of injection | 231 | 37.07 |
| Body and joint pain | 78 | 12.52 |
| Fever | 161 | 25.84 |
| Decreased appetite | 12 | 1.92 |
| Nausea | 18 | 2.88 |
| Dizziness | 36 | 5.77 |
| Irritation and burning sensation | 48 | 7.70 |
| Diarrhoea | 1 | 0.16 |
| Cough, sneezing | 1 | 0.16 |
| Anaphylaxis | 2 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, A.; Shahriar, S.; Tahsin, M.R.; Mim, S.R.; Fatema, K.R.; Saha, A.; Yesmin, F.; Bahar, N.B.; Samodder, M.; Mamun, M.A.H.; et al. A Retrospective Cross-Sectional Study Assessing Self-Reported Adverse Events following Immunization (AEFI) of the COVID-19 Vaccine in Bangladesh. Vaccines 2021, 9, 1090. https://doi.org/10.3390/vaccines9101090
Sultana A, Shahriar S, Tahsin MR, Mim SR, Fatema KR, Saha A, Yesmin F, Bahar NB, Samodder M, Mamun MAH, et al. A Retrospective Cross-Sectional Study Assessing Self-Reported Adverse Events following Immunization (AEFI) of the COVID-19 Vaccine in Bangladesh. Vaccines. 2021; 9(10):1090. https://doi.org/10.3390/vaccines9101090
Chicago/Turabian StyleSultana, Arifa, Saimon Shahriar, Md. Rafat Tahsin, Sabiha Rahman Mim, Kazi Rubiya Fatema, Ananya Saha, Fahmida Yesmin, Nasiba Binte Bahar, Mithun Samodder, Md. Ariful Haque Mamun, and et al. 2021. "A Retrospective Cross-Sectional Study Assessing Self-Reported Adverse Events following Immunization (AEFI) of the COVID-19 Vaccine in Bangladesh" Vaccines 9, no. 10: 1090. https://doi.org/10.3390/vaccines9101090
APA StyleSultana, A., Shahriar, S., Tahsin, M. R., Mim, S. R., Fatema, K. R., Saha, A., Yesmin, F., Bahar, N. B., Samodder, M., Mamun, M. A. H., Aknur Rahman, M., Ferdousy, S., Akter, T., Aktar, F., Kuddus, M. R., Rahman, M. M., Sarker, M. M. R., Büyüker, S. M., Chowdhury, J. A., ... Amran, M. S. (2021). A Retrospective Cross-Sectional Study Assessing Self-Reported Adverse Events following Immunization (AEFI) of the COVID-19 Vaccine in Bangladesh. Vaccines, 9(10), 1090. https://doi.org/10.3390/vaccines9101090














