Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment of Participants
- (a)
- no adverse reactions or only minor on the injection side;
- (b)
- moderate adverse reactions (not further classified);
- (c)
- severe adverse reactions—defined as any symptom(s) resulting in sick leave or would have resulted in sick leave in case the vaccination was followed by day(s) off.
2.2. SARS-CoV-2 Binding Antibodies
2.3. SARS-CoV-2 Neutralization Activity
2.4. SARS-CoV-2 T-Cell Response
2.5. Statistics
2.6. Ethical Issues
3. Results
3.1. Selection of Participants
3.2. Demography
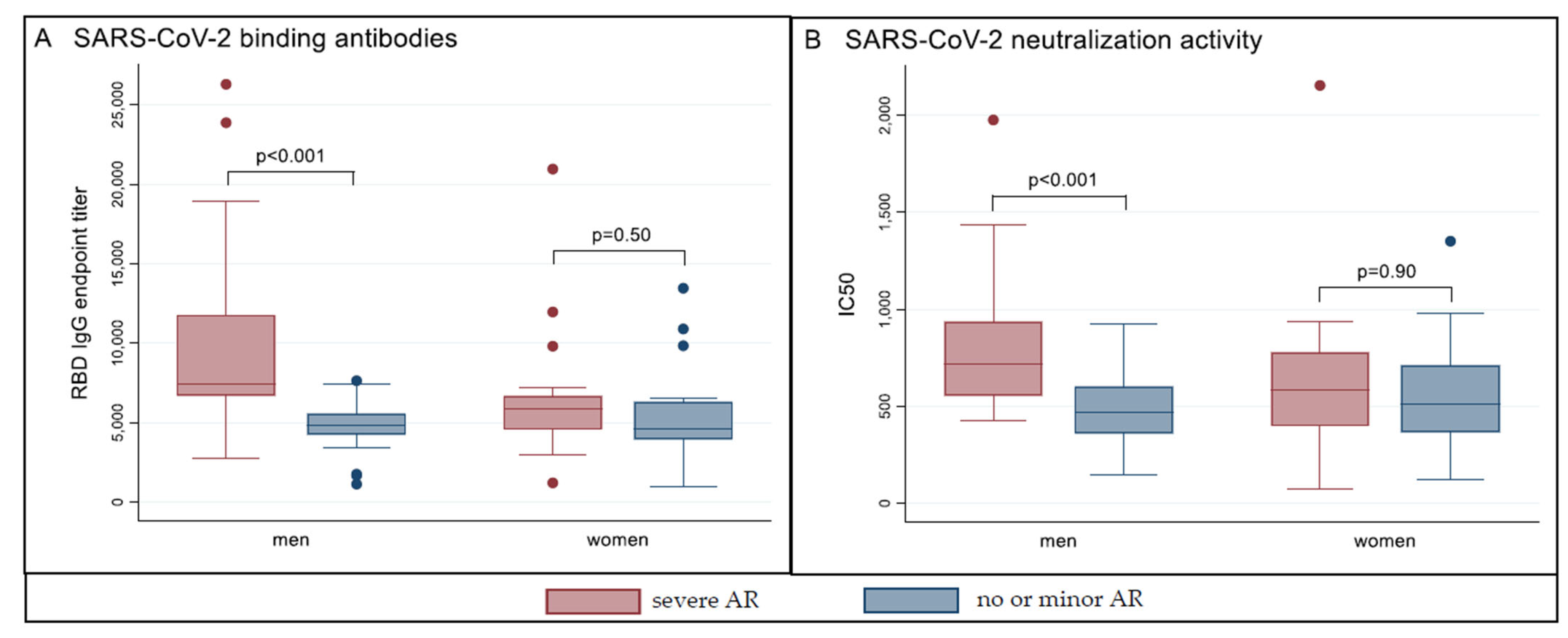
3.3. Humoral Immune Response
3.4. T-Cell Mediated Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Da Tavares Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- WHO Expert Committee on Biological Standardization. Guidelines on Clinical Evaluation of Vaccines: Regulatory Expectations; WHO: Geneve, Switzerland, 2017; Volume 924. [Google Scholar]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- Altmann, D.M.; Boyton, R.J. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci. Immunol. 2020, 5, eabd6160. [Google Scholar] [CrossRef] [PubMed]
- Ng, O.-W.; Chia, A.; Tan, A.T.; Jadi, R.S.; Leong, H.N.; Bertoletti, A.; Tan, Y.-J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 2016, 34, 2008–2014. [Google Scholar] [CrossRef]
- Peterhoff, D.; Glück, V.; Vogel, M.; Schuster, P.; Schütz, A.; Neubert, P.; Albert, V.; Frisch, S.; Kiessling, M.; Pervan, P.; et al. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection 2021, 49, 75–82. [Google Scholar] [CrossRef]
- Schmidt, F.; Weisblum, Y.; Muecksch, F.; Hoffmann, H.-H.; Michailidis, E.; Lorenzi, J.C.C.; Mendoza, P.; Rutkowska, M.; Bednarski, E.; Gaebler, C.; et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020, 217, e20201181. [Google Scholar] [CrossRef]
- Hähnel, V.; Peterhoff, D.; Bäuerlein, V.; Brosig, A.-M.; Pamler, I.; Johnson, C.; Bica, A.; Totir, M.; Ossner, T.; Stemmer, B.; et al. Manufacturing of convalescent plasma of COVID-19 patients: Aspects of quality. PLoS ONE 2020, 15, e0243967. [Google Scholar] [CrossRef] [PubMed]
- Burny, W.; Marchant, A.; Hervé, C.; Callegaro, A.; Caubet, M.; Fissette, L.; Gheyle, L.; Legrand, C.; Ndour, C.; Da Tavares Silva, F.; et al. Inflammatory parameters associated with systemic reactogenicity following vaccination with adjuvanted hepatitis B vaccines in humans. Vaccine 2019, 37, 2004–2015. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Casella, C.R. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr. Opin. Immunol. 2017, 47, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, P.; Maruste, R.; Kärner, J.; et al. Declined antibody responses to COVID-19 mRNA vaccine within first three months. medRxiv 2021. [Google Scholar] [CrossRef]
- Takeuchi, M.; Higa, Y.; Esaki, A.; Nabeshima, Y.; Nakazono, A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? medRxiv 2021. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Song, K.-H.; Choi, Y.; Go, S.; Choi, S.-J.; Jung, J.; Kang, C.K.; Choe, P.G.; Kim, N.-J.; Park, W.B.; et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Coggins, S.A.A.; Laing, E.D.; Olsen, C.H.; Goguet, E.; Moser, M.; Jackson-Thompson, B.M.; Samuels, E.C.; Pollett, S.D.; Tribble, D.R.; Davies, J.; et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. medRxiv 2021. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Boniol, M.; McIsaac, M.; Xu, L.; Wuliji, T.; Diallo, K.; Campbell, J. Gender Equity in the Health Workforce: Analysis of 104 Countries: Working Paper 1; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Jabal, K.A.; Ben-Amram, H.; Beiruti, K.; Batheesh, Y.; Sussan, C.; Zarka, S.; Edelstein, M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021, 26, 2100096. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; de Virgilio, A.; de Marco, F.; Di Domenico, E.G.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef]
- Bayart, J.-L.; Morimont, L.; Closset, M.; Wieërs, G.; Roy, T.; Gerin, V.; Elsen, M.; Eucher, C.; van Eeckhoudt, S.; Ausselet, N.; et al. Confounding Factors Influencing the Kinetics and Magnitude of Serological Response Following Administration of BNT162b2. Microorganisms 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Pekosz, A.; Park, H.-S.; Ursin, R.L.; Shapiro, J.R.; Benner, S.E.; Littlefield, K.; Kumar, S.; Naik, H.M.; Betenbaugh, M.J.; et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J. Clin. Investig. 2020, 130, 6141–6150. [Google Scholar] [CrossRef] [PubMed]
- Schlickeiser, S.; Schwarz, T.; Steiner, S.; Wittke, K.; Al Besher, N.; Meyer, O.; Kalus, U.; Pruß, A.; Kurth, F.; Zoller, T.; et al. Disease Severity, Fever, Age, and Sex Correlate With SARS-CoV-2 Neutralizing Antibody Responses. Front. Immunol. 2020, 11, 628971. [Google Scholar] [CrossRef] [PubMed]
- Karbiener, M.; Farcet, M.R.; Ilk, R.; Schreiner, J.; Lenart, J.; Powers, N.; Stewart, J.M.; Tallman, H.; Kreil, T.R. Longitudinal analysis of SARS-CoV-2 antibodies in 8000 U.S. first-time convalescent plasma donations. Transfusion 2021, 61, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. MedRxiv 2020. [Google Scholar] [CrossRef]

| Characteristic | All n = 735 | Male n = 190 | Female n = 545 |
|---|---|---|---|
| median age in years (range, IQR) | 38 (16–66, 25) | 42 (16–66, 26) | 36 (16–66, 23) |
| adverse reactions after 1st vaccination (%) | |||
| no/minor at injection side | 551 (75.0) | 155 (81.6) | 396 (72.7) |
| moderate | 152 (20.7) | 32 (16.8) | 120 (22.0) |
| severe | 32 (4.3) | 3 (1.6) | 29 (5.3) |
| adverse reactions after 2nd vaccination (%) | |||
| no/minor at injection side | 270 (36.7) | 87 (45.8) | 183 (33.6) |
| moderate | 216 (29.4) | 63 (33.2) | 153 (28.1) |
| severe | 249 (33.9) | 40 (21.0) | 209 (38.3) |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Reactions after 1st/2nd Vaccination | Severe Severe | Moderate Severe | Severe Moderate | No or Minor Severe | No or Minor No or Minor | |||||
| sex | male | female | male | female | male | female | male | female | male | female |
| all responders (n = 735) | 2 | 19 | 7 | 59 | 0 | 6 | 31 | 131 | 76 | 149 |
| participants included in analysis (n = 76) | 1 | 13 | 5 | 5 | - | - | 14 | - | 20 | 18 |
| Characteristic | Severe AR (n = 38) | No or minor AR (n = 38) | p-Value |
|---|---|---|---|
| male sex—no. (%) | 20 (52.6) | 20 (52.6) | 1.00 |
| median age in years (range, IQR) | 43 (23–64, 26) | 42.5 (23–63, 25) | 0.97 |
| adverse reactions after 1st vaccination | |||
| no/minor | 15 (39.5) | 38 (100) | <0.001 |
| moderate | 7 (18.4) | 0 | 0.01 |
| severe | 16 (42.1) | 0 | <0.001 |
| adverse reactions after 2nd vaccination | |||
| no/minor | 0 | 38 (100) | <0.001 |
| moderate | 0 | 0 | 1.00 |
| severe | 38 (100) | 0 | <0.001 |
| smoking | 4 (10.5) | 1 (2.6) | 0.36 |
| median BMI (range, IQR) | 23.7 (18.4–32.4, 4.7) | 24.8 (17.6–35.9, 6.0) | 0.40 |
| any chronic disease | 9 (23.7) | 10 (26.3) | 1.00 |
| immunosuppression | 0 | 0 | 1.00 |
| antipyretic medication before 1st vaccination | 1 (2.6) | 2 (5.3) | 1.00 |
| antipyretic medication before 2nd vaccination | 4 (10.5) | 1 (2.6) | 0.36 |
| median time interval between 1st and 2nd vaccination—days (range, IQR) | 33 (21–38, 12) | 33 (21–38, 12) | 0.84 |
| median time interval between 2nd vaccination and blood sample collection—days (range, IQR) | 49 (35–58, 5) | 49 (35–58, 7) | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauernfeind, S.; Salzberger, B.; Hitzenbichler, F.; Scigala, K.; Einhauser, S.; Wagner, R.; Gessner, A.; Koestler, J.; Peterhoff, D. Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2. Vaccines 2021, 9, 1089. https://doi.org/10.3390/vaccines9101089
Bauernfeind S, Salzberger B, Hitzenbichler F, Scigala K, Einhauser S, Wagner R, Gessner A, Koestler J, Peterhoff D. Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2. Vaccines. 2021; 9(10):1089. https://doi.org/10.3390/vaccines9101089
Chicago/Turabian StyleBauernfeind, Stilla, Bernd Salzberger, Florian Hitzenbichler, Karolina Scigala, Sebastian Einhauser, Ralf Wagner, André Gessner, Josef Koestler, and David Peterhoff. 2021. "Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2" Vaccines 9, no. 10: 1089. https://doi.org/10.3390/vaccines9101089
APA StyleBauernfeind, S., Salzberger, B., Hitzenbichler, F., Scigala, K., Einhauser, S., Wagner, R., Gessner, A., Koestler, J., & Peterhoff, D. (2021). Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2. Vaccines, 9(10), 1089. https://doi.org/10.3390/vaccines9101089






