Are We Facing a New Colposcopic Practice in the HPV Vaccination Era? Opportunities, Challenges, and New Perspectives
Abstract
:1. Introduction
- HPV vaccines are prophylactic;
- population coverage is not yet as expected (95%);
- vaccines do not protect against all HPV types that cause precancer and cancer, although the nine genotypes covered account for 90% of cancers;
- duration of vaccine protection and long-term efficacy is not known, but at least 12 years has already been demonstrated and lifetime protection assumed;
- vaccination effectiveness seems to be related to the interval of time between the first vaccination and the screening program. Younger age at immunization is associated with increasing vaccine effectiveness.
2. Referral Criteria for Colposcopy in the Vaccination Era: Changes and Challenges
2.1. Target Screening Populations and Colposcopy in the Vaccination Era
- cohorts not offered vaccination;
- vaccinated girls and women with different vaccine and at different age;
- unvaccinated girls and women within vaccinated cohorts (at different ages and with different vaccines);
- vaccinated cohorts in whom it is not known who has and has not been vaccinated and with which vaccine.
- women vaccinated across or after treatment for HSIL with an adjuvant intent.
- -
- Different vaccination status of the population;
- -
- different age cohorts in vaccinated and screened population;
- -
- different timing between vaccination and screening test;
- -
- different vaccine effectiveness;
- -
- Different primary screening tests according to target population age and vaccination status;
- -
- HR HPV partial genotyping as the primary screening test is used to refer only the highest risk group HPV 16/18 positive to direct colposcopy;
- -
- HR HPV extended genotyping as an additional or primary screening test repre-sents; an essential step of HPV-risk estimation even if the additional risk strati-fication needs further evidence.
2.2. Primary Screening Test and Colposcopy in the Vaccination Era
3. Colposcopic Practice in the Post-Vaccination Era
- Reduced the prevalence of HPV 16 (and other vaccine HPV types) infection and HPV 16 CIN2+;
- reduced the PPV of colposcopy for the detection of CIN2+;
- does not have a significant effect on commonly recognized colposcopic features; multiple, as opposed to single HR HPV infection, is associated with larger colposcopic lesions;
- modified pattern of referral to colposcopy: the proportion of LSIL increase, the proportion of HSIL decrease and therefore the number of referrals to colposcopy for HSIL decrease;
- modified colposcopic performance (increase in cases with absence of abnormal colposcopic features or those having no clinical interventions; decrease in the proportion of women with a colposcopic impression of high-grade SIL and those having diagnostic punch biopsy/biopsies or treatment) thus diagnostic and therapeutic procedures require major knowledge and skills;
- new screening guidelines recommend colposcopy only when lesions will likely be detected;
- modifications of colposcopic standards are required (more or less than 50 colposcopies/year for HSIL);
- higher-quality colposcopy service represents an essential component of future cervical cancer screening programs;
- new guidelines are moving from more operator-dependent morphology-based screening and diagnosis to less operator-dependent biomolecular management.
3.1. Reduction of HPV Vaccine-Types Prevalence and Disease in Italy
3.2. Reduction of HPV Vaccine-Types Squamous Disease (CIN2+) in Different Countries
3.3. Adenocarcinoma of the Cervix: Post Vaccination Incidence and Colposcopic Practice
- The relative reduction in cases of squamous lesions as a result of organized or opportunistic screening and HPV vaccine implementation;
- the impact of processing techniques (liquid-based cytology) modifications on cytodiagnosis and increasing of the proportion of AGC cervical smears;
- a change in the distribution of HPV types coupled with better recognition of glandular lesions by the pathologists;
- a higher prevalence of non-HPV vaccine genotypes infection in time.
3.4. Impact of Primary and Secondary Prevention on Colposcopic Practice
3.4.1. Colposcopic Features and HPV Genoptypes
3.4.2. Reduction of HPV Vaccine-Types Disease and Influence on Colposcopic PPV for CIN2+
3.4.3. Multiple Infections and Colposcopic Accuracy
3.4.4. Reduction in Number and Modification of Reasons for Referral to Colposcopy
3.4.5. Modifications of Colposcopic Performance and Procedures
- Increase in cases with absence of abnormal colposcopic features [i.e., no acetowhite, no capillary vessel patterns (mosaic and/or punctation), or no abnormal vessels];
- increase in the proportion of women having no clinical interventions (biopsy or treatment);
- decrease in the proportion of women with a high-grade CIN colposcopic impression;
- decrease in the proportion having diagnostic punch biopsy/biopsies or treatment (loop excision or cold coagulation) [58].
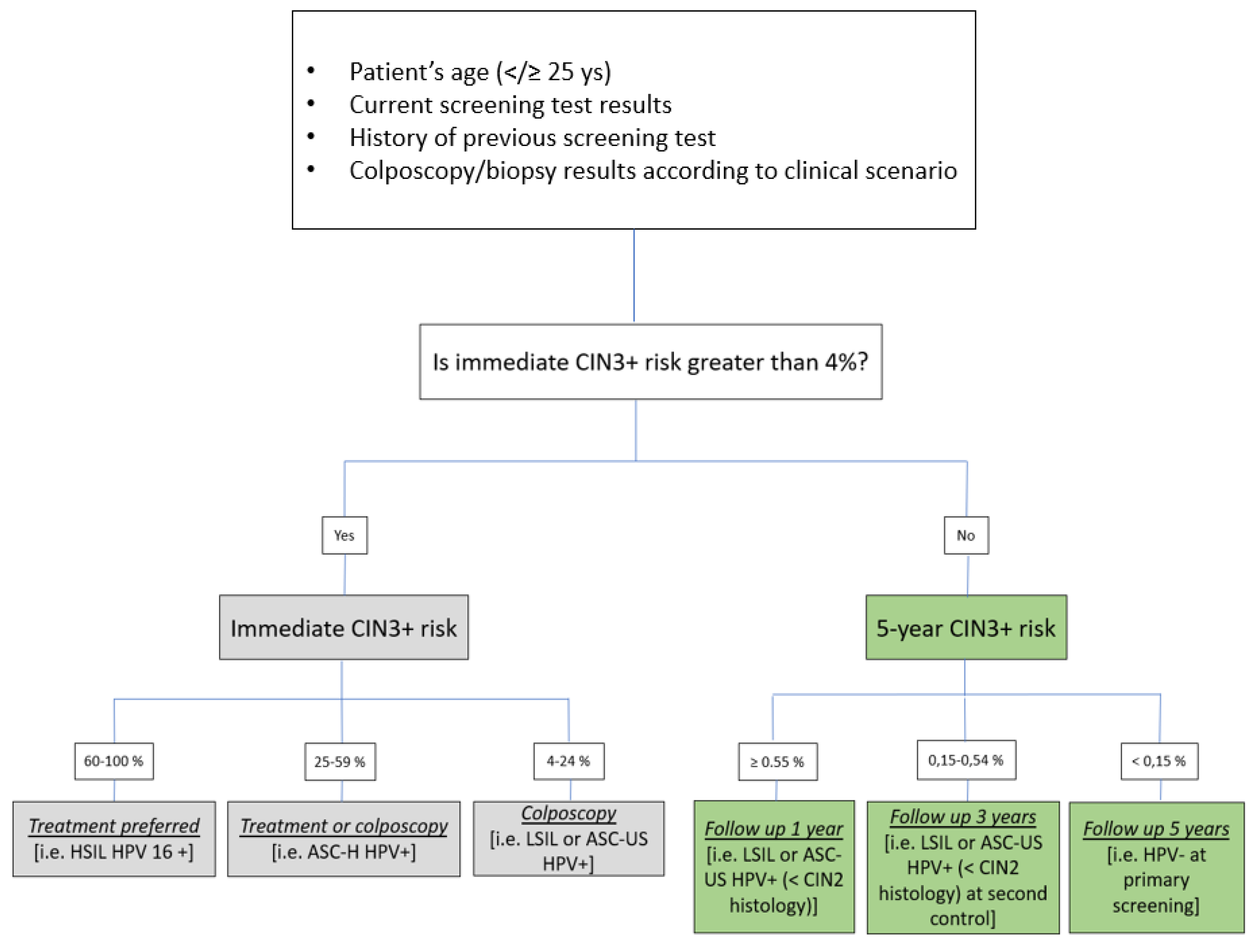
3.5. Strategies to Preserve the Diagnostic Accuracy of Colposcopy in the Vaccination Era
- (A)
- Risk stratification based on age (</≥25), vaccination status, current test results, and history of previous screening test and colposcopy/biopsy results and expressed in a percentage ranging from 0.15 to 100%;
- (B)
- risk groups based on the positivity of the single HR HPV genotype found adding partial or extensive genotyping to all positive HR HPV screening test cases;
- (C)
- combination of A + B.
3.6. Applying Risk-Based Stratification to the Italian Screening Setting
4. Conclusions
- Colposcopic practice is changing in the HPV vaccination era;
- high-quality colposcopy service remains an essential component of future cervical cancer screening programs;
- the colposcopist remains the clinical manager;
- colposcopic guidelines are moving towards patient risk stratification;
- risk stratification process will gradually include other factors (overage, current and previous tests, viral genotype) such as vaccination status, patient’s immune health status, microbiota vaginotype, genetic and epigenetic factors available in clinical evaluation, etc.;
- the reduction in colposcopic PPV is a point of concern; colposcopy will increasingly become an expert investigation;
- future strategies to maintain and increase expertise and informational role of colposcopy;
- the role of national and international Societies of colposcopy remains fundamental; national societies of colposopy should provide continuous, updated, available, and mandatory accreditation to all colposcopic professionals;
- application of artificial intelligence models to colposcopic practice to improve diagnostic accuracy can be the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canfell, K. Towards the global elimination of cervical cancer. Papillomavirus Res. 2019, 8, 100170. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, S.K.; Nygård, M.; Sundström, K.; Dillner, J.; Tryggvadottir, L.; Munk, C.; Berger, S.; Enerly, E.; Hortlund, M.; Ágústsson, Á.I.; et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four nordic countries. EClinicalMedicine 2020, 23, 100401. [Google Scholar] [CrossRef]
- Olsson, S.E.; Restrepo, J.A.; Reina, J.C.; Pitisuttithum, P.; Ulied, A.; Varman, M.; Van Damme, P.; Moreira, E.D., Jr.; Ferris, D.; Block, S.; et al. Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys 9 to 15 years of age: Interim analysis after 8 years of follow-up. Papillomavirus Res. 2020, 10, 100203. [Google Scholar] [CrossRef]
- Garland, S.M.; Kjaer, S.K.; Muñoz, N.; Block, S.L.; Brown, D.R.; DiNubile, M.J.; Lindsay, B.R.; Kuter, B.J.; Perez, G.; Dominiak-Felden, G.; et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Rev. Infect. Dis. 2016, 63, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Brotherton, J.M.; Pillsbury, A.; Jayasinghe, S.; Donovan, B.; Macartney, K.; Marshall, H. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: What additional disease burden will a nonavalent vaccine prevent? Eurosurveillance 2018, 23, 1700737. [Google Scholar] [CrossRef]
- Hariri, S.; Bennett, N.M.; Niccolai, L.M.; Schafer, S.; Park, I.U.; Bloch, K.C.; Unger, E.R.; Whitney, E.; Julian, P.; Scahill, M.W.; et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States—2008–2012. Vaccine 2015, 33, 1608–1613. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, L.; Yao, X.; Li, G.; Wang, Y.; Lin, Y. Progress in Vaccination of Prophylactic Human Papillomavirus Vaccine. Front. Immunol. 2020, 11, 1434. [Google Scholar] [CrossRef]
- Liverani, C.A.; Di Giuseppe, J.; Giannella, L.; Delli Carpini, G.; Ciavattini, A. Cervical Cancer Screening Guidelines in the Postvaccination Era: Review of the Literature. J. Oncol. 2020, 2020, 8887672. [Google Scholar] [CrossRef] [PubMed]
- Baio, G.; Capone, A.; Marcellusi, A.; Mennini, F.S.; Favato, G. Economic burden of human papillomavirus-related diseases in Italy. PLoS ONE 2012, 7, e49699. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Cofie, L.E.; Berenson, A.B. Cervical Cancer Incidence in Young U.S. Females after Human Papillomavirus Vaccine Introduction. Am. J. Prev. Med. 2018, 55, 197–204. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Mix, J.M.; Van Dyne, E.A.; Saraiya, M.; Hallowell, B.D.; Thomas, C.C. Assessing Impact of HPV Vaccination on Cervical Cancer Incidence among Women Aged 15–29 Years in the United States, 1999–2017: An Ecologic Study. Cancer Epidemiol. Prev. Biomark. 2021, 30, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Burger, E.A.; Nygård, M.; Kristiansen, I.S.; Kim, J.J. Adapting cervical cancer screening for women vaccinated against human papillomavirus infections: The value of stratifying guidelines. Eur. J. Cancer 2018, 91, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Giorgi Rossi, P.; Carozzi, F.; Federici, A.; Ronco, G.; Zappa, M.; Franceschi, S.; Italian Screening in HPV Vaccinated Girls Consensus Conference Group. Cervical cancer screening in women vaccinated against human papillomavirus infection: Recommendations from a consensus conference. Prev. Med. 2017, 98, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Landy, R.; Windridge, P.; Gillman, M.S.; Sasieni, P.D. What cervical screening is appropriate for women who have been vaccinated against high risk HPV? A simulation study. Int. J. Cancer 2018, 142, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef] [Green Version]
- Ministero Della Salute. Piano Nazionale Della Prevenzione 2010–2012. Azione Centrale Prioritaria Concernente la «Definizione di Documenti Tecnici di Sintesi Delle Evidenze Scientifiche» a Supporto Della Programmazione, Monitoraggio e Valutazione Degli Interventi di Prevenzione Oncologica Nella Popolazione a Rischio. Ministero Della Salute, DGPRE 001068-P-14/01/2013; pp. 1–22. Available online: http://www.osservatorionazionalescreening.it/sites/default/files/allegati/Screening.pdf (accessed on 20 September 2021).
- Ronco, G.; Biggeri, A.; Confortini, M.; Naldoni, C.; Segnan, N.; Sideri, M.; Zappa, M.; Zorzi, M.; Calvia, M.; Accetta, G.; et al. Health Technology assessment Report: Ricerca del DNA di Papillomavirus Umano (HPV) come test primario per lo screening dei precursori del cancro del collo dell’utero. HPV DNA based primary screening for cervical cancer precursors. Epidemiol. Prev. 2012, 36 (Suppl. 1), 1–72. [Google Scholar]
- Stoler, M.H.; Wright, T.C., Jr.; Parvu, V.; Yanson, K.; Cooper, C.K.; Andrews, J. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol. Oncol. 2019, 153, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Bonde, J.H.; Sandri, M.T.; Gary, D.S.; Andrews, J.C. Clinical Utility of Human Papillomavirus Genotyping in Cervical Cancer Screening: A Systematic Review. J. Low. Genit. Tract Dis. 2020, 24, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Demarco, M.; Hyun, N.; Carter-Pokras, O.; Raine-Bennett, T.R.; Cheung, L.; Chen, X.; Hammer, A.; Campos, N.; Kinney, W.; Gage, J.C.; et al. A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs. EClinicalMedicine 2020, 22, 100293. [Google Scholar] [CrossRef] [PubMed]
- Demarco, M.; Egemen, D.; Raine-Bennett, T.R.; Cheung, L.C.; Befano, B.; Poitras, N.E.; Lorey, T.S.; Chen, X.; Gage, J.C.; Castle, P.E.; et al. A Study of Partial Human Papillomavirus Genotyping in Support of the 2019 ASCCP Risk-Based Management Consensus Guidelines. J. Low. Genit. Tract Dis. 2020, 24, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Egemen, D.; Cheung, L.C.; Chen, X.; Demarco, M.; Perkins, R.B.; Kinney, W.; Poitras, N.; Befano, B.; Locke, A.; Guido, R.S.; et al. Risk Estimates Supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines. J. Low. Genit. Tract Dis. 2020, 24, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Liverani, C.A.; Bolis, G. Cervical Cancer Screening Strategies: Not the Test You Take, but the Decision You Make. Trends Gynecol. Oncol. 2016, 1, e102. [Google Scholar] [CrossRef]
- Massad, L.S.; Jeronimo, J.; Katki, H.A.; Schiffman, M.; National Institutes of Health/American Society for Colposcopy and Cervical Pathology Research Group. The accuracy of colposcopic grading for detection of high-grade cervical intraepithelial neoplasia. J. Low. Genit. Tract Dis. 2009, 13, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.I.; Andrews, J.; Cooper, C.K.; Gage, J.C.; Gold, M.A.; Khan, M.J.; Massad, L.S.; Parvu, V.; Perkins, R.B.; Schiffman, M.; et al. Risk of Cervical Intraepithelial Neoplasia 2 or Worse by Cytology, Human Papillomavirus 16/18, and Colposcopy Impression: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2018, 132, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, R.G.; Belinson, J.L.; Burchette, R.J.; Hu, S.; Zhang, X.; Qiao, Y.L. Regardless of skill, performing more biopsies increases the sensitivity of colposcopy. J. Low. Genit. Tract Dis. 2011, 15, 180–188. [Google Scholar] [CrossRef]
- Pretorius, R.G.; Belinson, J.L.; Azizi, F.; Peterson, P.C.; Belinson, S. Utility of random cervical biopsy and endocervical curettage in a low-risk population. J. Low. Genit. Tract Dis. 2012, 16, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Huh, W.K.; Sideri, M.; Stoler, M.; Zhang, G.; Feldman, R.; Behrens, C.M. Relevance of random biopsy at the transformation zone when colposcopy is negative. Obstet. Gynecol. 2014, 124, 670–678. [Google Scholar] [CrossRef]
- Stoler, M.H.; Vichnin, M.D.; Ferenczy, A.; Ferris, D.G.; Perez, G.; Paavonen, J.; Joura, E.A.; Djursing, H.; Sigurdsson, K.; Jefferson, L.; et al. The accuracy of colposcopic biopsy: Analyses from the placebo arm of the Gardasil clinical trials. Int. J. Cancer 2011, 128, 1354–1362. [Google Scholar] [CrossRef]
- Carozzi, F.M.; Ocello, C.; Burroni, E.; Faust, H.; Zappa, M.; Paci, E.; Iossa, A.; Bonanni, P.; Confortini, M.; Sani, C. Effectiveness of HPV vaccination in women reaching screening age in Italy. J. Clin. Virol. 2016, 84, 74–81. [Google Scholar] [CrossRef]
- Carozzi, F.; Puliti, D.; Ocello, C.; Anastasio, P.S.; Moliterni, E.A.; Perinetti, E.; Serradell, L.; Burroni, E.; Confortini, M.; Mantellini, P.; et al. Monitoring vaccine and non-vaccine HPV type prevalence in the post-vaccination era in women living in the Basilicata region, Italy. BMC Infect. Dis. 2018, 18, 38. [Google Scholar] [CrossRef] [Green Version]
- Bogani, G.; Serati, M.; Leone Roberti Maggiore, U.; Ditto, A.; Gardella, B.; Ferrero, S.; Spinillo, A.; Ghezzi, F.; Raspagliesi, F. Corrigendum to “Cervical intraepithelial neoplasia in women who had vaccination against HPV” [Int. J. Gynecol. Obstet. 2019, 147, 233–237]. Int. J. Gynecol. Obstet. 2020, 148, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acuti Martellucci, C.; Nomura, S.; Yoneoka, D.; Ueda, P.; Brotherton, J.M.L.; Canfell, K.; Palmer, M.; Manzoli, L.; Giorgi Rossi, P.; De Togni, A.; et al. Human papillomavirus vaccine effectiveness within a cervical cancer screening programme: Cohort study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, J.M.; Malloy, M.; Budd, A.C.; Saville, M.; Drennan, K.T.; Gertig, D.M. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: Observational cohort of young women in Australia. Papillomavirus Res. 2015, 1, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.M.; Strumpf, E.C.; Kaufman, J.S.; Lofters, A.; Schwandt, M.; Lévesque, L.E. The early benefits of human papillomavirus vaccination on cervical dysplasia and anogenital warts. Pediatrics 2015, 135, e1131–e1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, T.; Wallace, L.; Pollock, K.G.; Cuschieri, K.; Robertson, C.; Kavanagh, K.; Cruickshank, M. Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12–13 in Scotland: Retrospective population study. BMJ 2019, 365, l1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, K.G.J.; Kavanagh, K.; Potts, A.; Love, J.B.; Cuschieri, K.; Cubie, H.; Robertson, C.; Cruickshank, M.E.; Palmer, T.J.; Nicoll, S.M.; et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br. J. Cancer 2014, 111, 1824–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; Ali, H.; Boily, M.C.; Baldo, V.; Brassard, P.; Brotherton, J.M.L.; Callander, D.; et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Carstensen, B.; Møller, H.; Zappa, M.; Žakelj, M.P.; Lawrence, G.; Hakama, M.; Weiderpass, E. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol. Prev. Biomark. 2005, 14, 2191–2199. [Google Scholar] [CrossRef] [Green Version]
- Adegoke, O.; Kulasingam, S.; Virnig, B. Cervical Cancer Trends in the United States: A 35-Year Population-Based Analysis. J. Women’s Health 2012, 21, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Castanon, A.; Landy, R.; Sasieni, P.D. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int. J. Cancer 2016, 139, 1040–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirog, E.C.; RIS HPV TT Study Group; Lloveras, B.; Molijn, A.; Tous, S.; Guimerà, N.; Alejo, M.; Clavero, O.; Klaustermeier, J.; Jenkins, D.; et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod. Pathol. 2014, 27, 1559–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgson, A.; Park, K.J. Cervical Adenocarcinomas: A Heterogeneous Group of Tumors with Variable Etiologies and Clinical Outcomes. Arch. Pathol. Lab. Med. 2019, 143, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Gupta, R.; Gupta, S. Glandular cell abnormalities in cervical cytology: What has changed in this decade and what has not? Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 68–73. [Google Scholar] [CrossRef]
- Cleveland, A.A.; Gargano, J.W.; Park, I.U.; Griffin, M.R.; Niccolai, L.M.; Powell, M.; Bennett, N.M.; Saadeh, K.; Pemmaraju, M.; Higgins, K.; et al. Cervical adenocarcinoma in situ: Human papillomavirus types and incidence trends in five states, 2008–2015. Int. J. Cancer 2020, 146, 810–818. [Google Scholar] [CrossRef]
- Jeronimo, J.; Massad, L.S.; Schiffman, M.; Group, R.; National Institutes of Health. Visual appearance of the uterine cervix: Correlation with human papillomavirus detection and type. Am. J. Obstet. Gynecol. 2007, 197, 47.e1–47.e8. [Google Scholar] [CrossRef]
- Nam, K.; Kwak, J.; Kim, J.; Jeon, S. Human Papillomavirus Type 16 Causes Larger Colposcopic Lesions than Other HPV Types in Patients with Grade 3 Cervical Intraepithelial Neoplasia. J. Low. Genit. Tract Dis. 2013, 17, 1–5. [Google Scholar] [CrossRef]
- Spinillo, A.; Gardella, B.; Chiesa, A.; Cesari, S.; Alberizzi, P.; Silini, E.M. Diagnostic accuracy of colposcopy in relation to human papillomavirus genotypes and multiple infection. Gynecol. Oncol. 2014, 134, 527–533. [Google Scholar] [CrossRef]
- Spinillo, A.; Gardella, B.; Iacobone, A.D.; Cesari, S.; Alberizzi, P.; Silini, E.M. Multiple Papillomavirus Infection and Size of Colposcopic Lesions among Women with Cervical Intraepithelial Neoplasia. J. Low. Genit. Tract Dis. 2016, 20, 22–25. [Google Scholar] [CrossRef]
- Van Der Marel, J.; Van Baars, R.; Quint, W.; Berkhof, J.; Del Pino, M.; Torné, A.; Ordi, J.; Wentzensen, N.; Schiffman, M.; Van De Sandt, M.; et al. The impact of human papillomavirus genotype on colposcopic appearance: A cross-sectional analysis. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1117–1126. [Google Scholar] [CrossRef]
- Munro, A.; Gillespie, C.; Cotton, S.; Busby-Earle, C.; Kavanagh, K.; Cuschieri, K.; Cubie, H.; Robertson, C.; Smart, L.; Pollock, K.; et al. The impact of human papillomavirus type on colposcopy performance in women offered HPV immunisation in a catch-up vaccine programme: A two-centre observational study. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1394–1401. [Google Scholar] [CrossRef] [Green Version]
- Sherman, M.E.; Wang, S.S.; Tarone, R.; Rich, L.; Schiffman, M. Histopathologic extent of cervical intraepithelial neoplasia 3 lesions in the atypical squamous cells of undetermined significance low-grade squamous intraepithelial lesion triage study: Implications for subject safety and lead-time bias. Cancer Epidemiol. Prev. Biomark. 2003, 12, 372–379. [Google Scholar]
- Pretorius, R.G.; Zhang, W.-H.; Belinson, J.L.; Huang, M.-N.; Wu, L.-Y.; Zhang, X.; Qiao, Y.-L. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am. J. Obstet. Gynecol. 2004, 191, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Du, H.; Pretorius, R.G.; Wang, C.; Yang, B.; Wang, G.; Tang, J.; Belinson, J.L.; Wu, R. High-grade cervical intraepithelial neoplasia detected by colposcopy-directed or random biopsy relative to age, cytology, human papillomavirus 16, and lesion size. J. Low. Genit. Tract Dis. 2016, 20, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, M.E.; Pan, J.; Cotton, S.C.; Kavanagh, K.; Robertson, C.; Cuschieri, K.; Cubie, H.; Palmer, T.; Pollock, K.G. Reduction in colposcopy workload and associated clinical activity following human papillomavirus (HPV) catch-up vaccination programme in Scotland: An ecological study. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1386–1393. [Google Scholar] [CrossRef]
- Wright, T.C.; Stoler, M.H.; Parvu, V.; Yanson, K.; Cooper, C.; Andrews, J. Risk detection for high-grade cervical disease using Onclarity HPV extended genotyping in women, ≥21 years of age, with ASC-US or LSIL cytology. Gynecol. Oncol. 2019, 154, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Jach, R.; Mazurec, M.; Trzeszcz, M.; Bartosinska-Dyc, A.; Galarowicz, B.; Kedzia, W.; Nowakowski, A.; Pitynski, K.; Zimmer, M.; Marszalek, A.; et al. COLPOSCOPY 2020—COLPOSCOPY PROTOCOLS: A Summary of the Clinical Experts Consensus Guidelines of the Polish Society of Colposcopy and Cervical Pathophysiology and the Polish Society of Gynaecologists and Obstetricians. Ginekol. Pol. 2020, 91, 362371. [Google Scholar] [CrossRef]
- Brusselaers, N.; Shrestha, S.; Van De Wijgert, J.; Verstraelen, H. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 221, 9–18.e8. [Google Scholar] [CrossRef]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 171–180. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Ntritsos, G.; Smith, A.; Tsilidis, K.K.; Marchesi, J.R.; Bennett, P.R.; Moscicki, A.-B.; Kyrgiou, M. The vaginal microbiota associates with the re-gression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun. 2020, 11, 1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciavattini, A.; Giannella, L.; De Vincenzo, R.; Di Giuseppe, J.; Papiccio, M.; Lukic, A.; Carpini, G.D.; Perino, A.; Frega, A.; Sopracordevole, F.; et al. HPV Vaccination: The Position Paper of the Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV). Vaccines 2020, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Massad, L.S.; Mayeaux, E.J., Jr.; Khan, M.J.; Waxman, A.G.; Einstein, M.H.; Conageski, C.; Schiffman, M.H.; Gold, M.A.; Apgar, B.S.; et al. Evidence-based consensus recommendations for colposcopy practice for cer-vical cancer prevention in the United States. J. Low. Genit. Tract Dis. 2017, 21, 216–222. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukic, A.; De Vincenzo, R.; Ciavattini, A.; Ricci, C.; Senatori, R.; Ruscito, I.; Frega, A. Are We Facing a New Colposcopic Practice in the HPV Vaccination Era? Opportunities, Challenges, and New Perspectives. Vaccines 2021, 9, 1081. https://doi.org/10.3390/vaccines9101081
Lukic A, De Vincenzo R, Ciavattini A, Ricci C, Senatori R, Ruscito I, Frega A. Are We Facing a New Colposcopic Practice in the HPV Vaccination Era? Opportunities, Challenges, and New Perspectives. Vaccines. 2021; 9(10):1081. https://doi.org/10.3390/vaccines9101081
Chicago/Turabian StyleLukic, Ankica, Rosa De Vincenzo, Andrea Ciavattini, Caterina Ricci, Roberto Senatori, Ilary Ruscito, and Antonio Frega. 2021. "Are We Facing a New Colposcopic Practice in the HPV Vaccination Era? Opportunities, Challenges, and New Perspectives" Vaccines 9, no. 10: 1081. https://doi.org/10.3390/vaccines9101081
APA StyleLukic, A., De Vincenzo, R., Ciavattini, A., Ricci, C., Senatori, R., Ruscito, I., & Frega, A. (2021). Are We Facing a New Colposcopic Practice in the HPV Vaccination Era? Opportunities, Challenges, and New Perspectives. Vaccines, 9(10), 1081. https://doi.org/10.3390/vaccines9101081







