Febrile Seizures and Measles-Containing Vaccines in China: A Self-Controlled Case Series Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
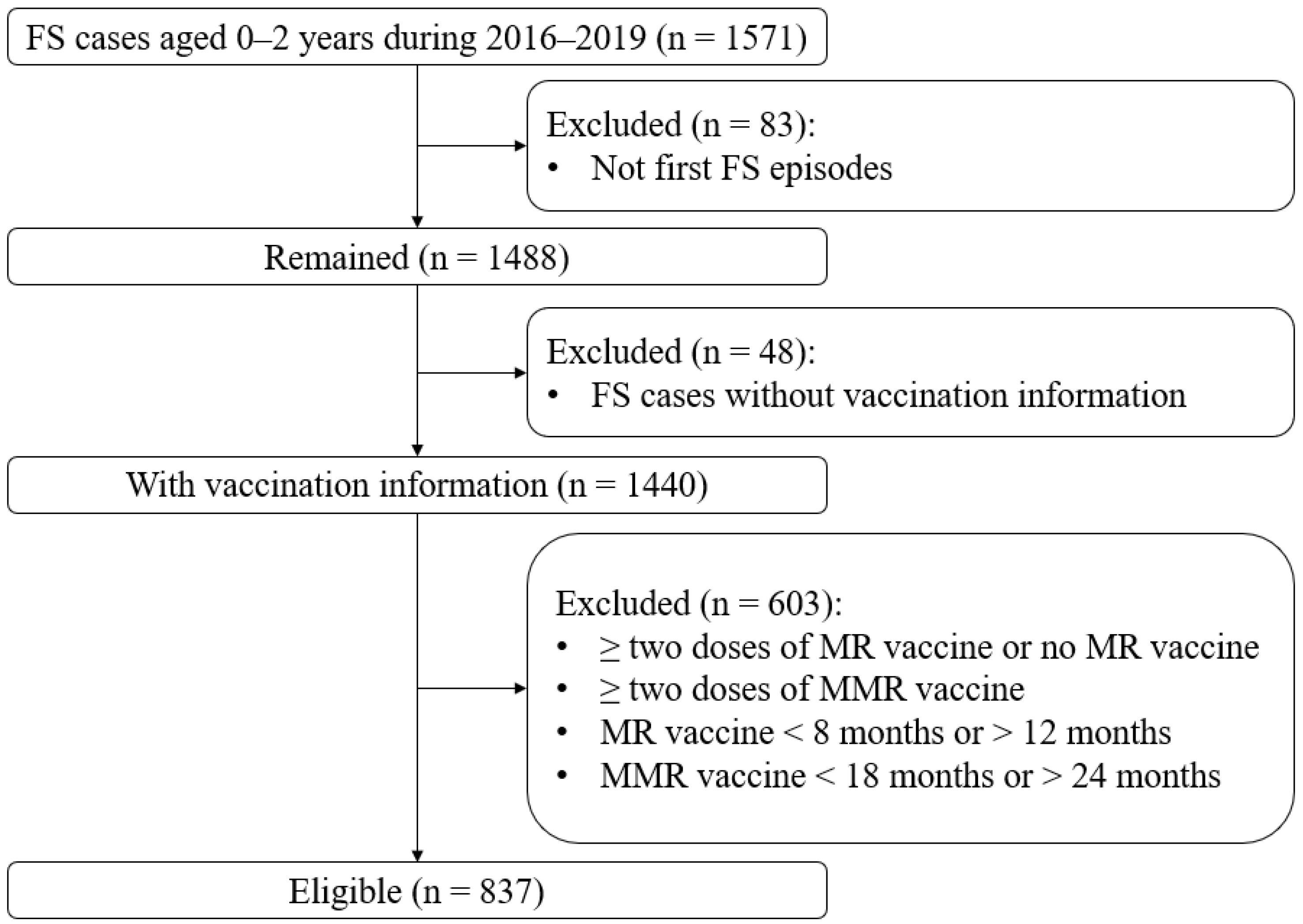
2.2. Study Population
2.3. Case Validation
2.4. Statistical Analysis
2.5. Sample Size
3. Results
3.1. Characteristics of the Children with FSs
3.2. Case Validation
3.3. Risk of FSs following MR and MMR Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Category of Condition | Condition | ICD-10 Code |
|---|---|---|
| Diseases of the nervous system: Inflammatory diseases of the central nervous system | Bacterial meningitis, not classified elsewhere | G00 |
| Meningitis in bacterial diseases classified elsewhere | G01 | |
| Meningitis in other infectious and parasitic diseases classified elsewhere | G02 | |
| Meningitis due to other and unspecified causes | G03 | |
| Encephalitis, myelitis and encephalomyelitis | G04 | |
| Encephalitis, myelitis and encephalomyelitis in diseases classified elsewhere | G05 | |
| Intracranial and intraspinal abscess and granuloma | G06 | |
| Intracranial and intraspinal abscess and granuloma in diseases classified elsewhere | G07 | |
| Intracranial and intraspinal phlebitis and thrombophlebitis | G08 | |
| Sequelae of inflammatory diseases of the central nervous system | G09 | |
| Diseases of the nervous system: Systemic atrophies primarily affecting the central nervous system | Huntington’s disease | G10 |
| Spinal muscular atrophy and related syndromes | G12 | |
| Diseases of the nervous system: Extrapyramidal and movement disorders | Other degenerative diseases of the basal ganglia | G23 |
| Dystonia | G24 | |
| Other extrapyramidal and movement disorders | G25 | |
| Extrapyramidal and movement disorders in diseases classified elsewhere | G26 | |
| Diseases of the nervous system: Other degenerative diseases of the nervous system | Other degenerative diseases of nervous system, not classified elsewhere | G31 |
| Other degenerative disorders of nervous system in diseases classified elsewhere | G32 | |
| Diseases of the nervous system: Episodic and paroxysmal disorders | Epilepsy | G40 |
| Diseases of the nervous system: Other disorders of the nervous system | Disorders of the autonomic nervous system | G90 |
| Hydrocephalus | G91 | |
| Toxic encephalopathy | G92 | |
| Other disorders of the brain | G93 | |
| Other disorders of the brain in diseases classified elsewhere | G94 | |
| Other disorders of the central nervous system | G96 | |
| Postprocedural disorders of the nervous system, not classified elsewhere | G97 | |
| Other disorders of the nervous system, not classified elsewhere | G98 | |
| Other disorders of the nervous system in diseases classified elsewhere | G99 | |
| Metabolic disorders | Sphingolipid metabolism disorders and other lipid storage disorders | E75 |
| Infectious and parasitic diseases | Tuberculosis of the nervous system | A17 |
| Atypical virus infections of the central nervous system | A81 | |
| Mosquito-borne viral encephalitis | A83 | |
| Tick-borne viral encephalitis | A84 | |
| Other viral encephalitis, not classified elsewhere | A85 | |
| Unspecified viral encephalitis | A86 | |
| Viral meningitis | A87 | |
| Other viral infections of the central nervous system, not classified elsewhere | A88 | |
| Unspecified viral infection of the central nervous system | A89 | |
| Other mosquito-borne viral fevers | A92 | |
| Other arthropod-borne viral fevers, not classified elsewhere | A93 | |
| Unspecified arthropod-borne viral fever | A94 | |
| Neoplasms | Malignant neoplasm of the meninges | C70 |
| Malignant neoplasm of the brain | C71 | |
| Malignant neoplasm of the spinal cord, cranial nerves and other parts of the central nervous system | C72 | |
| Benign neoplasm of the meninges | D32 | |
| Benign neoplasm of the brain and other parts of the central nervous system | D33 | |
| Injuries to the head | Fracture of the skull and facial bones | S02 |
| Intracranial injury | S06 | |
| Crushing injury of the head | S07 | |
| Traumatic amputation of part of the head | S08 | |
| Other and unspecified injuries of the head | S09 |
References
- Sawyer, M.H.; Simon, G.; Byington, C. Vaccines and febrile seizures: Quantifying the risk. Pediatrics 2016, 138, e20160976. [Google Scholar] [CrossRef] [Green Version]
- Principi, N.; Esposito, S. Vaccines and febrile seizures. Expert Rev. Vaccines 2013, 12, 885–892. [Google Scholar] [CrossRef]
- Di Pietrantonj, C.; Rivetti, A.; Marchione, P.; Debalini, M.G.; Demicheli, V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst. Rev. 2020, 4, Cd004407. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Hviid, A.; Madsen, K.M.; Wohlfahrt, J.; Thorsen, P.; Schendel, D.; Melbye, M.; Olsen, J. MMR vaccination and febrile seizures: Evaluation of susceptible subgroups and long-term prognosis. JAMA 2004, 292, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Barlow, W.E.; Davis, R.L.; Glasser, J.W.; Rhodes, P.H.; Thompson, R.S.; Mullooly, J.P.; Black, S.B.; Shinefield, H.R.; Ward, J.I.; Marcy, S.M.; et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N. Engl. J. Med. 2001, 345, 656–661. [Google Scholar] [CrossRef]
- Macartney, K.; Gidding, H.F.; Trinh, L.; Wang, H.; Dey, A.; Hull, B.; Orr, K.; McRae, J.; Richmond, P.; Gold, M.; et al. Evaluation of combination measles-mumps-rubella-varicella vaccine introduction in Australia. JAMA Pediatr. 2017, 171, 992–998. [Google Scholar] [CrossRef] [PubMed]
- McClure, D.L.; Jacobsen, S.J.; Klein, N.P.; Naleway, A.L.; Kharbanda, E.O.; Glanz, J.M.; Jackson, L.A.; Weintraub, E.S.; McLean, H.Q. Similar relative risks of seizures following measles containing vaccination in children born preterm compared to full-term without previous seizures or seizure-related disorders. Vaccine 2019, 37, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.N.; Bryant, N.J.; Andrews, N.J.; Bowley, J.S.; Ohrling, A.; Verity, C.M.; Ross, E.M.; Miller, E. Risk of serious neurologic disease after immunization of young children in Britain and Ireland. Pediatrics 2007, 120, 314–321. [Google Scholar] [CrossRef] [PubMed]
- A Picture to Understand the Children’s Immunization Program of National Immunization Program. Available online: http://www.chinacdc.cn/jkzt/ymyjz/zswd_10960/201904/t20190426_201664.html (accessed on 2 January 2020).
- Summary of WHO Position Papers—Recommended Routine Immunizations for Children. Available online: https://www.who.int/immunization/policy/Immunization_routine_table2.pdf (accessed on 20 June 2021).
- Recommended Child and Adolescent Immunization Schedule for Ages 18 Years or Younger, United States. 2020. Available online: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html (accessed on 9 January 2021).
- Vaccine Schedules in All Countries of the European Union. Available online: https://vaccine-schedule.ecdc.europa.eu/ (accessed on 9 January 2021).
- National Immunisation Program Schedule. Available online: https://www.health.gov.au/health-topics/immunisation/immunisation-throughout-life/national-immunisation-program-schedule (accessed on 9 January 2021).
- Patel, N.; Ram, D.; Swiderska, N.; Mewasingh, L.D.; Newton, R.W.; Offringa, M. Febrile seizures. BMJ 2015, 351, h4240. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S.M.; Abubakar, A.; Stein, A.; Marsh, K.; Newton, C. Prevalence, causes, and behavioral and emotional comorbidities of acute symptomatic seizures in Africa: A critical review. Epilepsia Open 2017, 2, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, L.; Yang, Y.; Meng, R.; Fang, T.; Dong, Y.; Li, N.; Xu, G.; Zhan, S. Active surveillance of adverse events following human papillomavirus vaccination: Feasibility pilot study based on the Regional Health Care Information Platform in the city of Ningbo, China. J. Med. Internet Res. 2020, 22, e17446. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, Y.; Tang, J.; Jia, H.; Qin, W.; Su, Y.; Wang, H.; Hao, L. Assessment of mumps-containing vaccine effectiveness during an outbreak: Importance to introduce the 2-dose schedule for China. Hum. Vaccine Immunother. 2018, 14, 1392–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonhoeffer, J.; Menkes, J.; Gold, M.S.; de Souza-Brito, G.; Fisher, M.C.; Halsey, N.; Vermeer, P. Generalized convulsive seizure as an adverse event following immunization: Case definition and guidelines for data collection, analysis, and presentation. Vaccine 2004, 22, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Marcy, S.M.; Kohl, K.S.; Dagan, R.; Nalin, D.; Blum, M.; Jones, M.C.; Hansen, J.; Labadie, J.; Lee, L.; Martin, B.L.; et al. Fever as an adverse event following immunization: Case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004, 22, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Farrington, C.P.; Whitaker, H.J.; Hocine, M.N. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics 2009, 10, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitaker, H.J.; Farrington, C.P.; Spiessens, B.; Musonda, P. Tutorial in biostatistics: The self-controlled case series method. Stat. Med. 2006, 25, 1768–1797. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, S.E.; Dover, D.C.; Simmonds, K.A.; Svenson, L.W. Risk of febrile seizures after first dose of measles-mumps-rubella-varicella vaccine: A population-based cohort study. CMAJ 2014, 186, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Rodewald, L.; Yang, J.; Qin, Y.; Pang, M.; Feng, L.; Yu, H. The landscape of vaccines in China: History, classification, supply, and price. BMC Infect. Dis. 2018, 18, 502. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.L.; Marcuse, E.; Black, S.; Shinefield, H.; Givens, B.; Schwalbe, J.; Ray, P.; Thompson, R.S.; Chen, R. MMR2 immunization at 4 to 5 years and 10 to 12 years of age: A comparison of adverse clinical events after immunization in the Vaccine Safety Datalink project. The Vaccine Safety Datalink Team. Pediatrics 1997, 100, 767–771. [Google Scholar] [CrossRef]
- Chen, R.T.; Moses, J.M.; Markowitz, L.E.; Orenstein, W.A. Adverse events following measles-mumps-rubella and measles vaccinations in college students. Vaccine 1991, 9, 297–299. [Google Scholar] [CrossRef]
- Deng, L.; Wood, N.; Danchin, M. Seizures following vaccination in children: Risks, outcomes and management of subsequent revaccination. Aust. J. Gen. Pract. 2020, 49, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Andrews, N.; Stowe, J.; Grant, A.; Waight, P.; Taylor, B. Risks of convulsion and aseptic meningitis following measles-mumps-rubella vaccination in the United Kingdom. Am. J. Epidemiol. 2007, 165, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, J.; Liu, Y.; Xu, Y.; Che, X.; Gu, W.; Du, J.; Zhang, X.; Xu, E. Survey of contraindications in children’s routine vaccination in Hangzhou, China. Hum. Vaccin. Immunother. 2017, 13, 1539–1543. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Maleq, N.; Guillermet, E.; Colombini, A.; Gessner, B.D. A systematic literature review of missed opportunities for immunization in low- and middle-income countries. Vaccine 2014, 32, 6870–6879. [Google Scholar] [CrossRef]
- Hawken, S.; Potter, B.K.; Little, J.; Benchimol, E.I.; Mahmud, S.; Ducharme, R.; Wilson, K. The use of relative incidence ratios in self-controlled case series studies: An overview. BMC Med. Res. Methodol. 2016, 16, 126. [Google Scholar] [CrossRef] [Green Version]
- Galazka, A.M.; Lauer, B.A.; Henderson, R.H.; Keja, J. Indications and contraindications for vaccines used in the Expanded Programme on Immunization. Bull. World Health Organ. 1984, 62, 357–366. [Google Scholar]
- Carazo Perez, S.; Bureau, A.; De Serres, G. Post-immunisation fever and the antibody response to measles-containing vaccines. Epidemiol. Infect. 2018, 146, 1584–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feenstra, B.; Pasternak, B.; Geller, F.; Carstensen, L.; Wang, T.; Huang, F.; Eitson, J.L.; Hollegaard, M.V.; Svanstrom, H.; Vestergaard, M.; et al. Common variants associated with general and MMR vaccine-related febrile seizures. Nat. Genet. 2014, 46, 1274–1282. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.K.; Hon, K.L.; Leung, T.N. Febrile seizures: An overview. Drugs Context 2018, 7, 212536. [Google Scholar] [CrossRef]
| Characteristic | Total | Male | Female |
|---|---|---|---|
| No. | 837 | 517 | 320 |
| Median onset age, days (IQR) | 515 (403, 614) | 516 (410, 622) | 510.5 (400, 606.5) |
| During the risk period after MR vaccination, No. (%) | 33 (3.94) | 23 (4.45) | 10 (3.13) |
| During the risk period after MMR vaccination, No. (%) | 92 (10.99) | 45 (8.70) | 47 (14.69) |
| Days since MR Vaccination | No. of FS Cases | Primary Analysis [RI (95% CI, p)] |
|---|---|---|
| 0 to 42 days | 33 | 1.17 (0.65 to 2.11, 0.609) |
| 0 to 6 days | 4 | 1.11 (0.33 to 3.70, 0.871) |
| 7 to 13 days | 3 | 0.80 (0.23 to 2.86, 0.737) |
| 14 to 27 days | 14 | 1.67 (0.81 to 3.42, 0.165) |
| 28 to 42 days | 12 | 1.02 (0.49 to 2.14, 0.954) |
| No. of FS Cases | Analysis [RI (95% CI, p)] | ||||
|---|---|---|---|---|---|
| Days Since MMR Vaccination | Primary Analysis or Sensitivity Analysis One a | Sensitivity Analysis Two b | Primary Analysis | Sensitivity Analysis One | Sensitivity Analysis Two |
| 0 to 42 days | 92 | 90 | 0.90 (0.64 to 1.27, 0.538) | 1.07 (0.81 to 1.41, 0.631) | 0.85 (0.60 to 1.20, 0.356) |
| 0 to 6 days | 16 | 16 | 0.99 (0.56 to 1.75, 0.976) | 1.15 (0.68 to 1.93, 0.604) | 0.94 (0.53 to 1.66, 0.819) |
| 7 to 13 days | 19 | 19 | 1.17 (0.68 to 2.01, 0.563) | 1.36 (0.83 to 2.23, 0.216) | 1.11 (0.64 to 1.90, 0.712) |
| 14 to 27 days | 28 | 26 | 0.87 (0.54 to 1.39, 0.554) | 1.01 (0.66 to 1.53, 0.978) | 0.77 (0.47 to 1.24, 0.278) |
| 28 to 42 days | 29 | 29 | 0.85 (0.54 to 1.34, 0.481) | 0.99 (0.66 to 1.48, 0.948) | 0.83 (0.53 to 1.31, 0.424) |
| Male | Female | |||
|---|---|---|---|---|
| Days Since MMR Vaccination | No. of FS Cases | Primary Analysis [RI (95% CI, p)] | No. of FS Cases | Primary Analysis [RI (95% CI, p)] |
| 0 to 42 days | 45 | 0.66 (0.42 to 1.05, 0.079) | 47 | 1.39 (0.79 to 2.44, 0.247) |
| 0 to 6 days | 7 | 0.64 (0.28 to 1.46, 0.290) | 9 | 1.84 (0.80 to 4.24, 0.153) |
| 7 to 13 days | 10 | 0.92 (0.44 to 1.91, 0.818) | 9 | 1.82 (0.79 to 4.18, 0.159) |
| 14 to 27 days | 15 | 0.70 (0.38 to 1.31, 0.264) | 13 | 1.29 (0.61 to 2.74, 0.505) |
| 28 to 42 days | 13 | 0.59 (0.31 to 1.13, 0.112) | 16 | 1.35 (0.68 to 2.69, 0.390) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Li, N.; Zhang, L.; Ma, R.; Fang, T.; Liu, Z.; Zhan, S. Febrile Seizures and Measles-Containing Vaccines in China: A Self-Controlled Case Series Study. Vaccines 2021, 9, 1073. https://doi.org/10.3390/vaccines9101073
Xu L, Li N, Zhang L, Ma R, Fang T, Liu Z, Zhan S. Febrile Seizures and Measles-Containing Vaccines in China: A Self-Controlled Case Series Study. Vaccines. 2021; 9(10):1073. https://doi.org/10.3390/vaccines9101073
Chicago/Turabian StyleXu, Lu, Ning Li, Liang Zhang, Rui Ma, Ting Fang, Zhike Liu, and Siyan Zhan. 2021. "Febrile Seizures and Measles-Containing Vaccines in China: A Self-Controlled Case Series Study" Vaccines 9, no. 10: 1073. https://doi.org/10.3390/vaccines9101073
APA StyleXu, L., Li, N., Zhang, L., Ma, R., Fang, T., Liu, Z., & Zhan, S. (2021). Febrile Seizures and Measles-Containing Vaccines in China: A Self-Controlled Case Series Study. Vaccines, 9(10), 1073. https://doi.org/10.3390/vaccines9101073






