Plasmodium falciparum Malaria Vaccines and Vaccine Adjuvants
Abstract
1. Introduction
2. Immunity against P. falciparum
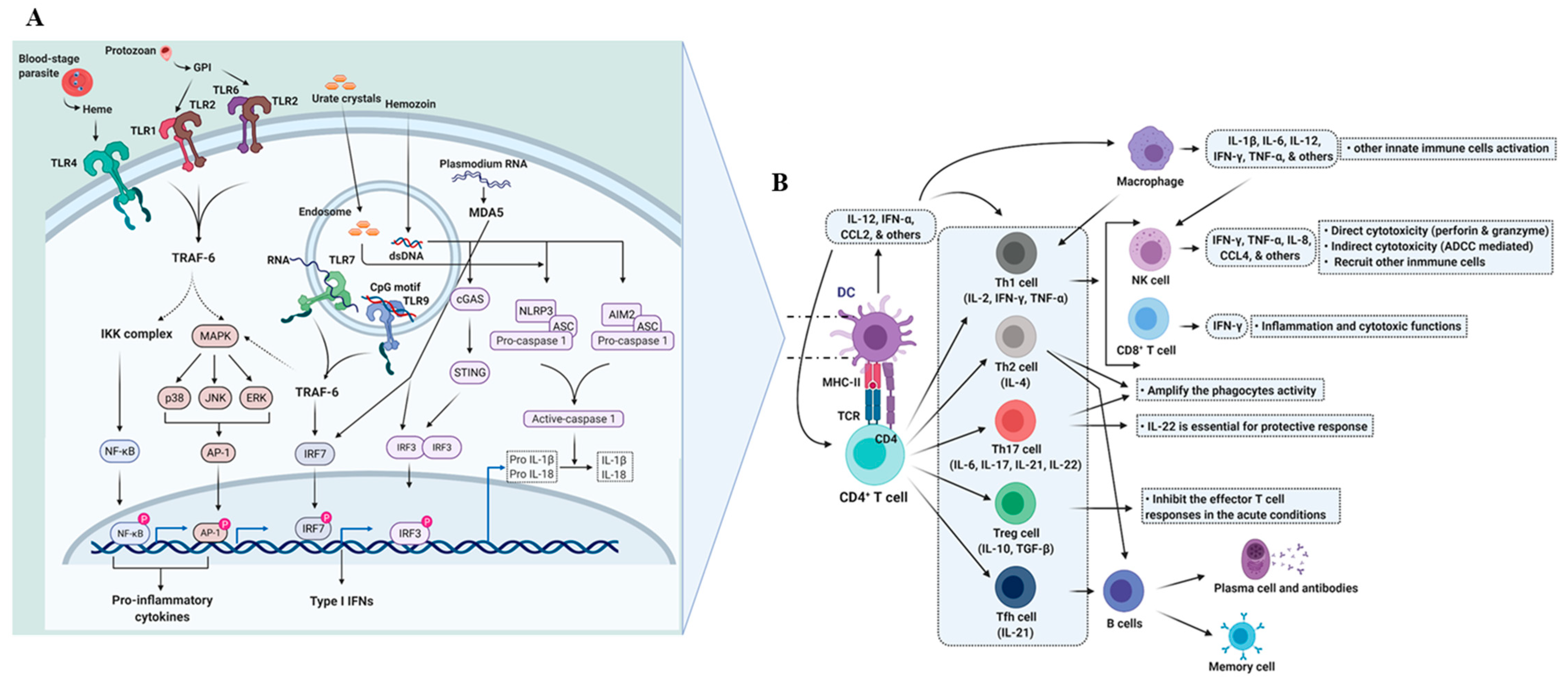
2.1. Dendritic Cells and the Initiation of the Immune Response
2.2. Adaptive Immunity
2.2.1. T Cell-Mediated Immunity
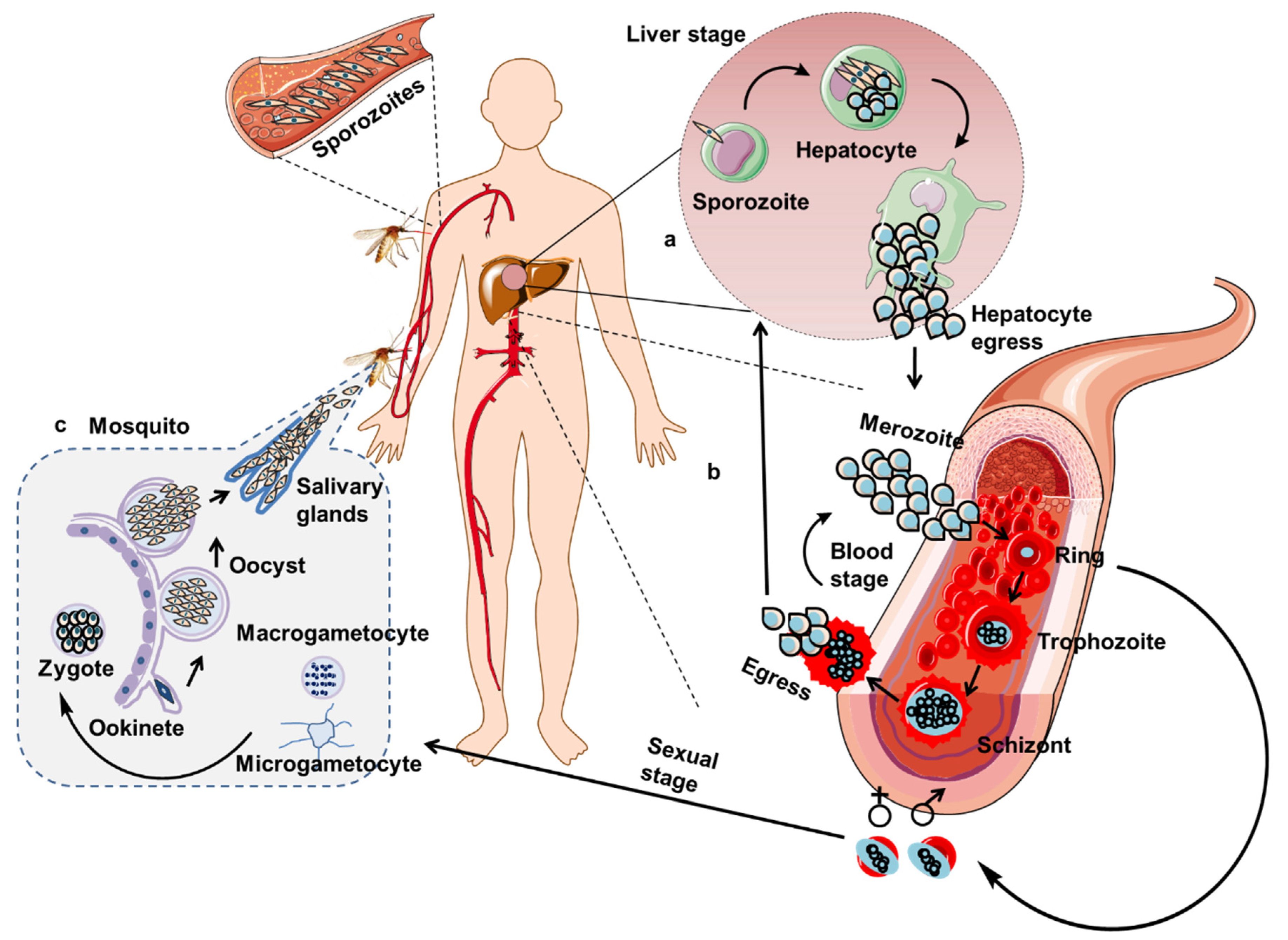
2.2.2. T Cell-Mediated Immunity against Liver-Stage Malaria Parasites
2.2.3. T Cell-Mediated Immunity against Asexual Blood-Stage Malaria Parasites
2.2.4. Antibody-Mediated Immunity
3. Vaccines
4. Vaccine Adjuvants
5. Adjuvants under Clinical Evaluation
5.1. Alum
5.2. Vaccine Delivery Systems/Formulations
5.2.1. Liposomes
5.2.2. AS01
5.2.3. Emulsions
5.2.4. AS02
5.2.5. Montanides (ISA 51, ISA 720)
5.3. Immune Potentiators or Immunomodulators
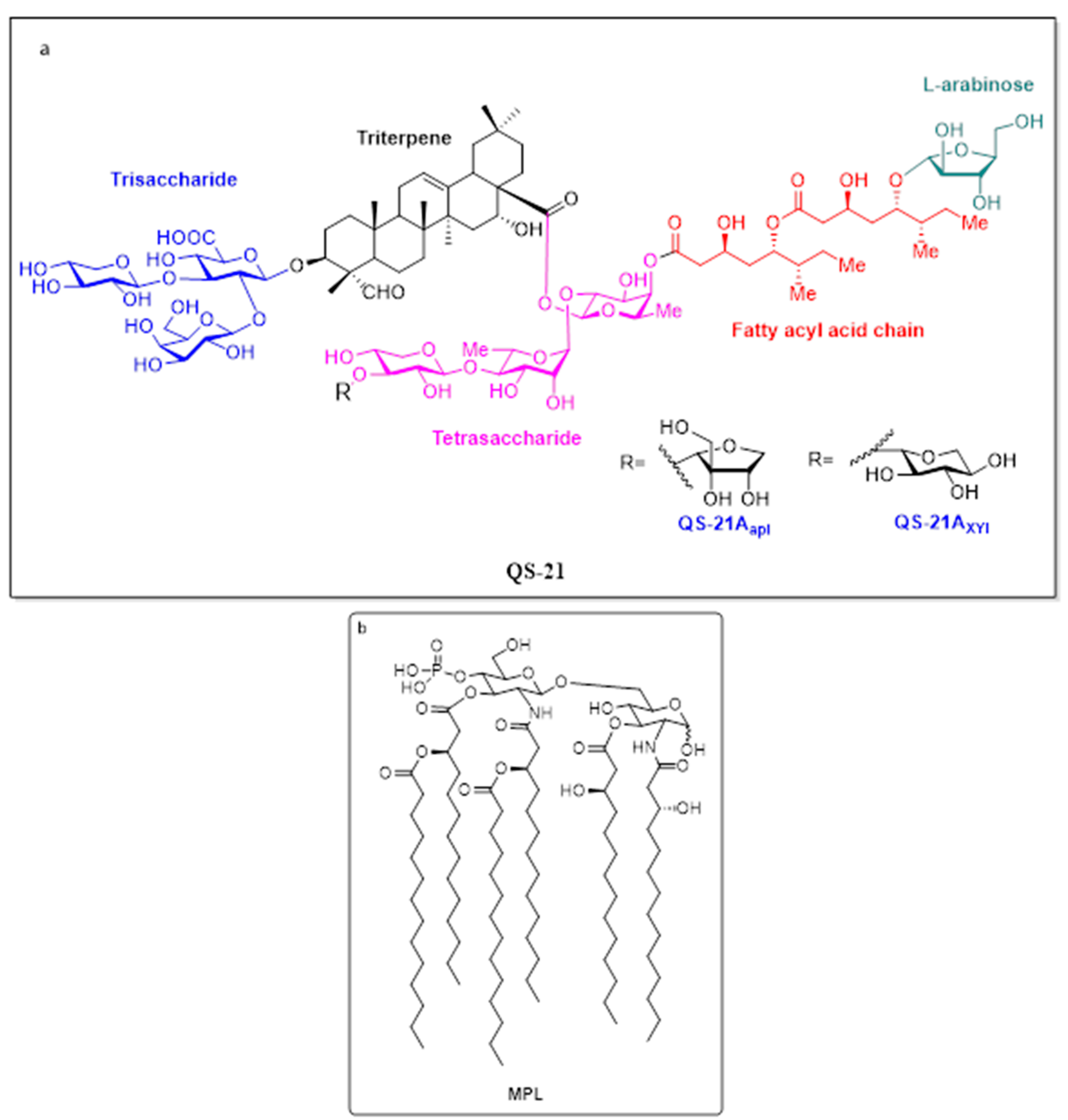
5.3.1. QS-21
5.3.2. Other Saponin Based Adjuvants (Quil-A and ISCOMs)
5.3.3. CpG ODN
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | antibody-dependent cell cytotoxicity |
| AIM2 | absent in melanoma 2 |
| AP-1 | activator protein 1 |
| ASC | apoptosis-associated speck-like protein containing a CARD (caspase activation and recruitment domains) |
| CCL2/MCP-1 | monocyte chemoattractant protein-1 |
| CCL4/MIP-1β | macrophage inflammatory protein-1β |
| cGAS | cyclic GMP-AMP synthase |
| DAMPs | damage-associated molecular patterns |
| DCs | dendritic cells |
| dsDNA | double-stranded DNA |
| ERK | extracellular signal-regulated kinases |
| GPI | glycosylphosphatidylinositol |
| IKK | IκB kinase |
| IRF | IFN (interferon) regulatory factor |
| IL | interleukin |
| JNK | c-Jun N-terminal kinases |
| MAPK | mitogen-activated protein kinases |
| MDA5 | melanoma differentiation-associated gene 5 |
| MHC | major histocompatibility complex |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cells | natural killer cells |
| NLRP3 | NOD-, LRR- and pyrin domain-containing 3 |
| PAMPs | pathogen-associated molecular patterns |
| PRRs | pathogen-recognition receptors |
| STING | stimulator of IFN genes |
| TCR | T cell receptor |
| Tfh | follicular T cell |
| TGF | transforming growth factor |
| Th | helper T cell |
| TLRs | toll-like receptors |
| TNF | tumor-necrosis factor |
| TRAF6 | tumor necrosis factor receptor-associated factor 6 |
| Treg | regulatory T cell |
Appendix A
Key Points in Malaria Vaccine Development
- Spatiotemporal relation between liver anatomy and sporozoite biology should be explored in detail [258].
- Unlike other infections, vaccine adjuvants, which induces both Th1 and Th2 mediated immunity is essential against P. falciparum malaria.
- Dominant MHC I-presenting antigen epitopes should be selected for cell-mediated, especially CD8+ T cells and tissue-resident memory T cells in the liver, which are essential to confer vaccine-induced protection [261].
- Protective vaccines (e.g., RTS,S) to pre-erythrocytic stage infections are greatly needed [264].
- Prime-boost and/or prime-target immunization strategies are probably better options to confer protection against liver-stage infections [266].
Appendix B
Appendix B.1. RTS, S Malaria Vaccine
- RTS, S is a genetically engineered vaccine where “RT” denotes C-terminal (190 amino acids) part of P. falciparum (strain NF54) circumsporozoite protein (CSP) protein, which contains both T and B cell epitopes (central repeat region). “S” is a surface antigen of hepatitis-B virus and the combination is expressed in Saccharomyces cerevisiae (S) [268].
- RTS, S was produced by Walter Reed Army Institute of Research (WRAIR) in collaboration with GlaxoSmithKline (GSK) biologicals and the Programs for Appropriate Technologies in Health (PATH) Malaria Vaccine Initiative (MVI).
- The idea behind the development of the RTS, S vaccine is, like other vaccines (e.g., ChAd63/MVA ME-TRAP), to inhibit sporozoite motility at pre-erythrocytic stage and preventing the entry to liver cells.
- The first licensed vaccine for children living in endemic areas in sub-Saharan Africa, and is also approved by European Medicines Agency (EMA) in 2015 [269].
- Presently under clinical evaluation in infants and children in Africa (Phase IV/NCT00866619). Previous regimens are partly effective, now four dose- schedule have been initiated in Kenya [270].
- Studies in infants and children vaccinated with RTS, S/AS01 confirmed the antibody (IgG subclass)-mediated protection against P. falciparum malaria by altering the natural acquired immunity [147].
- Safe, immunogenic and potent for individuals who experienced malaria.
- Like other majority vaccines, RTS, S also has hurdles in the stability maintenance of specified temperatures (2–8 °C), which is certainly expensive in developing countries.
- Adult vaccination regimen containing RTS,S/AS01B vaccine with ChAd63/MVA ME-TRAP vaccine has not shown interesting results [273].
Appendix B.2. Highlights
- Fine strategies to eradicate and eliminate malaria through drugs or vaccines have gained momentum.
- Fundamental functions of CD8+ T cells and NK cells have proven their effective role in preventing malaria transmission.
- Majority of prophylactic/therapeutic malaria vaccines are in the clinical evaluation.
- RTS, S/AS01 is the only licensed malaria vaccine.
- Novel delivery systems of malarial vaccines with QS-21 have yielded increased potency and reduced toxicity.
- Though subunit vaccines are an effective strategy, the search for novel approaches like live and/or whole parasite vaccinations should be evaluated in detail.
Appendix B.3. Glossary
Appendix B.4. Outstanding Questions: P. falciparum Vaccines and Vaccine Adjuvants
- Knowledge on the optimum combination of multi-stage malarial antigens for the vaccines
- Influence of natural immunity towards vaccine response and performance in endemic countries
- Fine incorporation of parameters of anti-disease immunity of the host to conceive effective and safe anti-malarial vaccines.
- The role of genetic background of the population in determining the vaccine response to malaria.
- Need of appropriate experimental models that closely mimic human for the pre-screening of malaria vaccine candidates.
References
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef] [PubMed]
- WHO. The “World Malaria Report 2019” at a Glance. Available online: https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019 (accessed on 1 July 2020).
- Key Points: World Malaria Report 2017. Available online: https://www.who.int/malaria/media/world-malaria-report-2017/en/ (accessed on 1 January 2021).
- World Health Organization. This Year’s World Malaria Report at a Glance. Available online: https://www.who.int/malaria/media/world-malaria-report-2018/en/ (accessed on 1 July 2020).
- Head, M.G.; Goss, S.; Gelister, Y.; Alegana, V.; Brown, R.J.; Clarke, S.C.; Fitchett, J.R.A.; Atun, R.; Scott, J.A.G.; Newell, M.L.; et al. Global funding trends for malaria research in sub-Saharan Africa: A systematic analysis. Lancet Glob. Health 2017, 5, e772–e781. [Google Scholar] [CrossRef]
- Kumar, A.; Valecha, N.; Jain, T.; Dash, A.P. Burden of malaria in india: Retrospective and prospective view. Am. J. Trop. Med. Hyg. 2007, 77, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.; Shretta, R.; Wells, T.N.; Bell, D.; Djimde, A.A.; Achee, N.; Qi, G. Tools and strategies for malaria control and elimination: What do we need to achieve a grand convergence in malaria? PLoS Biol. 2016, 14, e1002380. [Google Scholar] [CrossRef]
- Cox, F.E.G. History of the discovery of the malaria parasites and their vectors. Parasite Vector 2010, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Krettli, A.U.; Miller, L.H. Malaria: A sporozoite runs through it. Curr. Biol. 2001, 11, R409–R412. [Google Scholar] [CrossRef]
- Baer, K.; Klotz, C.; Kappe, S.H.; Schnieder, T.; Frevert, U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 2007, 3, e171. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Bhardwaj, J.; Rivera-Correa, J.; Freire-De-Lima, C.G.; Morrot, A. Immune escape strategies of malaria parasites. Front. Microbiol. 2016, 7, 1617. [Google Scholar] [CrossRef]
- Phillips, M.A.; Burrows, J.N.; Manyando, C. Nature reviews disease primers. Malaria 2017, 3, 17050. [Google Scholar] [CrossRef]
- Yap, X.Z.; Lundie, R.J.; Beeson, J.G.; O’Keeffe, M. Dendritic cell responses and function in malaria. Front. Immunol. 2019, 10, 357. [Google Scholar] [CrossRef]
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef]
- Thu, A.M.; Phyo, A.P.; Landier, J.; Parker, D.M.; Nosten, F.H. Combating multidrug-resistant Plasmodium falciparum malaria. FEBS J. 2017, 284, 2569–2578. [Google Scholar] [CrossRef]
- Ross, L.S.; Fidock, D.A. Elucidating mechanisms of drug-resistant Plasmodium falciparum. Cell Host Microbe 2019, 26, 35–47. [Google Scholar] [CrossRef]
- Dagen, M. History of malaria and its treatment. In Antimalarial Agents; Patrick, G.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–48. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Treatment of Malaria. Available online: https://apps.who.int/iris/bitstream/handle/10665/162441/9789241549127_eng.pdf (accessed on 1 July 2020).
- Blasco, B.; Leroy, D.; Fidock, D.A. Antimalarial drug resistance: Linking Plasmodium falciparum parasite biology to the clinic. Nat. Med. 2017, 23, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Lubell, Y.; Dondorp, A.; Guerin, P.J.; Drake, T.; Meek, S.; Ashley, E.; Day, N.P.; White, N.J.; White, L.J. Artemisinin resistance—Modelling the potential human and economic costs. Malar. J. 2014, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Plebanski, M.; Hannan, C.M.; Behboudi, S.; Flanagan, K.L.; Apostolopoulos, V.; Sinden, R.E.; Hill, A.V. Direct processing and presentation of antigen from malaria sporozoites by professional antigen-presenting cells in the induction of CD8 T-cell responses. Immunol. Cell Biol. 2005, 83, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Bousheri, S.; Cao, H. New insight into the role of dendritic cells in malaria immune pathogenesis. Trends Parasitol. 2008, 24, 199–200. [Google Scholar] [CrossRef]
- Draheim, M.; Wlodarczyk, M.F.; Crozat, K.; Saliou, J.M.; Alayi, T.D.; Tomavo, S.; Hassan, A.; Salvioni, A.; Demarta-Gatsi, C.; Sidney, J.; et al. Profiling mhc ii immunopeptidome of blood-stage malaria reveals that cDC1 control the functionality of parasite-specific CD4 T cells. EMBO Mol. Med. 2017, 9, 1605–1621. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Kalantari, P.; Fitzgerald, K.A.; Golenbock, D.T. Innate sensing of malaria parasites. Nat. Rev. Immunol. 2014, 14, 744–757. [Google Scholar] [CrossRef]
- Liehl, P.; Zuzarte-Luís, V.; Chan, J.; Zillinger, T.; Baptista, F.; Carapau, D.; Konert, M.; Hanson, K.K.; Carret, C.; Lassnig, C.; et al. Host-cell sensors for plasmodium activate innate immunity against liver-stage infection. Nat. Med. 2014, 20, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Osii, R.S.; Otto, T.D.; Garside, P.; Ndungu, F.M.; Brewer, J.M. The impact of malaria parasites on dendritic cell–T cell interaction. Front. Immunol. 2020, 11, 1597. [Google Scholar] [CrossRef]
- deWalick, S.; Amante, F.H.; McSweeney, K.A.; Randall, L.M.; Stanley, A.C.; Haque, A.; Kuns, R.D.; MacDonald, K.P.; Hill, G.R.; Engwerda, C.R. Cutting edge: Conventional dendritic cells are the critical required for the induction of experimental cerebral malaria. J. Immunol. 2007, 178, 6033–6037. [Google Scholar] [CrossRef] [PubMed]
- Claudianos, C.; Dessens, J.T.; Trueman, H.E.; Arai, M.; Mendoza, J.; Butcher, G.A.; Crompton, T.; Sinden, R.E. A malaria scavenger receptor-like protein essential for parasite development. Mol. Microbiol. 2002, 45, 1473–1484. [Google Scholar] [CrossRef]
- Gowda, D.C.; Wu, X. Parasite recognition and signaling mechanisms in innate immune responses to malaria. Front. Immunol. 2018, 9, 3006. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, P. The emerging role of pattern recognition receptors in the pathogenesis of malaria. Vaccines 2018, 6, 13. [Google Scholar] [CrossRef]
- McGuinness, D.H.; Dehal, P.K.; Pleass, R.J. Pattern recognition molecules and innate immunity to parasites. Trends Parasitol. 2003, 19, 312–319. [Google Scholar] [CrossRef]
- Jide, C.; Ying, H.; Wenyue, X.; Fusheng, H. Toll-like receptors, a double-edged sword in immunity to malaria. J. Med. Coll. PLA 2009, 24, 118–124. [Google Scholar] [CrossRef]
- Wykes, M.N.; Lewin, S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2018, 18, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Yap, X.Z.; Hustin, L.S.P.; Sauerwein, R.W. Th1-polarized tfh cells delay naturally-acquired immunity to malaria. Front. Immunol. 2019, 10, 1096. [Google Scholar] [CrossRef]
- Mauduit, M.; See, P.; Peng, K.; Renia, L.; Ginhoux, F. Dendritic cells and the malaria pre-erythrocytic stage. Immunol. Res. 2012, 53, 115–126. [Google Scholar] [CrossRef]
- Winkel, B.M.F.; Pelgrom, L.R.; van Schuijlenburg, R.; Baalbergen, E.; Ganesh, M.S.; Gerritsma, H.; de Korne, C.M.; Duszenko, N.; Langenberg, M.C.C.; Chevalley-Maurel, S.C.; et al. Plasmodium sporozoites induce regulatory macrophages. PLoS Pathog. 2020, 16, e1008799. [Google Scholar] [CrossRef]
- Leiriao, P.; Mota, M.M.; Rodriguez, A. Apoptotic plasmodium-infected hepatocytes provide antigens to liver dendritic cells. J. Infect. Dis. 2005, 191, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Cockburn, I.A.; Kuk, S.; Overstreet, M.G.; Sacci, J.B.; Zavala, F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 2007, 13, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Enea, V.; Morimoto, T.; Nussenzweig, V. Infectivity of Plasmodium berghei sporozoites measured with a DNA probe. Mol. Biochem. Parasitol. 1986, 19, 103–109. [Google Scholar] [CrossRef]
- Kurup, S.P.; Anthony, S.M.; Hancox, L.S.; Vijay, R.; Pewe, L.L.; Moioffer, S.J.; Sompallae, R.; Janse, C.J.; Khan, S.M.; Harty, J.T. Monocyte-derived CD11c+ cells acquire plasmodium from hepatocytes to prime CD8 T cell immunity to liver-stage malaria. Cell Host Microbe 2019, 25, 565–577.e6. [Google Scholar] [CrossRef] [PubMed]
- Rénia, L.; Marañón, C.; Hosmalin, A.; Grüner, A.C.; Silvie, O.; Snounou, G. Do apoptotic plasmodium-infected hepatocytes initiate protective immune responses? J. Infect. Dis. 2006, 193, 163–164. [Google Scholar] [CrossRef][Green Version]
- Nussenzweig, R.; Chen, D.F. Sporozoite-induced immunity in mammalian malaria. Am. J. Trop. Med. Hyg. 1972, 21, 722–728. [Google Scholar] [CrossRef]
- Seder, R.A.; Chang, L.J.; Enama, M.E.; Zephir, K.L.; Sarwar, U.N.; Gordon, I.J.; Holman, L.A.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013, 341, 1359–1365. [Google Scholar] [CrossRef]
- Yang, A.S.P.; O’Neill, M.T.; Jennison, C.; Lopaticki, S.; Allison, C.C.; Armistead, J.S.; Erickson, S.M.; Rogers, K.L.; Ellisdon, A.M.; Whisstock, J.C.; et al. Cell traversal activity is important for Plasmodium falciparum liver infection in humanized mice. Cell Rep. 2017, 18, 3105–3116. [Google Scholar] [CrossRef]
- Sack, B.K.; Mikolajczak, S.A.; Fishbaugher, M.; Vaughan, A.M.; Flannery, E.L.; Nguyen, T.; Betz, W.; Jane Navarro, M.; Foquet, L.; Steel, R.W.J.; et al. Humoral protection against mosquito bite-transmitted Plasmodium falciparum infection in humanized mice. NPJ Vaccines 2017, 2, 27. [Google Scholar] [CrossRef]
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for life—Properties of red blood cell deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Cooke, B.M.; Stuart, J.; Nash, G.B. The cellular and molecular rheology of malaria. Biorheology 2014, 51, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Buffet, P.A.; Safeukui, I.; Deplaine, G.; Brousse, V.; Prendki, V.; Thellier, M.; Turner, G.D.; Mercereau-Puijalon, O. The pathogenesis of Plasmodium falciparum malaria in humans: Insights from splenic physiology. Blood 2011, 117, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Russell, B.; Renia, L. Sticking for a cause: The falciparum malaria parasites cytoadherence paradigm. Front. Immunol. 2019, 10, 1444. [Google Scholar] [CrossRef]
- Urban, B.C.; Willcox, N.; Roberts, D.J. A role for CD36 in the regulation of dendritic cell function. Proc. Natl. Acad. Sci. USA 2001, 98, 8750–8755. [Google Scholar] [CrossRef]
- Urban, B.C.; Ferguson, D.J.; Pain, A.; Willcox, N.; Plebanski, M.; Austyn, J.M.; Roberts, D.J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 1999, 400, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Loharungsikul, S.; Troye-Blomberg, M.; Amoudruz, P.; Pichyangkul, S.; Yongvanitchit, K.; Looareesuwan, S.; Mahakunkijcharoen, Y.; Sarntivijai, S.; Khusmith, S. Expression of Toll-like receptors on antigen-presenting cells in patients with falciparum malaria. Acta Trop. 2008, 105, 10–15. [Google Scholar] [CrossRef]
- Pinzon-Charry, A.; Woodberry, T.; Kienzle, V.; McPhun, V.; Minigo, G.; Lampah, D.A.; Kenangalem, E.; Engwerda, C.; Lopez, J.A.; Anstey, N.M.; et al. Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J. Exp. Med. 2013, 210, 1635–1646. [Google Scholar] [CrossRef]
- Van Kooyk, Y. Next-generation malarial vaccines. Nat. Mater. 2019, 18, 94–96. [Google Scholar] [CrossRef]
- Brown, J.; Smalley, M.E. Specific antibody-dependent cellular cytotoxicity in human malaria. Clin. Exp. Immunol. 1980, 41, 423–429. [Google Scholar] [PubMed]
- Arora, G.; Hart, G.T.; Manzella-Lapeira, J.; Doritchamou, J.Y.; Narum, D.L.; Thomas, L.M.; Brzostowski, J.; Rajagopalan, S.; Doumbo, O.K.; Traore, B. NK cells inhibit Plasmodium falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. eLife 2018, 7, e36806. [Google Scholar] [CrossRef]
- Wolf, A.-S.; Sherratt, S.; Riley, E.M. NK cells: Uncertain allies against malaria. Front. Immunol. 2017, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Amaladoss, A.; Ye, W.; Liu, M.; Dummler, S.; Kong, F.; Wong, L.H.; Loo, H.L.; Loh, E.; Tan, S.Q. Human natural killer cells control Plasmodium falciparum infection by eliminating infected red blood cells. Proc. Natl. Acad. Sci. USA 2014, 111, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Hart, G.T.; Tran, T.M.; Theorell, J.; Schlums, H.; Arora, G.; Rajagopalan, S.; Sangala, A.J.; Welsh, K.J.; Traore, B.; Pierce, S.K. Adaptive NK cells in people exposed to Plasmodium falciparum correlate with protection from malaria. J. Exp. Med. 2019, 216, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Kurup, S.P.; Butler, N.S.; Harty, J.T. T cell-mediated immunity to malaria. Nat. Rev. Immunol. 2019, 19, 457–471. [Google Scholar] [CrossRef]
- Schofield, L.; Villaquiran, J.; Ferreira, A.; Schellekens, H.; Nussenzweig, R.; Nussenzweig, V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 1987, 330, 664–666. [Google Scholar] [CrossRef]
- Weiss, W.R.; Sedegah, M.; Beaudoin, R.L.; Miller, L.H.; Good, M.F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. USA 1988, 85, 573–576. [Google Scholar] [CrossRef]
- Silvie, O.; Amino, R.; Hafalla, J.C. Tissue-specific cellular immune responses to malaria pre-erythrocytic stages. Curr. Opin. Microbiol. 2017, 40, 160–167. [Google Scholar] [CrossRef]
- Druilhe, P.; Rénia, L.; Fidock, D. Immunity to Liver Stages. In Malaria: Parasite Biology, Pathogenesis, and Protection; Sherman, I., Ed.; ASM Press: Washington, DC, USA, 1998. [Google Scholar]
- Rénia, L.; Marussig, M.S.; Grillot, D.; Pied, S.; Corradin, G.; Miltgen, F.; Del Giudice, G.; Mazier, D. In vitro activity of CD4+ and CD8+ T lymphocytes from mice immunized with a synthetic malaria peptide. Proc. Natl. Acad. Sci. USA 1991, 88, 7963–7967. [Google Scholar] [CrossRef]
- Renia, L.; Grillot, D.; Marussig, M.; Corradin, G.; Miltgen, F.; Lambert, P.H.; Mazier, D.; Delgiudice, G. Effector functions of circumsporozoite peptide-primed CD4+ T-cell clones against plasmodium-yoelii liver stages. J. Immunol. 1993, 150, 1471–1478. [Google Scholar] [PubMed]
- Bongfen, S.E.; Torgler, R.; Romero, J.F.; Renia, L.; Corradin, G. Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J. Immunol. 2007, 178, 7054–7063. [Google Scholar] [CrossRef]
- Doll, K.L.; Pewe, L.L.; Kurup, S.P.; Harty, J.T. Discriminating protective from nonprotective plasmodium-specific CD8+ T cell responses. J. Immunol. 2016, 196, 4253–4262. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, I.A.; Amino, R.; Kelemen, R.K.; Kuo, S.C.; Tse, S.-W.; Radtke, A.; Mac-Daniel, L.; Ganusov, V.V.; Zavala, F.; Ménard, R. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proc. Natl. Acad. Sci. USA 2013, 110, 9090–9095. [Google Scholar] [CrossRef] [PubMed]
- Nussler, A.K.; Beger, H.G.; Liu, Z.Z.; Billiar, T.R. Nitric oxide, hepatocytes and inflammation. Res. Immunol. 1995, 146, 671–677. [Google Scholar] [CrossRef]
- Belnoue, E.; Costa, F.T.; Frankenberg, T.; Vigario, A.M.; Voza, T.; Leroy, N.; Rodrigues, M.M.; Landau, I.; Snounou, G.; Renia, L. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 2004, 172, 2487–2495. [Google Scholar] [CrossRef]
- Belnoue, E.; Voza, T.; Costa, F.T.; Gruner, A.C.; Mauduit, M.; Rosa, D.S.; Depinay, N.; Kayibanda, M.; Vigario, A.M.; Mazier, D.; et al. Vaccination with live Plasmodium yoelii blood stage parasites under chloroquine cover induces cross-stage immunity against malaria liver stage. J. Immunol. 2008, 181, 8552–8558. [Google Scholar] [CrossRef]
- Trimnell, A.; Takagi, A.; Gupta, M.; Richie, T.L.; Kappe, S.H.; Wang, R. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J. Immunol. 2009, 183, 5870–5878. [Google Scholar] [CrossRef]
- Carvalho, L.H.; Sano, G.; Hafalla, J.C.; Morrot, A.; de Lafaille, M.A.C.; Zavala, F. Il-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 2002, 8, 166–170. [Google Scholar] [CrossRef]
- Lefebvre, M.N.; Harty, J.T. You shall not pass: Memory CD8 T cells in liver-stage malaria. Trends Parasitol. 2020, 36, 147–157. [Google Scholar] [CrossRef]
- Valencia-Hernandez, A.M.; Ng, W.Y.; Ghazanfari, N.; Ghilas, S.; de Menezes, M.N.; Holz, L.E.; Huang, C.; English, K.; Naung, M.; Tan, P.S.; et al. A natural peptide antigen within the Plasmodium ribosomal protein RPL6 confers liver TRM cell-mediated immunity against malaria in mice. Cell Host Microbe 2020, 27, 950–962.e7. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, D.; Ng, W.Y.; Holz, L.E.; Ma, J.Z.; Zaid, A.; Wong, Y.C.; Lau, L.S.; Mollard, V.; Cozijnsen, A.; Collins, N.; et al. Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity 2016, 45, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.E.; Tewari, K.; Lyke, K.E.; Sim, B.K.; Billingsley, P.F.; Laurens, M.B.; Gunasekera, A.; Chakravarty, S.; James, E.R.; Sedegah, M.; et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 2011, 334, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, I.A.; Seder, R.A. Malaria prevention: From immunological concepts to effective vaccines and protective antibodies. Nat. Immunol. 2018, 19, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Gola, A.; Silman, D.; Walters, A.A.; Sridhar, S.; Uderhardt, S.; Salman, A.M.; Halbroth, B.R.; Bellamy, D.; Bowyer, G.; Powlson, J.; et al. Prime and target immunization protects against liver-stage malaria in mice. Sci. Transl. Med. 2018, 10, eaap9128. [Google Scholar] [CrossRef]
- Hill, A.V.; Reyes-Sandoval, A.; O’Hara, G.; Ewer, K.; Lawrie, A.; Goodman, A.; Nicosia, A.; Folgori, A.; Colloca, S.; Cortese, R.; et al. Prime-boost vectored malaria vaccines: Progress and prospects. Hum Vaccin 2010, 6, 78–83. [Google Scholar] [CrossRef]
- Rutishauser, T.; Lepore, M.; Di Blasi, D.; Dangy, J.-P.; Abdulla, S.; Jongo, S.; Ramadhani, K.; Sim, B.K.L.; Hoffman, S.L.; Tanner, M.; et al. Activation of tcr vδ1+ and vδ1−vδ2− γδ T cells upon controlled infection with Plasmodium falciparum in tanzanian volunteers. J. Immunol. 2019. [Google Scholar] [CrossRef]
- Mpina, M.; Maurice, N.J.; Yajima, M.; Slichter, C.K.; Miller, H.W.; Dutta, M.; McElrath, M.J.; Stuart, K.D.; De Rosa, S.C.; McNevin, J.P.; et al. Controlled human malaria infection leads to long-lasting changes in innate and innate-like lymphocyte populations. J. Immunol. 2017, 199, 107–118. [Google Scholar] [CrossRef]
- Perez-Mazliah, D.; Langhorne, J. CD4 T-cell subsets in malaria: Th1/th2 revisited. Front. Immunol. 2014, 5, 671. [Google Scholar] [CrossRef]
- Perez-Mazliah, D.; Nguyen, M.P.; Hosking, C.; McLaughlin, S.; Lewis, M.D.; Tumwine, I.; Levy, P.; Langhorne, J. Follicular helper T cells are essential for the elimination of plasmodium infection. EBioMedicine 2017, 24, 216–230. [Google Scholar] [CrossRef]
- Soon, M.S.F.; Lee, H.J.; Engel, J.A.; Straube, J.; Thomas, B.S.; Pernold, C.P.S.; Clarke, L.S.; Laohamonthonkul, P.; Haldar, R.N.; Williams, C.G.; et al. Transcriptome dynamics of CD4+ T cells during malaria maps gradual transit from effector to memory. Nat. Immunol. 2020, 21, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Deroost, K.; Langhorne, J. Gamma/delta T cells and their role in protection against malaria. Front. Immunol. 2018, 9, 2973. [Google Scholar] [CrossRef] [PubMed]
- Goodier, M.R.; Wolf, A.S.; Riley, E.M. Differentiation and adaptation of natural killer cells for anti-malarial immunity. Immunol. Rev. 2020, 293, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Horne-Debets, J.M.; Faleiro, R.; Karunarathne, D.S.; Liu, X.Q.; Lineburg, K.E.; Poh, C.M.; Grotenbreg, G.M.; Hill, G.R.; MacDonald, K.P.; Good, M.F.; et al. Pd-1 dependent exhaustion of CD8+ T cells drives chronic malaria. Cell Rep. 2013, 5, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Horne-Debets, J.M.; Karunarathne, D.S.; Faleiro, R.J.; Poh, C.M.; Renia, L.; Wykes, M.N. Mice lacking programmed cell death-1 show a role for CD8+ T cells in long-term immunity against blood-stage malaria. Sci. Rep. 2016, 6, 26210. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Ishida, H.; Suzue, K.; Taniguchi, T.; Okada, H.; Shimokawa, C.; Hisaeda, H. Cytotoxic activities of CD8+ T cells collaborate with macrophages to protect against blood-stage murine malaria. eLife 2015, 4, e04232. [Google Scholar] [CrossRef]
- Kaminski, L.C.; Riehn, M.; Abel, A.; Steeg, C.; Yar, D.D.; Addai-Mensah, O.; Aminkiah, F.; Owusu Dabo, E.; Jacobs, T.; Mackroth, M.S. Cytotoxic T cell-derived granzyme B is increased in severe Plasmodium falciparum malaria. Front. Immunol. 2019, 10, 2917. [Google Scholar] [CrossRef]
- Riggle, B.A.; Manglani, M.; Maric, D.; Johnson, K.R.; Lee, M.H.; Neto, O.L.A.; Taylor, T.E.; Seydel, K.B.; Nath, A.; Miller, L.H.; et al. CD8+ T cells target cerebrovasculature in children with cerebral malaria. J. Clin. Investig. 2020, 130, 1128–1138. [Google Scholar] [CrossRef]
- Belnoue, E.; Kayibanda, M.; Vigario, A.M.; Deschemin, J.C.; van Rooijen, N.; Viguier, M.; Snounou, G.; Renia, L. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J. Immunol. 2002, 169, 6369–6375. [Google Scholar] [CrossRef]
- Engwerda, C.; Belnoue, E.; Gruner, A.C.; Renia, L. Experimental models of cerebral malaria. Curr. Top. Microbiol. Immunol. 2005, 297, 103–143. [Google Scholar]
- Renia, L.; Goh, Y.S. Malaria parasites: The great escape. Front. Immunol. 2016, 7, 463. [Google Scholar] [CrossRef]
- Scholzen, A.; Mittag, D.; Rogerson, S.J.; Cooke, B.M.; Plebanski, M. Plasmodium falciparum-mediated induction of human CD25hiFoxp3hi CD4 T cells is independent of direct tcr stimulation and requires IL-2, IL-10 and TGFβ. PLoS Pathog. 2009, 5, e1000543. [Google Scholar] [CrossRef] [PubMed]
- Kurup, S.P.; Obeng-Adjei, N.; Anthony, S.M.; Traore, B.; Doumbo, O.K.; Butler, N.S.; Crompton, P.D.; Harty, J.T. Regulatory T cells impede acute and long-term immunity to blood-stage malaria through CTLA-4. Nat. Med. 2017, 23, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Nyirenda, T.S.; Molyneux, M.E.; Kenefeck, R.; Walker, L.S.; MacLennan, C.A.; Heyderman, R.S.; Mandala, W.L. T-regulatory cells and inflammatory and inhibitory cytokines in malawian children residing in an area of high and an area of low malaria transmission during acute uncomplicated malaria and in convalescence. J. Pediatr. Infect. Dis. Soc. 2015, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.M.; Salmazi, K.C.; Santos, B.A.; Bastos, M.S.; Rocha, S.C.; Boscardin, S.B.; Silber, A.M.; Kallas, E.G.; Ferreira, M.U.; Scopel, K.K. CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: Do different parasite species elicit similar host responses? Infect. Immun. 2010, 78, 4763–4772. [Google Scholar] [CrossRef] [PubMed]
- Todryk, S.M.; Bejon, P.; Mwangi, T.; Plebanski, M.; Urban, B.; Marsh, K.; Hill, A.V.; Flanagan, K.L. Correlation of memory T cell responses against trap with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE 2008, 3, e2027. [Google Scholar] [CrossRef]
- Illingworth, J.; Butler, N.S.; Roetynck, S.; Mwacharo, J.; Pierce, S.K.; Bejon, P.; Crompton, P.D.; Marsh, K.; Ndungu, F.M. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J. Immunol. 2013, 190, 1038–1047. [Google Scholar] [CrossRef]
- Van Braeckel-Budimir, N.; Kurup, S.P.; Harty, J.T. Regulatory issues in immunity to liver and blood-stage malaria. Curr. Opin. Immunol. 2016, 42, 91–97. [Google Scholar] [CrossRef]
- Boyle, M.J.; Jagannathan, P.; Farrington, L.A.; Eccles-James, I.; Wamala, S.; McIntyre, T.I.; Vance, H.M.; Bowen, K.; Nankya, F.; Auma, A.; et al. Decline of Foxp3+ regulatory CD4 T cells in peripheral blood of children heavily exposed to malaria. PLoS Pathog. 2015, 11, e1005041. [Google Scholar] [CrossRef]
- Dups, J.N.; Pepper, M.; Cockburn, I.A. Antibody and B cell responses to Plasmodium sporozoites. Front. Microbiol. 2014, 5, 625. [Google Scholar] [CrossRef]
- Blank, A.; Fürle, K.; Jäschke, A.; Mikus, G.; Lehmann, M.; Hüsing, J.; Heiss, K.; Giese, T.; Carter, D.; Böhnlein, E.; et al. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. NPJ Vaccines 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Aitken, E.H.; Mahanty, S.; Rogerson, S.J. Antibody effector functions in malaria and other parasitic diseases: A few needles and many haystacks. Immunol. Cell Biol. 2020, 98, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, C.C.; Verhage, D.F.; Telgt, D.S.; Teelen, K.; Bousema, J.T.; Roestenberg, M.; Bolad, A.; Berzins, K.; Corradin, G.; Leroy, O. Glutamate-rich protein (GLURP) induces antibodies that inhibit in vitro growth of Plasmodium falciparum in a phase 1 malaria vaccine trial. Vaccine 2007, 25, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Plebanski, M.; Locke, E.; Kazura, J.W.; Coppel, R.L. Malaria vaccines: Into a mirror, darkly? Trends Parasitol. 2008, 24, 532–536. [Google Scholar] [CrossRef]
- Gupta, S.; Snow, R.W.; Donnelly, C.A.; Marsh, K.; Newbold, C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 1999, 5, 340–343. [Google Scholar] [CrossRef]
- Julien, J.-P.; Wardemann, H. Antibodies against Plasmodium falciparum malaria at the molecular level. Nat. Rev. Immunol. 2019, 19, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Vanderberg, J.P.; Frevert, U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 2004, 34, 991–996. [Google Scholar] [CrossRef]
- Aliprandini, E.; Tavares, J.; Panatieri, R.H.; Thiberge, S.; Yamamoto, M.M.; Silvie, O.; Ishino, T.; Yuda, M.; Dartevelle, S.; Traincard, F.; et al. Cytotoxic anti-circumsporozoite antibodies target malaria sporozoites in the host skin. Nat. Microbiol. 2018, 3, 1224–1233. [Google Scholar] [CrossRef]
- Schwenk, R.; Lumsden, J.M.; Rein, L.E.; Juompan, L.; Kester, K.E.; Heppner, D.G.; Krzych, U. Immunization with the RTS,S/AS malaria vaccine induces IFN-γ+ CD4 T cells that recognize only discrete regions of the circumsporozoite protein and these specificities are maintained following booster immunizations and challenge. Vaccine 2011, 29, 8847–8854. [Google Scholar] [CrossRef]
- Nudelman, S.; Renia, L.; Charoenvit, Y.; Yuan, L.; Miltgen, F.; Beaudoin, R.L.; Mazier, D. Dual action of anti-sporozoite antibodies in vitro. J. Immunol. 1989, 143, 996–1000. [Google Scholar]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Boyle, M.J.; Reiling, L.; Feng, G.; Langer, C.; Osier, F.H.; Aspeling-Jones, H.; Cheng, Y.S.; Stubbs, J.; Tetteh, K.K.; Conway, D.J.; et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015, 42, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Osier, F.H.A.; Feng, G.; Boyle, M.J.; Langer, C.; Zhou, J.; Richards, J.S.; McCallum, F.J.; Reiling, L.; Jaworowski, A.; Anders, R.F.; et al. Opsonic phagocytosis of Plasmodium falciparummerozoites: Mechanism in human immunity and a correlate of protection against malaria. BMC Med. 2014, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.J.; Chan, J.A.; Handayuni, I.; Reiling, L.; Feng, G.; Hilton, A.; Kurtovic, L.; Oyong, D.; Piera, K.A.; Barber, B.E.; et al. IgM in human immunity to Plasmodium falciparum malaria. Sci. Adv. 2019, 5, eaax4489. [Google Scholar] [CrossRef]
- Barfod, L.; Dalgaard, M.B.; Pleman, S.T.; Ofori, M.F.; Pleass, R.J.; Hviid, L. Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed pfemp1. Proc. Natl. Acad. Sci. USA 2011, 108, 12485–12490. [Google Scholar] [CrossRef]
- Hopp, C.S.; Diouf, A.; Miura, K.; Boswell, K.; Sekar, P.; Skinner, J.; Tipton, C.M.; Chambers, M.; Andrews, S.; Tan, J.; et al. Plasmodium falciparum-specific IgM B cells dominate in children, expand with malaria and produce parasite inhibitory IgM. bioRxiv 2020. [Google Scholar] [CrossRef]
- Renia, L.; Mattei, D.; Goma, J.; Pied, S.; Dubois, P.; Miltgen, F.; Nussler, A.; Matile, H.; Menegaux, F.; Gentilini, M.; et al. A malaria heat-shock-like determinant expressed on the infected hepatocyte surface is the target of antibody-dependent cell-mediated cytotoxic mechanisms by nonparenchymal liver cells. Eur. J. Immunol. 1990, 20, 1445–1449. [Google Scholar] [CrossRef]
- Dutta, S.; Kaushal, D.C.; Ware, L.A.; Puri, S.K.; Kaushal, N.A.; Narula, A.; Upadhyaya, D.S.; Lanar, D.E. Merozoite surface protein 1 of plasmodium vivax induces a protective response against plasmodium cynomolgi challenge in rhesus monkeys. Infect. Immun. 2005, 73, 5936–5944. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; López-Barragán, M.J.; Jiang, H.; Mu, J.; Gaur, D.; Zhao, K.; Felsenfeld, G.; Miller, L.H. Epigenetic control of the variable expression of a Plasmodium falciparum receptor protein for erythrocyte invasion. Proc. Natl. Acad. Sci. USA 2010, 107, 2224–2229. [Google Scholar] [CrossRef]
- Michon, P.; Fraser, T.; Adams, J.H. Naturally acquired and vaccine-elicited antibodies block erythrocyte cytoadherence of the plasmodium vivax duffy binding protein. Infect. Immun. 2000, 68, 3164–3171. [Google Scholar] [CrossRef]
- Marsh, K.; Kinyanjui, S. Immune effector mechanisms in malaria. Parasite Immunol. 2006, 28, 51–60. [Google Scholar] [CrossRef]
- Bouharoun-Tayoun, H.; Oeuvray, C.; Lunel, F.; Druilhe, P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 1995, 182, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Bouharoun-Tayoun, H.; Attanath, P.; Sabchareon, A.; Chongsuphajaisiddhi, T.; Druilhe, P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 1990, 172, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Z.; Heatwole, V.M.; Wertheimer, S.P.; Guinet, F.; Herrfeldt, J.A.; Peterson, D.S.; Ravetch, J.A.; Wellems, T.E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 1995, 82, 89–100. [Google Scholar] [CrossRef]
- Cheng, Q.; Cloonan, N.; Fischer, K.; Thompson, J.; Waine, G.; Lanzer, M.; Saul, A. Stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol. Biochem. Parasitol. 1998, 97, 161–176. [Google Scholar] [CrossRef]
- Kyes, S.A.; Rowe, J.A.; Kriek, N.; Newbold, C.I. Rifins: A second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1999, 96, 9333–9338. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Fowkes, F.J.; Beeson, J.G. Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell. Mol. Life Sci. 2014, 71, 3633–3657. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Pieper, K.; Piccoli, L.; Abdi, A.; Perez, M.F.; Geiger, R.; Tully, C.M.; Jarrossay, D.; Maina Ndungu, F.; Wambua, J.; et al. A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens. Nature 2016, 529, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Van Den Ham, K.; Shio, M.T.; Kassa, F.A.; Fougeray, S. Malarial pigment hemozoin and the innate inflammatory response. Front. Immunol. 2014, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Schofield, L.; Tachado, S.D. Regulation of host cell function by glycosylphosphatidylinositols of the parasitic protozoa. Immunol. Cell Biol. 1996, 74, 555–563. [Google Scholar] [CrossRef]
- Chang, Z.; Jiang, N.; Zhang, Y.; Lu, H.; Yin, J.; Wahlgren, M.; Cheng, X.; Cao, Y.; Chen, Q. The tatd-like dnase of plasmodium is a virulence factor and a potential malaria vaccine candidate. Nat. Commun. 2016, 7, 11537. [Google Scholar] [CrossRef]
- Bhatt, T.K.; Khan, S.; Dwivedi, V.P.; Banday, M.M.; Sharma, A.; Chandele, A.; Camacho, N.; Ribas de Pouplana, L.; Wu, Y.; Craig, A.G.; et al. Malaria parasite tyrosyl-tRNA synthetase secretion triggers pro-inflammatory responses. Nat. Commun. 2011, 2, 530. [Google Scholar] [CrossRef]
- Schofield, L.; Hewitt, M.C.; Evans, K.; Siomos, M.A.; Seeberger, P.H. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature 2002, 418, 785–789. [Google Scholar] [CrossRef] [PubMed]
- De Jong, R.M.; Tebeje, S.K.; Meerstein-Kessel, L.; Tadesse, F.G.; Jore, M.M.; Stone, W.; Bousema, T. Immunity against sexual stage Plasmodium falciparum and plasmodium vivax parasites. Immunol. Rev. 2020, 293, 190–215. [Google Scholar] [CrossRef] [PubMed]
- Dantzler, K.W.; Ma, S.; Ngotho, P.; Stone, W.J.R.; Tao, D.; Rijpma, S.; De Niz, M.; Nilsson Bark, S.K.; Jore, M.M.; Raaijmakers, T.K.; et al. Naturally acquired immunity against immature Plasmodium falciparum gametocytes. Sci. Transl. Med. 2019, 11, eaav3963. [Google Scholar] [CrossRef] [PubMed]
- Read, D.; Lensen, A.H.; Begarnie, S.; Haley, S.; Raza, A.; Carter, R. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 1994, 16, 511–519. [Google Scholar] [CrossRef]
- Blagborough, A.M.; Sinden, R.E. Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine 2009, 27, 5187–5194. [Google Scholar] [CrossRef]
- Volohonsky, G.; Steinert, S.; Levashina, E.A. Focusing on complement in the antiparasitic defense of mosquitoes. Trends Parasitol. 2010, 26, 1–3. [Google Scholar] [CrossRef]
- Sauerwein, R.W.; Richie, T.L. Malaria vaccines getting close to clinical reality. Vaccine 2015, 33, 7423–7424. [Google Scholar] [CrossRef]
- Kwenti, T.E.; Kukwah, T.A.; Kwenti, T.D.B.; Nyassa, B.R.; Dilonga, M.H.; Enow-Orock, G.; Tendongfor, N.; Anong, N.D.; Wanji, S.; Njunda, L.A.; et al. Comparative analysis of IgG and IgG subclasses against Plasmodium falciparum MSP-119 in children from five contrasting bioecological zones of cameroon. Malar. J. 2019, 18, 16. [Google Scholar] [CrossRef]
- Dobano, C.; Santano, R.; Vidal, M.; Jimenez, A.; Jairoce, C.; Ubillos, I.; Dosoo, D.; Aguilar, R.; Williams, N.A.; Diez-Padrisa, N.; et al. Differential patterns of IgG subclass responses to Plasmodium falciparum antigens in relation to malaria protection and RTS,S vaccination. Front. Immunol. 2019, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; Richards, J.S.; McCallum, F.J.; Michon, P.; King, C.L.; Schoepflin, S.; Gilson, P.R.; Murphy, V.J.; Anders, R.F.; Mueller, I.; et al. Immunoglobulin g subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect. Immun. 2009, 77, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Abagna, H.B.; Acquah, F.K.; Okonu, R.; Aryee, N.A.; Theisen, M.; Amoah, L.E. Assessment of the quality and quantity of naturally induced antibody responses to EBA175RIII–V in ghanaian children living in two communities with varying malaria transmission patterns. Malar. J. 2018, 17, 14. [Google Scholar] [CrossRef]
- Kana, I.H.; Garcia-Senosiain, A.; Singh, S.K.; Tiendrebeogo, R.W.; Chourasia, B.K.; Malhotra, P.; Sharma, S.K.; Das, M.K.; Singh, S.; Adu, B.; et al. Cytophilic antibodies against key Plasmodium falciparum blood stage antigens contribute to protection against clinical malaria in a high transmission region of eastern India. J. Infect. Dis. 2018, 218, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Lusingu, J.P.A.; Vestergaard, L.S.; Alifrangis, M.; Mmbando, B.P.; Theisen, M.; Kitua, A.Y.; Lemnge, M.M.; Theander, T.G. Cytophilic antibodies to Plasmodium falciparum glutamate rich protein are associated with malaria protection in an area of holoendemic transmission. Malar. J. 2005, 4, 48. [Google Scholar] [CrossRef]
- Courtin, D.; Oesterholt, M.; Huismans, H.; Kusi, K.; Milet, J.; Badaut, C.; Gaye, O.; Roeffen, W.; Remarque, E.J.; Sauerwein, R.; et al. The quantity and quality of african children’s IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS ONE 2009, 4, e7590. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B. Protective immunity against malaria after vaccination. Parasite Immunol. 2014, 36, 131–139. [Google Scholar] [CrossRef]
- Pinkevych, M.; Petravic, J.; Chelimo, K.; Kazura, J.W.; Moormann, A.M.; Davenport, M.P. The dynamics of naturally acquired immunity to Plasmodium falciparum infection. PLoS Comp. Biol. 2012, 8, e1002729. [Google Scholar] [CrossRef]
- John, C.C.; Moormann, A.M.; Pregibon, D.C.; Sumba, P.O.; McHugh, M.M.; Narum, D.L.; Lanar, D.E.; Schluchter, M.D.; Kazura, J.W. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am. J. Trop. Med. Hyg. 2005, 73, 222–228. [Google Scholar] [CrossRef]
- John, C.C.; Tande, A.J.; Moormann, A.M.; Sumba, P.O.; Lanar, D.E.; Min, X.M.; Kazura, J.W. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J. Infect. Dis. 2008, 197, 519–526. [Google Scholar] [CrossRef]
- Dobbs, K.R.; Dent, A.E. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology 2016, 143, 129–138. [Google Scholar] [CrossRef]
- Osier, F.H.; Fegan, G.; Polley, S.D.; Murungi, L.; Verra, F.; Tetteh, K.K.; Lowe, B.; Mwangi, T.; Bull, P.C.; Thomas, A.W.; et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun. 2008, 76, 2240–2248. [Google Scholar] [CrossRef]
- Dent, A.E.; Nakajima, R.; Liang, L.; Baum, E.; Moormann, A.M.; Sumba, P.O.; Vulule, J.; Babineau, D.; Randall, A.; Davies, D.H.; et al. Plasmodium falciparum protein microarray antibody profiles correlate with protection from symptomatic malaria in Kenya. J. Infect. Dis. 2015, 212, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; Fowkes, F.J.; Koinari, M.; Javati, S.; Lin, E.; Kiniboro, B.; Richards, J.S.; Robinson, L.J.; Schofield, L.; Kazura, J.W.; et al. Acquisition of antibodies against Plasmodium falciparum merozoites and malaria immunity in young children and the influence of age, force of infection, and magnitude of response. Infect. Immun. 2015, 83, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Kusi, K.A.; Manu, E.A.; Manful Gwira, T.; Kyei-Baafour, E.; Dickson, E.K.; Amponsah, J.A.; Remarque, E.J.; Faber, B.W.; Kocken, C.H.M.; Dodoo, D.; et al. Variations in the quality of malaria-specific antibodies with transmission intensity in a seasonal malaria transmission area of northern Ghana. PLoS ONE 2017, 12, e0185303. [Google Scholar] [CrossRef] [PubMed]
- McGregor, I.A. The Passive Transfer of Human Malarial Immunity. Am. J. Trop. Med. Hyg. 1964, 13, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; McGregor, I.A.; Carrington, S. Gamma-globulin and acquired immunity to human malaria. Nature 1961, 192, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Brown, G.V.; Genton, B.; Moorthy, V.S. A review of malaria vaccine clinical projects based on the who rainbow table. Malar. J. 2012, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Genton, B. Malaria vaccines: A toy for travelers or a tool for eradication? Expert Rev. Vaccines 2008, 7, 597–611. [Google Scholar] [CrossRef]
- Draper, S.J.; Angov, E.; Horii, T.; Miller, L.H.; Srinivasan, P.; Theisen, M.; Biswas, S. Recent advances in recombinant protein-based malaria vaccines. Vaccine 2015, 33, 7433–7443. [Google Scholar] [CrossRef]
- Coppel, R.L. Vaccinating with the genome: A sisyphean task? Trends Parasitol. 2009, 25, 205–212. [Google Scholar] [CrossRef]
- Florens, L.; Washburn, M.P.; Raine, J.D.; Anthony, R.M.; Grainger, M.; Haynes, J.D.; Moch, J.K.; Muster, N.; Sacci, J.B.; Tabb, D.L.; et al. A proteomic view of the Plasmodium falciparum life cycle. Nature 2002, 419, 520–526. [Google Scholar] [CrossRef]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria vaccines: Recent advances and new horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2003, 2, 727–735. [Google Scholar] [CrossRef]
- Guy, B. The perfect mix: Recent progress in adjuvant research. Nat. Rev. Microbiol. 2007, 5, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Coelho, C.H.; Doritchamou, J.Y.A.; Zaidi, I.; Duffy, P.E. Advances in malaria vaccine development: Report from the 2017 malaria vaccine symposium. NPJ Vaccines 2017, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef]
- Hansen, D.S.; Obeng-Adjei, N.; Ly, A.; Ioannidis, L.J.; Crompton, P.D. Emerging concepts in t follicular helper cell responses to malaria. Int. J. Parasitol. 2017, 47, 105–110. [Google Scholar] [CrossRef]
- Radtke, A.J.; Anderson, C.F.; Riteau, N.; Rausch, K.; Scaria, P.; Kelnhofer, E.R.; Howard, R.F.; Sher, A.; Germain, R.N.; Duffy, P. Adjuvant and carrier protein-dependent t-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Sci. Rep. 2017, 7, 40312. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.M.; Costa, P.A.C.; Diniz, S.Q.; Henriques, P.M.; Kano, F.S.; Tada, M.S.; Pereira, D.B.; Soares, I.S.; Martins-Filho, O.A.; Jankovic, D.; et al. T follicular helper cells regulate the activation of B lymphocytes and antibody production during plasmodium vivax infection. PLoS Pathog. 2017, 13, e1006484. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Li, A.V.; Ockenhouse, C.F.; Yadava, A.; Irvine, D.J. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. USA 2012, 109, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.S.; Peng, K.; Chia, W.N.; Siau, A.; Chotivanich, K.; Gruner, A.C.; Preiser, P.; Mayxay, M.; Pukrittayakamee, S.; Sriprawat, K.; et al. Neutralizing antibodies against Plasmodium falciparum associated with successful cure after drug therapy. PLoS ONE 2016, 11, e0159347. [Google Scholar] [CrossRef]
- Kurtovic, L.; Behet, M.C.; Feng, G.; Reiling, L.; Chelimo, K.; Dent, A.E.; Mueller, I.; Kazura, J.W.; Sauerwein, R.W.; Fowkes, F.J.I.; et al. Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med. 2018, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Radosevic, K.; Rodriguez, A.; Lemckert, A.A.; van der Meer, M.; Gillissen, G.; Warnar, C.; von Eyben, R.; Pau, M.G.; Goudsmit, J. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clin. Vaccine Immunol. 2010, 17, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Tom, J.K.; Albin, T.J.; Manna, S.; Moser, B.A.; Steinhardt, R.C.; Esser-Kahn, A.P. Applications of immunomodulatory immune synergies to adjuvant discovery and vaccine development. Trends Biotechnol. 2019, 37, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Garcon, N.; Di Pasquale, A. From discovery to licensure, the adjuvant system story. Hum. Vaccin. Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Draper, S.J.; Moore, A.C.; Goodman, A.L.; Long, C.A.; Holder, A.A.; Gilbert, S.C.; Hill, F.; Hill, A.V. Effective induction of high-titer antibodies by viral vector vaccines. Nat. Med. 2008, 14, 819–821. [Google Scholar] [CrossRef]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. NPJ Vaccines 2018, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Haensler, J.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol. Sci. 2009, 30, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Mata, E.; Salvador, A.; Igartua, M.; Hernandez, R.M.; Pedraz, J.L. Malaria vaccine adjuvants: Latest update and challenges in preclinical and clinical research. Biomed Res. Int. 2013, 2013, 282913. [Google Scholar] [CrossRef]
- Near, K.A.; Stowers, A.W.; Jankovic, D.; Kaslow, D.C. Improved immunogenicity and efficacy of the recombinant 19-kilodalton merozoite surface protein 1 by the addition of oligodeoxynucleotide and aluminum hydroxide gel in a murine malaria vaccine model. Infect. Immun. 2002, 70, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Roeffen, W.; Theisen, M.; van de Vegte-Bolmer, M.; van Gemert, G.; Arens, T.; Andersen, G.; Christiansen, M.; Sevargave, L.; Singh, S.K.; Kaviraj, S.; et al. Transmission-blocking activity of antibodies to Plasmodium falciparum GLURP.10C chimeric protein formulated in different adjuvants. Malar. J. 2015, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Nalla, N.; Pallavi, P.; Reddy, B.S.; Miryala, S.; Kumar, V.N.; Mahboob, M.; Halmuthur, M.S.K. Design, synthesis and immunological evaluation of 1,2,3-triazole-tethered carbohydrate–Pam3Cys conjugates as TLR2 agonists. Biorg. Med. Chem. 2015, 23, 5846–5855. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, D.; Pallavi, P.M.; Bonam, S.R.; Reddy, S.A.; Verma, Y.; Halmuthur, M.S.K. Design, synthesis, and evaluation of novel 1,2,3-triazole-tethered glycolipids as vaccine adjuvants. Arch. Pharm. 2015, 348, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Chioato, A.; Noseda, E.; Felix, S.; Stevens, M.; Del Giudice, G.; Fitoussi, S.; Kleinschmidt, A. Influenza and meningococcal vaccinations are effective in healthy subjects treated with the interleukin-1β-blocking antibody canakinumab: Results of an open-label, parallel group, randomized, single-center study. Clin. Vaccine Immunol. 2010, 17, 1952–1957. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Sandgren, K.J.; Thompson, E.A.; Francica, J.R.; Seubert, A.; De Gregorio, E.; Barnett, S.; O’Hagan, D.T.; Sullivan, N.J.; et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 2017, 9, eaal2094. [Google Scholar] [CrossRef]
- López, J.A.; Weilenman, C.; Audran, R.; Roggero, M.A.; Bonelo, A.; Tiercy, J.-M.; Spertini, F.; Corradin, G. A synthetic malaria vaccine elicits a potent CD8+ and CD4+ T lymphocyte immune response in humans. Implications for vaccination strategies. Eur. J. Immunol. 2001, 31, 1989–1998. [Google Scholar] [CrossRef]
- Sirima, S.B.; Richert, L.; Chene, A.; Konate, A.T.; Campion, C.; Dechavanne, S.; Semblat, J.P.; Benhamouda, N.; Bahuaud, M.; Loulergue, P.; et al. Primvac vaccine adjuvanted with alhydrogel or GLA-SE to prevent placental malaria: A first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect. Dis. 2020, 20, 585–597. [Google Scholar] [CrossRef]
- Carter, R.; Mendis, K.N.; Miller, L.H.; Molineaux, L.; Saul, A. Malaria transmission-blocking vaccines—How can their development be supported? Nat. Med. 2000, 6, 241–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sagara, I.; Healy, S.A.; Assadou, M.H.; Gabriel, E.E.; Kone, M.; Sissoko, K.; Tembine, I.; Guindo, M.A.; Doucoure, M.; Niare, K.; et al. Safety and immunogenicity of Pfs25h-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: A randomised, double-blind, comparator-controlled, dose-escalation study in healthy malian adults. Lancet Infect. Dis. 2018, 18, 969–982. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Fox, C.B. New generation adjuvants–from empiricism to rational design. Vaccine 2015, 33, B14–B20. [Google Scholar] [CrossRef]
- Gregoriadis, G. Immunological adjuvants: A role for liposomes. Immunol. Today 1990, 11, 89–97. [Google Scholar] [CrossRef]
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrands, I.; Vingsbo-Lundberg, C.; Bundgaard, T.J.; Lindenstrom, T.; Enouf, V.; van der Werf, S.; Andersen, P.; Agger, E.M. Enhanced humoral and cell-mediated immune responses after immunization with trivalent influenza vaccine adjuvanted with cationic liposomes. Vaccine 2011, 29, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupeze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garcon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Coccia, M.; Collignon, C.; Herve, C.; Chalon, A.; Welsby, I.; Detienne, S.; van Helden, M.J.; Dutta, S.; Genito, C.J.; Waters, N.C.; et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early ifngamma response promoting vaccine immunogenicity. NPJ Vaccines 2017, 2, 25. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Collignon, C.; Bourguignon, P.; Wouters, S.; Fierens, K.; Fochesato, M.; Dendouga, N.; Langlet, C.; Malissen, B.; Lambrecht, B.N.; et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. 2014, 193, 1920–1930. [Google Scholar] [CrossRef]
- Leroux-Roels, I.; Forgus, S.; De Boever, F.; Clement, F.; Demoitie, M.A.; Mettens, P.; Moris, P.; Ledent, E.; Leroux-Roels, G.; Ofori-Anyinam, O.; et al. Improved CD4+ T cell responses to mycobacterium tuberculosis in ppd-negative adults by M72/AS01 as compared to the M72/AS02 and MTB72F/AS02 tuberculosis candidate vaccine formulations: A randomized trial. Vaccine 2013, 31, 2196–2206. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.B.; Baldwin, S.L.; Vedvick, T.S.; Angov, E.; Reed, S.G. Effects on immunogenicity by formulations of emulsion-based adjuvants for malaria vaccines. Clin. Vaccine Immunol. 2012, 19, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Pallikkuth, S.; Chaudhury, S.; Lu, P.; Pan, L.; Jongert, E.; Wille-Reece, U.; Pahwa, S. A delayed fractionated dose RTS,S AS01 vaccine regimen mediates protection via improved t follicular helper and B cell responses. eLife 2020, 9, e51889. [Google Scholar] [CrossRef] [PubMed]
- Marty-Roix, R.; Vladimer, G.I.; Pouliot, K.; Weng, D.; Buglione-Corbett, R.; West, K.; MacMicking, J.D.; Chee, J.D.; Wang, S.X.; Lu, S.; et al. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J. Biol. Chem. 2016, 291, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Regules, J.A.; Cummings, J.F.; Ockenhouse, C.F. The RTS,S vaccine candidate for malaria. Expert Rev. Vaccines 2011, 10, 589–599. [Google Scholar] [CrossRef]
- Coler, R.N.; Bertholet, S.; Moutaftsi, M.; Guderian, J.A.; Windish, H.P.; Baldwin, S.L.; Laughlin, E.M.; Duthie, M.S.; Fox, C.B.; Carter, D.; et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS ONE 2011, 6, e16333. [Google Scholar] [CrossRef]
- Sravanthi, V.; Pallavi, M.C.P.; Bonam, S.R.; Sathyabama, S.; Kumar, H.M.S. Oleic acid nanoemulsion for nasal vaccination: Impact on adjuvanticity based immune response. J. Drug Deliv. Sci. Technol. 2015, 28, 56–63. [Google Scholar] [CrossRef]
- Kantipakala, R.; Bonam, S.R.; Vemireddy, S.; Miryala, S.; Halmuthur, M.S. Squalane-based emulsion vaccine delivery system: Composition with murabutide activate Th1 response. Pharm. Dev. Technol. 2019, 24, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Tritto, E.; Muzzi, A.; Monaci, E.; Bagnoli, F.; Iavarone, C.; O’Hagan, D.; Rappuoli, R.; De Gregorio, E. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2008, 105, 10501–10506. [Google Scholar] [CrossRef]
- Ott, G.; Barchfeld, G.L.; Chernoff, D.; Radhakrishnan, R.; van Hoogevest, P.; Van Nest, G. Vaccine Design; Springer: Berlin, Germany, 1995; pp. 277–296. [Google Scholar]
- Corradin, G.; Giudice, G.D. Novel adjuvants for vaccines. Curr. Med. Chem. 2005, 4, 185–191. [Google Scholar] [CrossRef]
- Wack, A.; Baudner, B.C.; Hilbert, A.K.; Manini, I.; Nuti, S.; Tavarini, S.; Scheffczik, H.; Ugozzoli, M.; Singh, M.; Kazzaz, J.; et al. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine 2008, 26, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Seubert, A.; Monaci, E.; Pizza, M.; O’Hagan, D.T.; Wack, A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J. Immunol. 2008, 180, 5402–5412. [Google Scholar] [CrossRef]
- Baudner, B.C.; Ronconi, V.; Casini, D.; Tortoli, M.; Kazzaz, J.; Singh, M.; Hawkins, L.D.; Wack, A.; O’Hagan, D.T. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020). Pharm. Res. 2009, 26, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Roestenberg, M.; Remarque, E.; de Jonge, E.; Hermsen, R.; Blythman, H.; Leroy, O.; Imoukhuede, E.; Jepsen, S.; Ofori-Anyinam, O.; Faber, B.; et al. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with alhydrogel™, montanide ISA 720 or AS02. PLoS ONE 2008, 3, e3960. [Google Scholar] [CrossRef]
- Brando, C.; Ware, L.A.; Freyberger, H.; Kathcart, A.; Barbosa, A.; Cayphas, S.; Demoitie, M.A.; Mettens, P.; Heppner, D.G.; Lanar, D.E. Murine immune responses to liver-stage antigen 1 protein FMP011, a malaria vaccine candidate, delivered with adjuvant AS01B or AS02A. Infect. Immun. 2007, 75, 838–845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Audran, R.; Lurati-Ruiz, F.; Genton, B.; Blythman, H.E.; Ofori-Anyinam, O.; Reymond, C.; Corradin, G.; Spertini, F. The synthetic Plasmodium falciparum circumsporozoite peptide PFCS102 as a malaria vaccine candidate: A randomized controlled phase I trial. PLoS ONE 2009, 4, e7304. [Google Scholar] [CrossRef] [PubMed]
- Kester, K.E.; Cummings, J.F.; Ofori-Anyinam, O.; Ockenhouse, C.F.; Krzych, U.; Moris, P.; Schwenk, R.; Nielsen, R.A.; Debebe, Z.; Pinelis, E.; et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: Safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 2009, 200, 337–346. [Google Scholar] [CrossRef]
- Polhemus, M.E.; Remich, S.A.; Ogutu, B.R.; Waitumbi, J.N.; Otieno, L.; Apollo, S.; Cummings, J.F.; Kester, K.E.; Ockenhouse, C.F.; Stewart, A.; et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS ONE 2009, 4, e6465. [Google Scholar] [CrossRef]
- Kester, K.E.; Cummings, J.F.; Ockenhouse, C.F.; Nielsen, R.; Hall, B.T.; Gordon, D.M.; Schwenk, R.J.; Krzych, U.; Holland, C.A.; Richmond, G.; et al. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the walter reed army institute of research. Vaccine 2008, 26, 2191–2202. [Google Scholar] [CrossRef]
- Alonso, P.L.; Sacarlal, J.; Aponte, J.J.; Leach, A.; Macete, E.; Aide, P.; Sigauque, B.; Milman, J.; Mandomando, I.; Bassat, Q.; et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in mozambican children: Single-blind extended follow-up of a randomised controlled trial. Lancet 2005, 366, 2012–2018. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Leroux-Roels, I.; Clement, F.; Ofori-Anyinam, O.; Lievens, M.; Jongert, E.; Moris, P.; Ballou, W.R.; Cohen, J. Evaluation of the immune response to RTS,S/AS01 and RTS,S/AS02 adjuvanted vaccines: Randomized, double-blind study in malaria-naive adults. Hum. Vaccines Immunother. 2014, 10, 2211–2219. [Google Scholar] [CrossRef]
- Stoute, J.A.; Kester, K.E.; Krzych, U.; Wellde, B.T.; Hall, T.; White, K.; Glenn, G.; Ockenhouse, C.F.; Garcon, N.; Schwenk, R.; et al. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J. Infect. Dis. 1998, 178, 1139–1144. [Google Scholar] [CrossRef]
- Bojang, K.A.; Olodude, F.; Pinder, M.; Ofori-Anyinam, O.; Vigneron, L.; Fitzpatrick, S.; Njie, F.; Kassanga, A.; Leach, A.; Milman, J.; et al. Safety and immunogenicty of RTS,S/AS02A candidate malaria vaccine in gambian children. Vaccine 2005, 23, 4148–4157. [Google Scholar] [CrossRef]
- Wu, Y.; Ellis, R.D.; Shaffer, D.; Fontes, E.; Malkin, E.M.; Mahanty, S.; Fay, M.P.; Narum, D.; Rausch, K.; Miles, A.P.; et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE 2008, 3, e2636. [Google Scholar] [CrossRef]
- Aucouturier, J.; Dupuis, L.; Deville, S.; Ascarateil, S.; Ganne, V. Montanide ISA 720 and 51: A new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev. Vaccines 2002, 1, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Verma, Y.K.; Reddy, B.S.; Pawar, M.S.; Bhunia, D.; Sampath Kumar, H.M. Design, synthesis, and immunological evaluation of benzyloxyalkyl-substituted 1,2,3-triazolyl alpha-galcer analogues. ACS Med. Chem. Lett. 2016, 7, 172–176. [Google Scholar] [CrossRef]
- Sandeep, A.; Reddy, B.S.; Hyder, I.; Kumar, H.M.S. Synthesis of a new class of glycolipids and the evaluation of their immunogenicity using murine splenocytes. J. Carbohydr. Chem. 2016, 35, 326–343. [Google Scholar] [CrossRef]
- Chirke, S.S.; Krishna, J.S.; Rathod, B.B.; Bonam, S.R.; Khedkar, V.M.; Rao, B.V.; Sampath Kumar, H.M.; Shetty, P.R. Synthesis of triazole derivatives of 9-ethyl-9H-carbazole and dibenzo[b,d]furan and evaluation of their antimycobacterial and immunomodulatory activity. ChemistrySelect 2017, 2, 7309–7318. [Google Scholar] [CrossRef]
- Lingamurthy, M.; Nalliboina, G.R.; Rao, M.V.; Rao, B.V.; Reddy, B.S.; Kumar, H.M.S. Ddq mediated stereoselective intermolecular benzylic cn bond formation: Synthesis of (−)-cytoxazone,(−)-4-epi-cytoxazone and their analogues and immunological evaluation of their cytokine modulating activity. Tetrahedron 2017, 73, 1473–1481. [Google Scholar] [CrossRef]
- Reddy Bonam, S.; Naidu Gorantla, J.; Thangarasu, A.K.; Lankalapalli, R.S.; Sampath Kumar, H.M. Polyhydroxy-n-alkyl-2-pyrrolidinones as a new class of glycolipid analogues with immune modulation potential. J. Carbohydr. Chem. 2018, 37, 30–43. [Google Scholar] [CrossRef]
- Bonam, S.R.; Bhunia, D.; Muller, S.; Nerella, S.G.; Alvala, M.; Halmuthur Mahabalarao, S.K. Novel trisaccharide based phospholipids as immunomodulators. Int. Immunopharmacol. 2019, 74, 105684. [Google Scholar] [CrossRef] [PubMed]
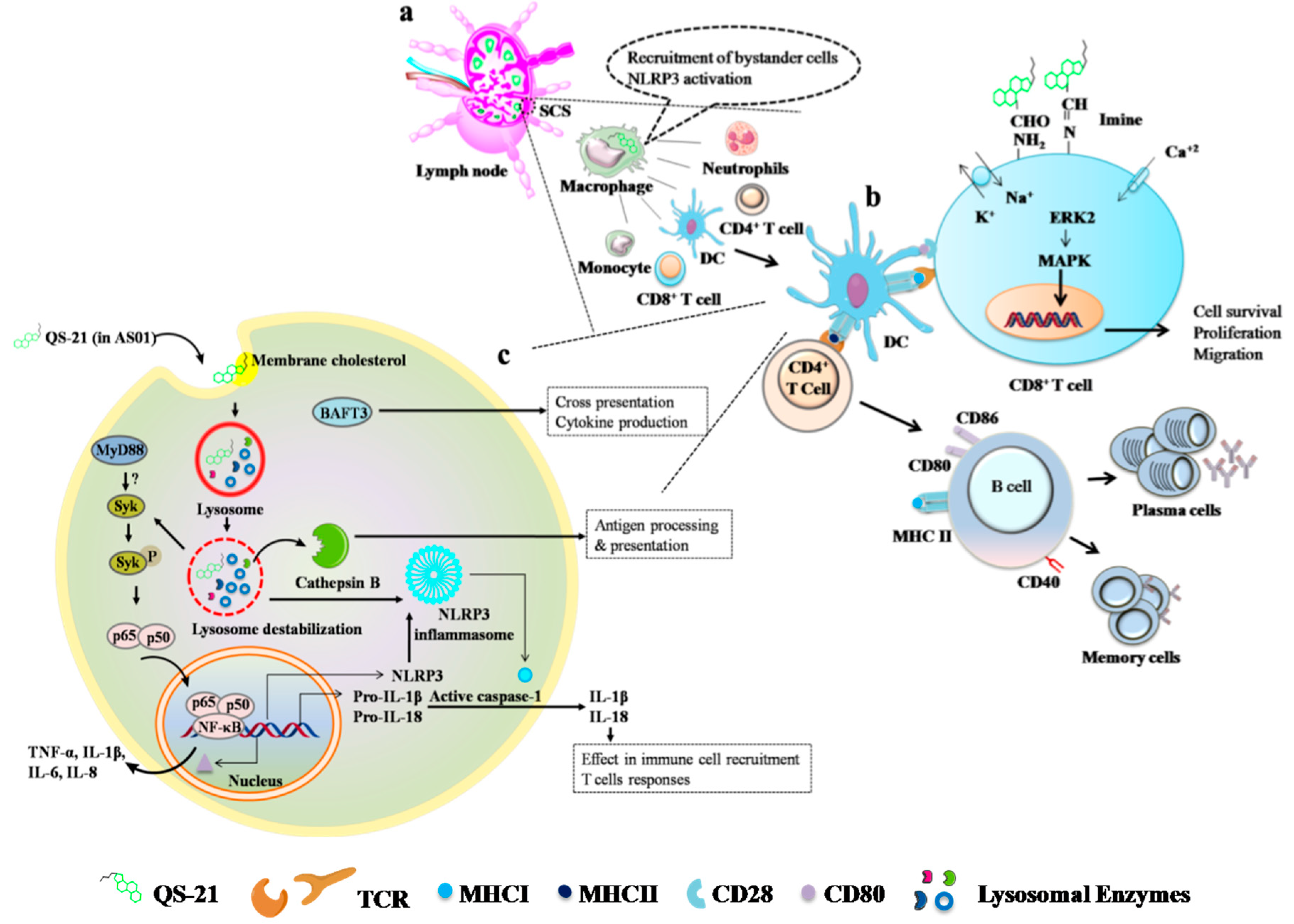
- Detienne, S.; Welsby, I.; Collignon, C.; Wouters, S.; Coccia, M.; Delhaye, S.; Van Maele, L.; Thomas, S.; Swertvaegher, M.; Detavernier, A.; et al. Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Sci. Rep. 2016, 6, 39475. [Google Scholar] [CrossRef]
- Welsby, I.; Detienne, S.; N’Kuli, F.; Thomas, S.; Wouters, S.; Bechtold, V.; De Wit, D.; Gineste, R.; Reinheckel, T.; Elouahabi, A.; et al. Lysosome-dependent activation of human dendritic cells by the vaccine adjuvant QS-21. Front. Immunol. 2016, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.I.; Fernando, G.J.P.; Depelsenaire, A.C.I.; Kendall, M.A.F. Potent response of QS-21 as a vaccine adjuvant in the skin when delivered with the nanopatch, resulted in adjuvant dose sparing. Sci. Rep. 2016, 6, 29368. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef]
- Coler, R.N.; Carter, D.; Friede, M.; Reed, S.G. Adjuvants for malaria vaccines. Parasite Immunol. 2009, 31, 520–528. [Google Scholar] [CrossRef]
- Petrovsky, N. Comparative safety of vaccine adjuvants: A summary of current evidence and future needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef]
- Klinman, D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Pirahmadi, S.; Zakeri, S.; Mehrizi, A.A.; Djadid, N.D.; Raz, A.A.; Sani, J.J. Combining monophosphoryl lipid a (MPL), CpG oligodeoxynucleotide (ODN), and QS-21 adjuvants induces strong and persistent functional antibodies and T cell responses against cell-traversal protein for ookinetes and sporozoites (CelTOS) of Plasmodium falciparum in BALB/c mice. Infect. Immun. 2019, 87, e00911-18. [Google Scholar] [CrossRef]
- Mullen, G.E.; Ellis, R.D.; Miura, K.; Malkin, E.; Nolan, C.; Hay, M.; Fay, M.P.; Saul, A.; Zhu, D.; Rausch, K.; et al. Phase 1 trial of AMA1-C1/alhydrogel plus CpG 7909: An asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS ONE 2008, 3, e2940. [Google Scholar] [CrossRef]
- Traore, B.; Kone, Y.; Doumbo, S.; Doumtabe, D.; Traore, A.; Crompton, P.D.; Mircetic, M.; Huang, C.Y.; Kayentao, K.; Dicko, A.; et al. The TLR9 agonist CpG fails to enhance the acquisition of Plasmodium falciparum-specific memory B cells in semi-immune adults in mali. Vaccine 2009, 27, 7299–7303. [Google Scholar] [CrossRef]
- Horii, T.; Shirai, H.; Jie, L.; Ishii, K.J.; Palacpac, N.Q.; Tougan, T.; Hato, M.; Ohta, N.; Bobogare, A.; Arakaki, N.; et al. Evidences of protection against blood-stage infection of Plasmodium falciparum by the novel protein vaccine SE36. Parasitol. Int. 2010, 59, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Tougan, T.; Aoshi, T.; Coban, C.; Katakai, Y.; Kai, C.; Yasutomi, Y.; Ishii, K.J.; Horii, T. TLR9 adjuvants enhance immunogenicity and protective efficacy of the SE36/AHG malaria vaccine in nonhuman primate models. Hum. Vaccines Immunother. 2013, 9, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.J.; Sheehy, S.H.; Ewer, K.J.; Douglas, A.D.; Collins, K.A.; Halstead, F.D.; Elias, S.C.; Lillie, P.J.; Rausch, K.; Aebig, J.; et al. Impact on malaria parasite multiplication rates in infected volunteers of the protein-in-adjuvant vaccine AMA1-C1/alhydrogel+CpG 7909. PLoS ONE 2011, 6, e22271. [Google Scholar] [CrossRef]
- Antony, H.A.; Parija, S.C. Antimalarial drug resistance: An overview. Trop. Parasitol. 2016, 6, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Aderem, A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature 2011, 473, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Voepel, N.; Boes, A.; Edgue, G.; Beiss, V.; Kapelski, S.; Reimann, A.; Schillberg, S.; Pradel, G.; Fendel, R.; Scheuermayer, M. Malaria vaccine candidate antigen targeting the pre-erythrocytic stage of Plasmodium falciparum produced at high level in plants. Biotechnol. J. 2014, 9, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.A.; Coccia, M.; Ballou, W.R.; Kester, K.E.; Ockenhouse, C.F.; Vekemans, J.; Jongert, E.; Didierlaurent, A.M.; van der Most, R.G. Predicting RTS,S vaccine-mediated protection from transcriptomes in a malaria-challenge clinical trial. Front. Immunol. 2017, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, R.W.; Roestenberg, M.; Moorthy, V.S. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat. Rev. Immunol. 2011, 11, 57–64. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Kappe, S.H.I. Malaria parasite liver infection and exoerythrocytic biology. Cold Spring Harb. Perspect. Med. 2017, 7, a025486. [Google Scholar] [CrossRef]
- Paul, A.S.; Egan, E.S.; Duraisingh, M.T. Host–parasite interactions that guide red blood cell invasion by malaria parasites. Curr. Opin. Hematol. 2015, 22. [Google Scholar] [CrossRef] [PubMed]
- Sinnis, P.; Sim, B.K. Cell invasion by the vertebrate stages of plasmodium. Trends Microbiol. 1997, 5, 52–58. [Google Scholar] [CrossRef]
- Holz, L.E.; Prier, J.E.; Freestone, D.; Steiner, T.M.; English, K.; Johnson, D.N.; Mollard, V.; Cozijnsen, A.; Davey, G.M.; Godfrey, D.I.; et al. CD8+ T cell activation leads to constitutive formation of liver tissue-resident memory T cells that seed a large and flexible niche in the liver. Cell Rep. 2018, 25, 68–79.e4. [Google Scholar] [CrossRef]
- Weaver, R.; Reiling, L.; Feng, G.; Drew, D.R.; Mueller, I.; Siba, P.M.; Tsuboi, T.; Richards, J.S.; Fowkes, F.J.I.; Beeson, J.G. The association between naturally acquired IgG subclass specific antibodies to the pfrh5 invasion complex and protection from Plasmodium falciparum malaria. Sci. Rep. 2016, 6, 33094. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.A.; Gottlieb, E.R.; Kouriba, B.; Diarra, I.; Thera, M.A.; Dutta, S.; Coulibaly, D.; Ouattara, A.; Niangaly, A.; Kone, A.K.; et al. Immunoglobulin G subclass and antibody avidity responses in Malian children immunized with Plasmodium falciparum apical membrane antigen 1 vaccine candidate FMP2.1/AS02A. Malar. J. 2019, 18, 13. [Google Scholar] [CrossRef]
- Ndungu, F.M.; Mwacharo, J.; Wambua, J.; Njuguna, P.; Marsh, K.; Drakeley, C.; Bejon, P. A seven-year study on the effect of the pre-erythrocytic malaria vaccine candidate RTS,S/AS01E on blood stage immunity in young Kenyan children. Wellcome Open Res. 2019, 4, 42. [Google Scholar] [CrossRef]
- Horowitz, A.; Hafalla, J.C.; King, E.; Lusingu, J.; Dekker, D.; Leach, A.; Moris, P.; Cohen, J.; Vekemans, J.; Villafana, T.; et al. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of tanzanian children with the RTS,S/AS01 malaria vaccine. J. Immunol. 2012, 188, 5054–5062. [Google Scholar] [CrossRef]
- Dunachie, S.J.; Hill, A.V. Prime-boost strategies for malaria vaccine development. J. Exp. Biol. 2003, 206, 3771–3779. [Google Scholar] [CrossRef]
- Hoffman, S.L.; Vekemans, J.; Richie, T.L.; Duffy, P.E. The march toward malaria vaccines. Vaccine 2015, 33 (Suppl. 4), D13–D23. [Google Scholar] [CrossRef]
- López, C.; Yepes-Pérez, Y.; Hincapié-Escobar, N.; Díaz-Arévalo, D.; Patarroyo, M.A. What is known about the immune response induced by plasmodium vivax malaria vaccine candidates? Front. Immunol. 2017, 8, 126. [Google Scholar] [CrossRef]
- Nega, D.; Alemu, A.; Tasew, G. Malaria vaccine development: Recent advances alongside the barriers. J. Bacteriol. Parasitol. 2016, 7, 300. [Google Scholar] [CrossRef]
- Villa, E.; Marchetti, S.; Ricci, J.E. No parkin zone: Mitophagy without parkin. Trends Cell Biol. 2018, 28, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Bejon, P.; Cook, J.; Bergmann-Leitner, E.; Olotu, A.; Lusingu, J.; Mwacharo, J.; Vekemans, J.; Njuguna, P.; Leach, A.; Lievens, M.; et al. Effect of the pre-erythrocytic candidate malaria vaccine RTS,S/AS01E on blood stage immunity in young children. J. Infect. Dis. 2011, 204, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, S.; Salim, N.; Machera, F.; Kamata, R.; Juma, O.; Shomari, M.; Kubhoja, S.; Mohammed, A.; Mwangoka, G.; Aebi, T.; et al. Randomized, controlled trial of the long term safety, immunogenicity and efficacy of RTS,S/AS02D malaria vaccine in infants living in a malaria-endemic region. Malar. J. 2013, 12, 11. [Google Scholar] [CrossRef]
- Rampling, T.; Ewer, K.J.; Bowyer, G.; Edwards, N.J.; Wright, D.; Sridhar, S.; Payne, R.; Powlson, J.; Bliss, C.; Venkatraman, N.; et al. Safety and efficacy of novel malaria vaccine regimens of RTS,S/AS01B alone, or with concomitant ChAd63-MVA-vectored vaccines expressing ME-TRAP. NPJ Vaccines 2018, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Berendt, A.R.; Tumer, G.D.; Newbold, C.I. Cerebral malaria: The sequestration hypothesis. Parasitol. Today 1994, 10, 412–414. [Google Scholar] [CrossRef]
- Clark, I.A.; Rockett, K.A. The cytokine theory of human cerebral malaria. Parasitol. Today 1994, 10, 410–412. [Google Scholar] [CrossRef]
- Vekemans, J.; Ballou, W.R. Plasmodium falciparum malaria vaccines in development. Expert Rev. Vaccines 2008, 7, 223–240. [Google Scholar] [CrossRef]
| Malaria Vaccine | Clinical Trial Identifier | Current Stage |
|---|---|---|
| ChAd63 RH5 (chimpanzee adenovirus serotype 63 reticulocyte-binding protein homolog 5) | NCT02181088 | Phase 1 |
| MVA (modified vaccinia virus Ankara) RH5 | NCT02181088 | Phase 1 |
| PEBS-POC1 (synthetic protein containing 131 amino acids) | NCT01605786 | Phase 1 |
| ChAd63-METRAP (multiple epitope string and thrombospondin-related adhesion protein) | NCT03084289 | Phase 1 |
| MVA METRAP | NCT03084289 | Phase 1 |
| DNA-Ad (contains a combination of circumsporozoite (CS) protein and AMA1) | NCT00870987 | Phase 2 |
| PfSPZ (P. falciparum (Pf) sporozoite (SPZ)) | NCT02601716 | Phase 2 |
| Ad35.CS.01 (P. falciparum CS surface antigen is inserted in a replication deficient Adenovirus 35 backbone) | NCT01018459 | Phase 1 |
| CS protein expressed either in MVA, or an attenuated Fowl pox virus strain (FP9). | NCT00121771 | Phase 1 |
| AdCh63-MSP1 (merozoite surface protein-1) and MVA-MSP1 | NCT01003314 | Phase 2 |
| GMZ2 (recombinant hybrid of the glutamate rich protein (GLURP) and the merozoite surface protein 3 (MSP 3)) | NCT00424944 | Phase 1 |
| FP9-PP and MVA-PP (FP9 polyprotein, modified virus Ankara polyprotein) | NCT00374998 | Phase 1 |
| p52-p36-GAP (genetically attenuated parasite malaria vaccine) | NCT01024686 | Phase 2 |
| PfSPZ-GA1 (genetically attenuated PfSPZ) | NCT03163121 | Phase 1 |
| ChAdOx1 LS2 (malaria liver-stage dual antigen LS2 (LSA1 and LSAP2) fused with the transmembrane domain from shark invariant chain) and MVA LS2 | NCT03203421 | Phase 2/Phase 1 |
| DNA-Ad (it contains a liver-stage antigen (circumsporozoite protein) and an antigen (apical membrane antigen 1)) | NCT00870987 | Phase 1 |
| NMRC-M3V-Ad-PfCA (NMRC + multi-antigen multi-stage, malaria vaccine + adenovectored + P. falciparum CSP and AMA1 antigens), is a combination of two recombinant adenovirus-derived constructs (adenovectors) | NCT00392015 | Phase 1 |
| Adjuvant | Vaccine | Life-Cycle Stage | Clinical Trial Identifier | Current Stage |
|---|---|---|---|---|
| Aluminium hydroxide/Alhydrogel® | Lyophilized PEBS synthetic protein (PfPEBS) (synthetic protein containing 131 amino acids) | Pre-erythrocytic and blood stage | NCT01605786 | Phase 2 |
| AMA1-C1 (combination of the 3D7 and FVO alleles of P. falciparum apical membrane antigen-1 (AMA1)) | Blood stage | NCT00984763 NCT00114010 | Phase 2 Phase 1 | |
| PRIMVAC (VAR2CSA protein) | Blood stage | NCT02658253 | Phase 1 | |
| P27A protein | Blood stage | NCT01949909 | Phase 1 | |
| Pfs25-EPA (Pfs25 has been conjugated to Pseudomonas aeruginosa ExoProtein A) (EPA) | Sexual stage | NCT01434381 | Phase 1 | |
| Pfs25 VLP | Sexual stage | NCT02013687 | Phase 1 | |
| AMA1-DiCo | Blood stage | NCT02014727 | Phase 1 | |
| Pfs25M-EPA, Pfs230D1M-EPA | Sexual stage | NCT02334462 | Phase 1 | |
| AdCh63 AMA1 + MVA AMA1 + AMA1-C1 | Blood stage | NCT01351948 | Phase 1 | |
| BSAM-2 (mixture of two proteins found on the surface of merozoites, AMA1 and MSP1 (42)) | Blood stage | NCT00889616 | Phase 1 | |
| ICC-1132 | Pre-erythrocytic stage | NCT00587249 | Phase 1 | |
| PAMVAC | Blood stage | NCT02647489 | Phase 1 | |
| MSP3-LSP | Blood stage | NCT01341704 | Phase 2 | |
| MSP1 42-C1 | Blood stage | NCT00320658 | Phase 1 | |
| Aluminum phosphate | Erythrocyte-binding antigen 175 kDA region II-non-glycosylated (EBA-175 RII-NG) | Blood stage | NCT01026246 | Phase 1 |
| AS01 | RH5.1 (protein ectodomain of the PfRH5 (amino acids E26—Q526) antigen) | Blood stage | NCT02927145 | Phase 2 |
| Pfs25M-EPA, Pfs230D1M-EPA (PfS230D1M conjugated to Pseudomonas aeruginosa ExoProtein A (EPA)) | Sexual stage | NCT02942277 | Phase 1 | |
| AS01B | R21 (RTS, S-like vaccine) | Pre-erythrocytic stage | NCT02600975 | Phase 1 |
| FMP012 (Escherichia coli-expressed P. falciparum cell-traversal protein for ookinetes and sporozoites (PfCelTOS)) | Sexual stage | NCT02174978 | Phase 1 | |
| P. falciparum malaria protein (FMP)010 | Blood stage | NCT00666380 | Phase 1 | |
| FMP2.1 (AMA1 malaria antigen) | Blood stage | NCT00385047 | Phase 1 | |
| AS01E | RTS, S | Pre-erythrocytic stage | NCT00380393 | Phase 2 |
| AS02A | Falciparum Merozoite Protein-1 (FMP1) | Blood stage | NCT00308061 | Phase 1 |
| FMP2.1 (AMA1 malaria antigen) | Blood stage | NCT00385047 | Phase 2 | |
| Falciparum Malaria Protein 11 | Pre-erythrocytic stage | NCT00312702 | Phase 2 | |
| AS02D | RTS, S | Pre-erythrocytic stage | NCT00289185 | Phase 2 |
| ODN 2006 (7909) | MSP1 42-C1 | Blood stage | NCT00320658 | Phase 1 |
| AMA1-C1 (combination of the 3D7 and FVO alleles of P. falciparum apical membrane antigen-1 (AMA1)) | Blood stage | NCT00984763 | Phase 2 | |
| AdCh63 AMA1 + MVA AMA1 + AMA1-C1 | Blood stage | NCT01351948 | Phase 1 | |
| BSAM-2 | Blood stage | NCT00889616 | Phase 1 | |
| Glucopyranosyl Lipid Adjuvant-Liposome-QS-21 Formulation (GLA-LSQ) | PAMVAC | Blood stage | NCT02647489 | Phase 1 |
| Recombinant circumsporozoite protein (rCSP) malaria vaccine administered with and without AP 10-602 (GLA-LSQ) | Pre-erythrocytic stage | NCT03589794 | Phase 1 | |
| Glucopyranosyl Lipid Adjuvant-Stable Emulsion (GLA-SE) | PAMVAC | Blood stage | NCT02647489 | Phase 1 |
| PRIMVAC (VAR2CSA protein) | Blood stage | NCT02658253 | Phase 1 | |
| P27A protein | Blood stage | NCT01949909 | Phase 1 | |
| AMA1-DiCo | Blood stage | NCT02014727 | Phase 1 | |
| FMP012 | Sexual stage | NCT01540474 | Phase 1 | |
| Matrix-M1 (a bifunctional matrix protein of influenza virus) | R21 | Pre-erythrocytic stage | NCT02925403 | Phase 1 |
| Montanide ISA51 | PpPfs25 | Sexual stage | NCT00295581 | Phase 1 |
| Montanide ISA 720 | PfCS102 (antigen of the sporozoite protein) | Pre-erythrocytic stage | NCT01031524 | Phase 1 |
| Virosomes | PEV301 & 302 (it includes two antigens (CSP and AMA1-derived)) | Pre-erythrocytic stage and blood stage | NCT00513669 | Phase 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonam, S.R.; Rénia, L.; Tadepalli, G.; Bayry, J.; Kumar, H.M.S. Plasmodium falciparum Malaria Vaccines and Vaccine Adjuvants. Vaccines 2021, 9, 1072. https://doi.org/10.3390/vaccines9101072
Bonam SR, Rénia L, Tadepalli G, Bayry J, Kumar HMS. Plasmodium falciparum Malaria Vaccines and Vaccine Adjuvants. Vaccines. 2021; 9(10):1072. https://doi.org/10.3390/vaccines9101072
Chicago/Turabian StyleBonam, Srinivasa Reddy, Laurent Rénia, Ganesh Tadepalli, Jagadeesh Bayry, and Halmuthur Mahabalarao Sampath Kumar. 2021. "Plasmodium falciparum Malaria Vaccines and Vaccine Adjuvants" Vaccines 9, no. 10: 1072. https://doi.org/10.3390/vaccines9101072
APA StyleBonam, S. R., Rénia, L., Tadepalli, G., Bayry, J., & Kumar, H. M. S. (2021). Plasmodium falciparum Malaria Vaccines and Vaccine Adjuvants. Vaccines, 9(10), 1072. https://doi.org/10.3390/vaccines9101072








