SnoRNAs and miRNAs Networks Underlying COVID-19 Disease Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. RNA Isolation and Quality Control
2.3. Microarray and Data Analysis
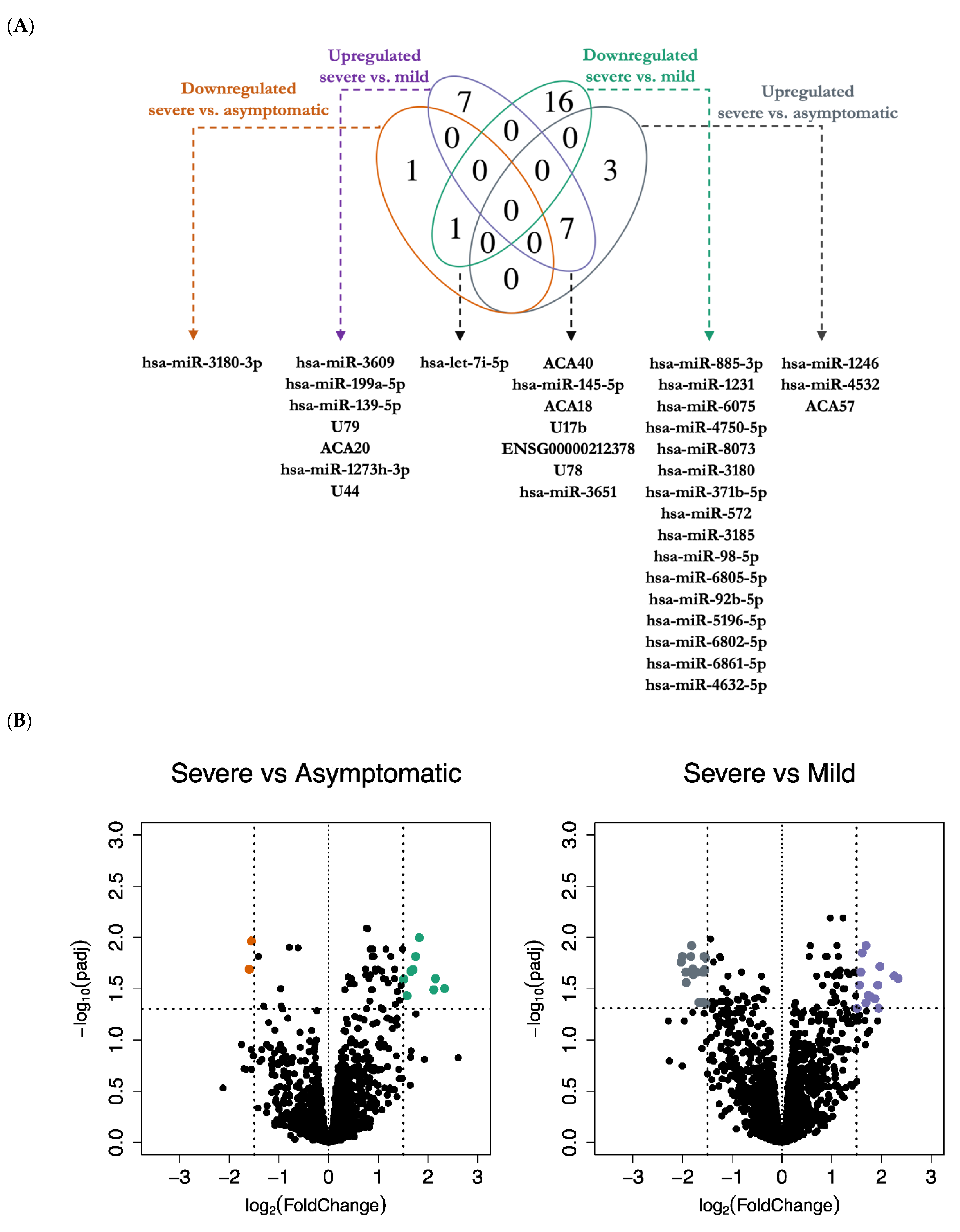
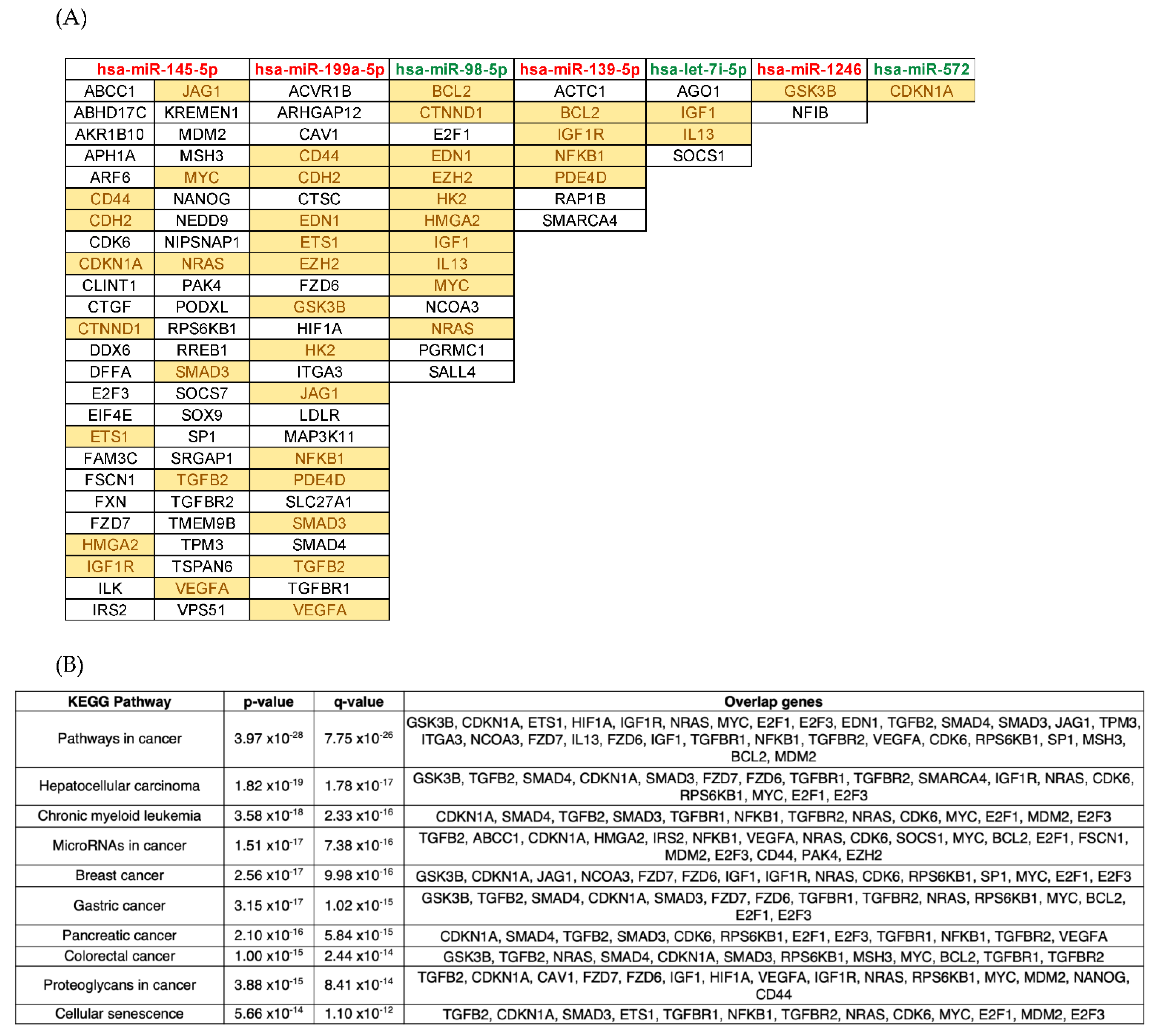
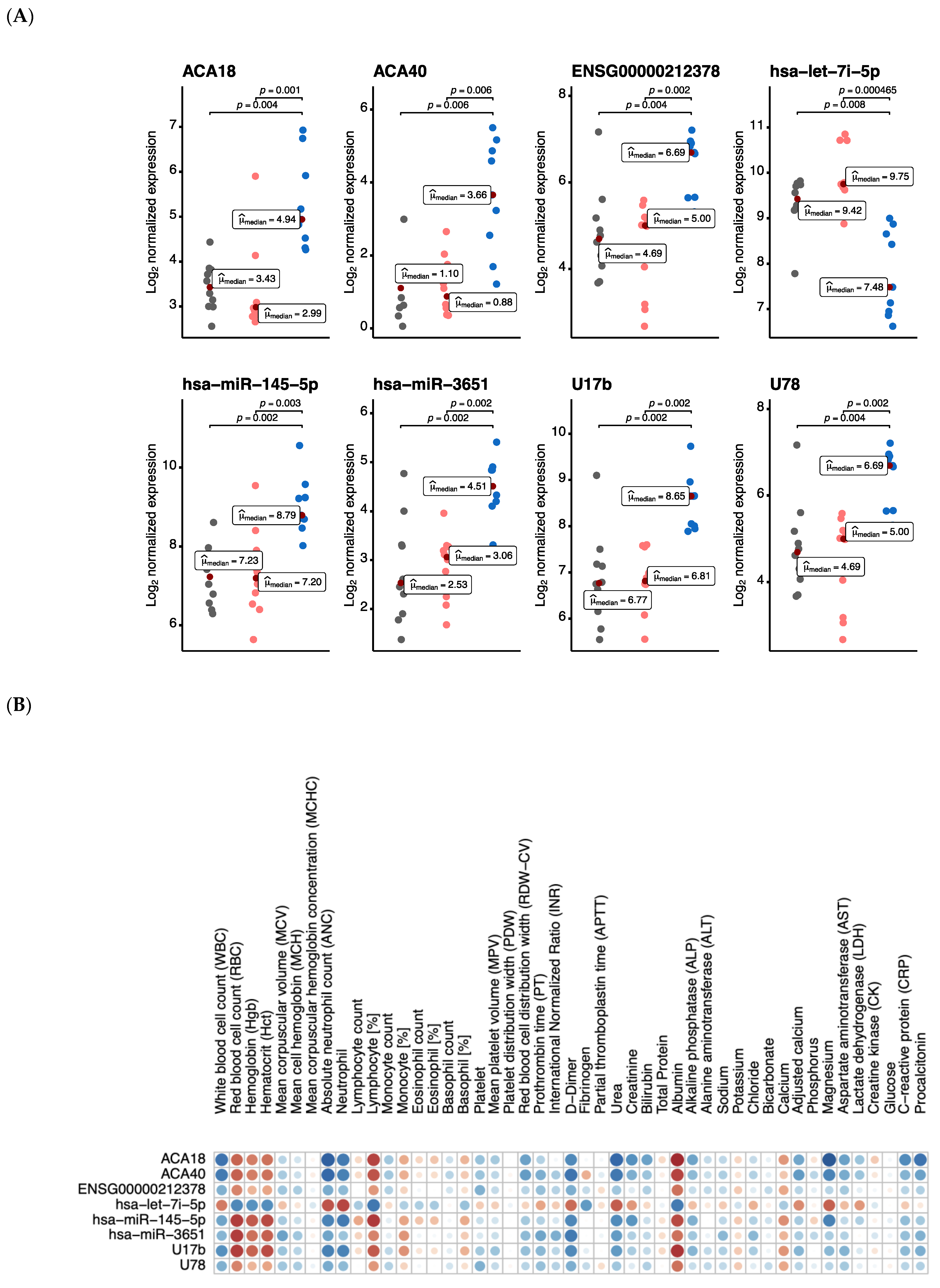
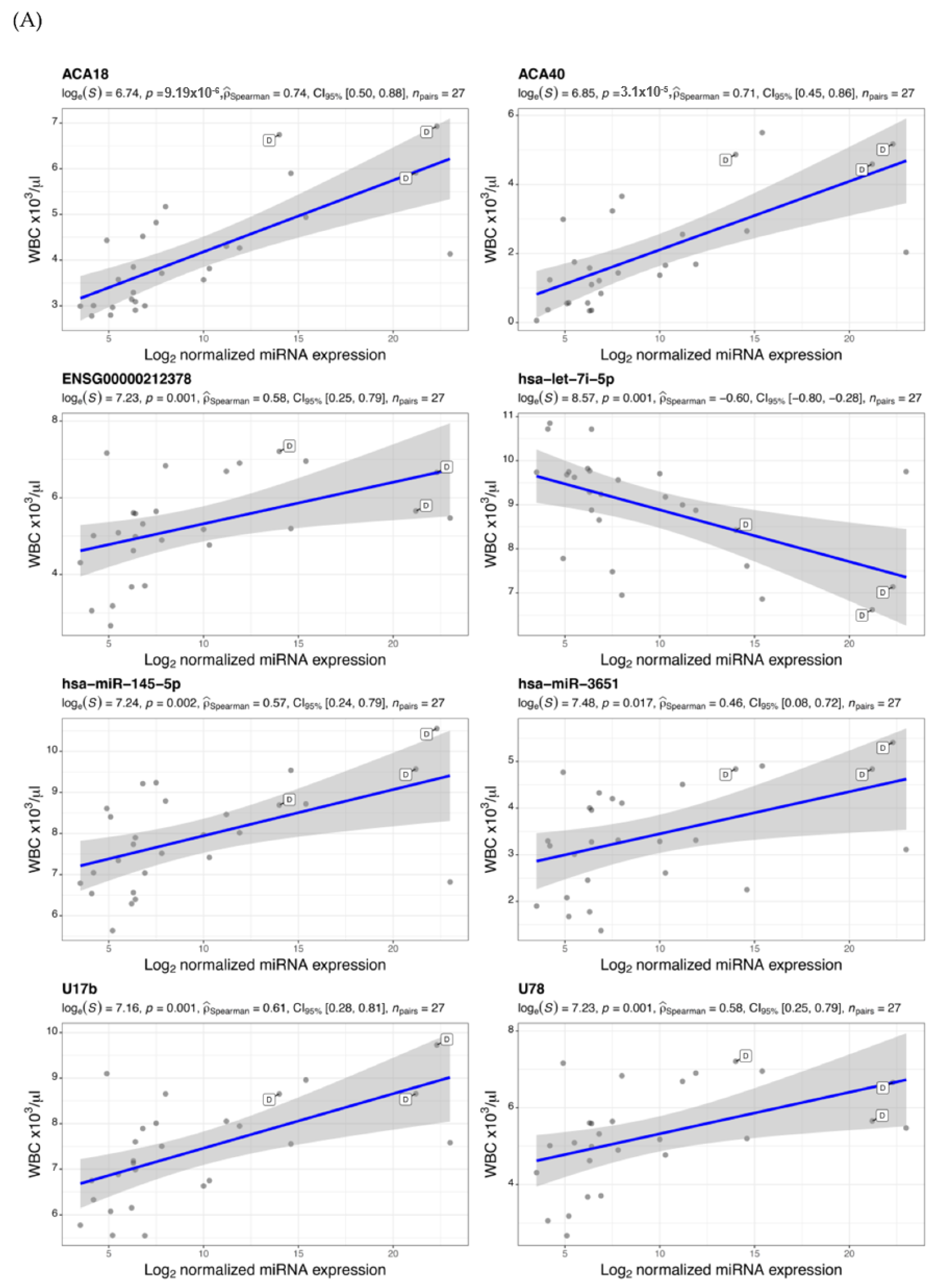
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Gao, Y.; Li, Z.; Miao, Y.; Huang, Z.; Liu, X.; Xie, L.; Li, H.; Wen, W.; Zheng, Y.; et al. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Transl. Med. 2020, 10, e200. [Google Scholar] [CrossRef] [PubMed]
- Drury, R.E.; O’Connor, D.; Pollard, A.J. The clinical application of micrornas in infectious disease. Front. Immunol. 2017, 8, 1182. [Google Scholar] [CrossRef]
- Fulzele, S.; Sahay, B.; Yusufu, I.; Lee, T.J.; Sharma, A.; Kolhe, R.; Isales, C.M. Covid-19 virulence in aged patients might be impacted by the host cellular micrornas abundance/profile. Aging Dis. 2020, 11, 509–522. [Google Scholar] [CrossRef]
- Girardi, E.; López, P.; Pfeffer, S. On the importance of host micrornas during viral infection. Front. Genet. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalo-Calvo, D.; Benítez, I.D.; Pinilla, L.; Carratalá, A.; Moncusí-Moix, A.; Gort-Paniello, C.; Molinero, M.; González, J.; Torres, G.; Bernal, M.; et al. Circulating microRNA profiles predict the severity of Covid-19 in hospitalized patients. Transl. Res. 2021, 236, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Henzinger, H.; Barth, D.A.; Klec, C.; Pichler, M. Non-coding RNAs and SARS-related coronaviruses. Viruses 2020, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Sany, M.; Us, R.; Islam, M.; Islam, A.B.M.M. Epigenetic regulator miRNA pattern differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 world-wide isolates delineated the mystery behind the epic pathogenicity and distinct clinical characteristics of pandemic COVID-19. Front. Genet. 2020, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Shaath, H.; Alajez, N.M. Identification of PBMC-based molecular signature associational with COVID-19 disease severity. Heliyon 2021, 7, e06866. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Barbu, M.G.; Condrat, C.E.; Thompson, D.C.; Bugnar, O.L.; Cretoiu, D.; Toader, O.D.; Suciu, N.; Voinea, S.C. MicroRNA involvement in signaling pathways during viral infection. Front. Cell Dev. Biol. 2020, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Bernier, A.; Sagan, S.M. The diverse roles of microRNAs at the host-virus interface. Viruses 2018, 10, 440. [Google Scholar] [CrossRef] [Green Version]
- Hum, C.; Loiselle, J.; Ahmed, N.; Shaw, T.A.; Toudic, C.; Pezacki, J.P. MicroRNA mimics or inhibitors as antiviral therapeutic approaches against COVID-19. Drugs 2021, 81, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, L.; Parca, L.; Mazza, T.; Weber, C.; Virgili, F.; Fratantonio, D. MicroRNAs and long non-coding RNAs as potential candidates to target specific motifs of SARS-CoV-2. Non-Coding RNA 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Amahong, K.; Sun, X.; Lian, X.; Liu, J.; Sun, H.; Lou, Y.; Zhu, F.; Qiu, Y. The miRNA: A small but powerful RNA for COVID-19. Brief. Bioinform. 2021, 22, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Hutzinger, R.; Feederle, R.; Mrazek, J.; Schiefermeier-Mach, N.; Balwierz, P.J.; Zavolan, M.; Polacek, N.; Delecluse, H.-J.; Hüttenhofer, A. Expression and processing of a small nucleolar RNA from the epstein-barr virus genome. PLoS Pathog. 2009, 5, e1000547. [Google Scholar] [CrossRef] [Green Version]
- Stamm, S.; Lodmell, J.S. C/D box snoRNAs in viral infections: RNA viruses use old dogs for new tricks. Non-Coding RNA Res. 2019, 4, 46–53. [Google Scholar] [CrossRef]
- Bratkovič, T.; Božič, J.; Rogelj, B. Functional diversity of small nucleolar RNAs. Nucleic Acids Res. 2020, 48, 1627–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, B.S.; Irizarry, R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.I.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef]
- Haunsberger, S.J.; Connolly, N.M.C.; Prehn, J.H.M. MiRNAmeConverter: An R/bioconductor package for translating mature miRNA names to different miRBase versions. Bioinformatics 2016, 33, 592–593. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lin, Y.C.D.; Li, J.; Huang, K.Y.; Shrestha, S.; Hong, H.C.; Tang, Y.; Chen, Y.G.; Jin, C.N.; Yu, Y.; et al. MiRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licursi, V.; Conte, F.; Fiscon, G.; Paci, P. Mienturnet: An interactive web tool for microRNA-target enrichment and network-based analysis. BMC Bioinform. 2019, 20, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef] [PubMed]
- Marchi, R.; Sugita, B.; Centa, A.; Fonseca, A.S.; Bortoletto, S.; Fiorentin, K.; Ferreira, S.; Cavalli, L.R. The role of microRNAs in modulating SARS-CoV-2 infection in human cells: A systematic review. Infect. Genet. Evol. 2021, 91, 104832. [Google Scholar] [CrossRef] [PubMed]
- Nersisyan, S.; Shkurnikov, M.; Turchinovich, A.; Knyazev, E.; Tonevitsky, A. Integrative analysis of miRNA and mRNA sequencing data reveals potential regulatory mechanisms of ACE2 and TMPRSS2. PLoS ONE 2020, 15, e0235987. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad-Farsangi, S.; Jazi, M.M.; Rostamzadeh, F.; Hadizadeh, M. High affinity of host human microRNAs to SARS-CoV-2 genome: An In Silico analysis. Non-Coding RNA Res. 2020, 5, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.B.; Simion, V.; Icli, B.; Pérez-Cremades, D.; Cheng, H.S.; Feinberg, M.W. Computational analysis of targeting SARS-CoV-2, viral entry proteins ACE2 and TMPRSS2, and interferon genes by host MicroRNAs. Genes 2020, 11, 1354. [Google Scholar] [CrossRef]
- Saini, S.; Saini, A.; Thakur, C.J.; Kumar, V.; Gupta, R.D.; Sharma, J.K. Genome-wide computational prediction of miRNAs in severe acute respiratory syn-drome coronavirus 2 (SARS-CoV-2) revealed target genes involved in pulmonary vasculature and antiviral innate immunity. Mol. Biol. Res. Commun. 2020, 9, 83–91. [Google Scholar]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 In Vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Yin, J.; Zhang, J.; Jiang, Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: A challenge for cancer therapy. J. Hematol. Oncol. 2020, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Shin, E.C. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 2021, 53, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Gomes, M.; Kruglov, A.; Durek, P.; Heinrich, F.; Tizian, C.; Heinz, G.A.; Pascual-Reguant, A.; Du, W.; Mothes, R.; Fan, C.; et al. SARS-CoV-2 in severe Covid-19 induces a TGF-beta-dominated chronic immune response that does not target itself. Nat. Commun. 2021, 12, 1961. [Google Scholar] [CrossRef]



| Characteristic * | N | Asymptomatic | Mild | Severe | p-Value |
|---|---|---|---|---|---|
| Age [years] | 29 | 54.50 ± 4.58 | 49.60 ± 9.18 | 58.22 ± 12.88 | 0.078 |
| COVID-19 average CT | 21 | 22.9 (21.5–29.2) | 26.7 (23.4–29.4) | 33.8 (26.8–34.1) | 0.3 |
| Diabetes Mellitus | 29 | 6 (20.68%) | 6 (20.68%) | 6 (20.68%) | >0.9 |
| Glucose [mmol/L] | 20 | 7.00 (6.25–14.40) | 6.80 (6.27–8.12) | 6.60 (6.40–10.60) | 0.9 |
| WBC [×103/μL] | 27 | 6.3 (6.2–7.8) | 5.5 (5.1–6.4) | 11.9 (8.0–15.4) | 0.014 |
| Lymphocyte count [×103/μL] | 27 | 2.10 (1.20–2.50) | 1.30 (1.20–1.61) | 1.10 (0.90–1.40) | 0.2 |
| Lymphocyte [%] | 27 | 29 (24–33) | 24 (21–31) | 9 (5–14) | 0.004 |
| ANC [×103/μL] | 27 | 3.6 (2.9–6.1) | 3.6 (2.7–4.7) | 7.7 (6.7–13.1) | 0.005 |
| Neutrophil [%] | 21 | 56 (51–62) | 66 (58–69) | 83 (80–86) | 0.012 |
| Eosinophil count [×103/μL] | 27 | 0.10 (0.00–0.27) | 0.00 (0.00–0.00) | 0.00 (0.00–0.10) | 0.2 |
| Eosinophil [%] | 27 | 2.50 (0.90–3.40) | 0.10 (0.00–0.80) | 0.10 (0.00–0.80) | 0.029 |
| Monocyte count [×103/μL] | 27 | 0.60 (0.60–0.70) | 0.47 (0.40–0.50) | 0.90 (0.80–1.00) | 0.11 |
| Monocyte [%] | 27 | 8.70 (6.80–10.90) | 9.00 (5.10–9.40) | 5.60 (4.80–6.60) | 0.14 |
| Basophil count [×103/μL] | 27 | 0.030 (0.030–0.040) | 0.020 (0.020–0.050) | 0.030 (0.010–0.040) | 0.6 |
| Basophil [%] | 27 | 0.50 (0.40–0.80) | 0.40 (0.20–0.50) | 0.20 (0.10–0.40) | 0.026 |
| RBC [×106/μL] | 27 | 5.10 (4.90–5.30) | 5.00 (4.70–5.70) | 3.80 (3.50–4.20) | 0.011 |
| Hgb [g/dL] | 27 | 13.60 (13.50–15.20) | 14.20 (13.60–16.00) | 11.20 (9.90–12.60) | 0.036 |
| HbA1C [%] | 18 | 8.70 (6.05–11.80) | 7.00 (6.40–7.50) | 6.00 (5.90–6.40) | 0.4 |
| Hct [%] | 27 | 42 (40–43) | 42 (40–47) | 33 (30–38) | 0.03 |
| MCV [fL] | 27 | 81.9 (81.0–87.0) | 85.4 (82.2–91.2) | 90.6 (86.4–91.2) | 0.031 |
| MCH [pg] | 27 | 27.30 (26.50–29.50) | 28.10 (27.90–30.50) | 30.00 (29.40–30.30) | 0.11 |
| MCHC [g/dL] | 27 | 33.30 (32.40–34.00) | 33.60 (32.90–34.10) | 33.50 (32.50–34.10) | 0.8 |
| RDW-CV [%] | 27 | 13.00 (11.90–14.30) | 14.20 (12.70–14.90) | 13.90 (12.50–16.20) | 0.3 |
| MPV [fl] | 27 | 10.20 (9.60–11.30) | 10.80 (9.90–12.00) | 10.40 (9.80–11.50) | 0.8 |
| Platelet [×109/L] | 27 | 226 (200–251) | 187 (161–324) | 342 (223–363) | 0.2 |
| Ferritin [μg/L] | 18 | 574 (574–574) | 449 (210–804) | 1131 (700–1856) | 0.079 |
| PDW [fl] | 19 | 15.10 (15.10–15.10) | 13.90 (12.00–15.80) | 11.70 (11.00–14.20) | 0.5 |
| Fibrinogen [g/L] | 11 | 6.80 (6.80–6.80) | 4.60 (4.60–4.60) | 5.10 (3.30–5.60) | 0.5 |
| D-Dimer [mg/L FEU] | 16 | 1.55 (1.55–1.55) | 0.40 (0.32–0.60) | 2.65 (2.15–5.16) | 0.036 |
| APTT [second] | 15 | 26 (26–26) | 32 (29–35) | 31 (31–36) | 0.4 |
| PT [second] | 15 | 12.60 (12.60–12.60) | 11.30 (11.10–11.50) | 13.10 (12.60–13.50) | 0.047 |
| INR | 15 | 1.10 (1.10–1.10) | 1.00 (1.00–1.00) | 1.10 (1.10–1.10) | 0.057 |
| CRP [mg/L] | 27 | 3 (2–6) | 21 (5–56) | 26 (5–78) | 0.063 |
| IL-6 [pg/mL] | 9 | - | 32 (20–34) | 306 (137–476) | 0.2 |
| Total protein [g/L] | 24 | 76 (66–77) | 73 (69–75) | 71 (68–72) | 0.8 |
| Albumin [g/L] | 26 | 40 (36–42) | 37 (35–39) | 26 (24–29) | 0.001 |
| Chloride [mmol/L] | 25 | 100.0 (97.5–101.5) | 100.7 (98.0–103.0) | 104.0 (102.0–107.0) | 0.079 |
| Magnesium [mmol/L] | 14 | 0.85 (0.85–0.85) | 0.79 (0.76–1.00) | 0.94 (0.89–1.09) | 0.4 |
| Potassium [mmol/L] | 26 | 4.80 (4.55–5.05) | 4.10 (3.80–4.40) | 4.20 (3.60–4.60) | 0.11 |
| Sodium [mmol/L] | 27 | 136 (134–138) | 138 (136–140) | 139 (137–148) | 0.2 |
| Bicarbonate [mmol/L] | 23 | 25.0 (24.0–26.0) | 26.0 (21.5–27.0) | 23.0 (22.0–28.0) | >0.9 |
| Calcium [mmol/L] | 26 | 2.32 (2.25–2.37) | 2.38 (2.34–2.44) | 2.19 (2.14–2.30) | 0.093 |
| Adjusted calcium [mmol/L] | 26 | 2.29 (2.24–2.39) | 2.46 (2.35–2.46) | 2.46 (2.45–2.52) | 0.039 |
| Vitamin D [ng/mL] | 14 | 23 (20–24) | 23 (14–32) | 32 (23–39) | 0.6 |
| Procalcitonin [ng/mL] | 13 | 0.25 (0.25–0.25) | 0.38 (0.22–0.38) | 0.26 (0.10–0.72) | >0.9 |
| Bilirubin [mg/dL] | 25 | 5 (5–16) | 9 (5–12) | 10 (7–30) | 0.4 |
| Urea [mmol/L] | 27 | 4 (4–6) | 4 (4–5) | 16 (10–20) | <0.001 |
| Uric acid [µmol/L] | 19 | 295 (282–311) | 229 (210–270) | 355 (337–374) | 0.2 |
| Creatinine [µmol/L] | 27 | 85 (73–99) | 78 (69–100) | 115 (83–168) | 0.3 |
| ALT [U/L] | 23 | 18 (17–25) | 34 (20–55) | 59 (20–134) | 0.2 |
| AST [U/L] | 20 | 23 (18–33) | 27 (24–34) | 34 (28–110) | 0.2 |
| CK [U/L] | 14 | 129 (129–129) | 97 (62–173) | 103 (62–239) | 0.8 |
| ALP [U/L] | 25 | 79 (66–115) | 66 (61–93) | 78 (45–143) | 0.9 |
| LDH [U/L] | 14 | 183 (183–183) | 337 (226–343) | 332 (294–375) | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parray, A.; Mir, F.A.; Doudin, A.; Iskandarani, A.; Danjuma, I.M.M.; Kuni, R.A.T.; Abdelmajid, A.; Abdelhafez, I.; Arif, R.; Mulhim, M.; et al. SnoRNAs and miRNAs Networks Underlying COVID-19 Disease Severity. Vaccines 2021, 9, 1056. https://doi.org/10.3390/vaccines9101056
Parray A, Mir FA, Doudin A, Iskandarani A, Danjuma IMM, Kuni RAT, Abdelmajid A, Abdelhafez I, Arif R, Mulhim M, et al. SnoRNAs and miRNAs Networks Underlying COVID-19 Disease Severity. Vaccines. 2021; 9(10):1056. https://doi.org/10.3390/vaccines9101056
Chicago/Turabian StyleParray, Aijaz, Fayaz Ahmad Mir, Asmma Doudin, Ahmad Iskandarani, Ibn Mohammed Masud Danjuma, Rahim Ayadathil Thazhhe Kuni, Alaaedin Abdelmajid, Ibrahim Abdelhafez, Rida Arif, Mohammad Mulhim, and et al. 2021. "SnoRNAs and miRNAs Networks Underlying COVID-19 Disease Severity" Vaccines 9, no. 10: 1056. https://doi.org/10.3390/vaccines9101056
APA StyleParray, A., Mir, F. A., Doudin, A., Iskandarani, A., Danjuma, I. M. M., Kuni, R. A. T., Abdelmajid, A., Abdelhafez, I., Arif, R., Mulhim, M., Abukhattab, M., Dar, S. R., Moustafa, A.-E. A., Elkord, E., Al Khal, A. L., Elzouki, A.-N., & Cyprian, F. (2021). SnoRNAs and miRNAs Networks Underlying COVID-19 Disease Severity. Vaccines, 9(10), 1056. https://doi.org/10.3390/vaccines9101056








