Passive Immunization with Recombinant Antibody VLRB-PirAvp/PirBvp—Enriched Feeds against Vibrio parahaemolyticus Infection in Litopenaeus vannamei Shrimp
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Toxin Plasmids
2.2. Expression and Purification of Recombinant Toxins
2.3. Screening of VLRB Library
2.4. Establishment of Cell Line Secreting Anti-PirAvp/PirBvp Recombinant VLRBs
2.5. Bacterial Challenge Test
2.6. Statistical Analysis
3. Results
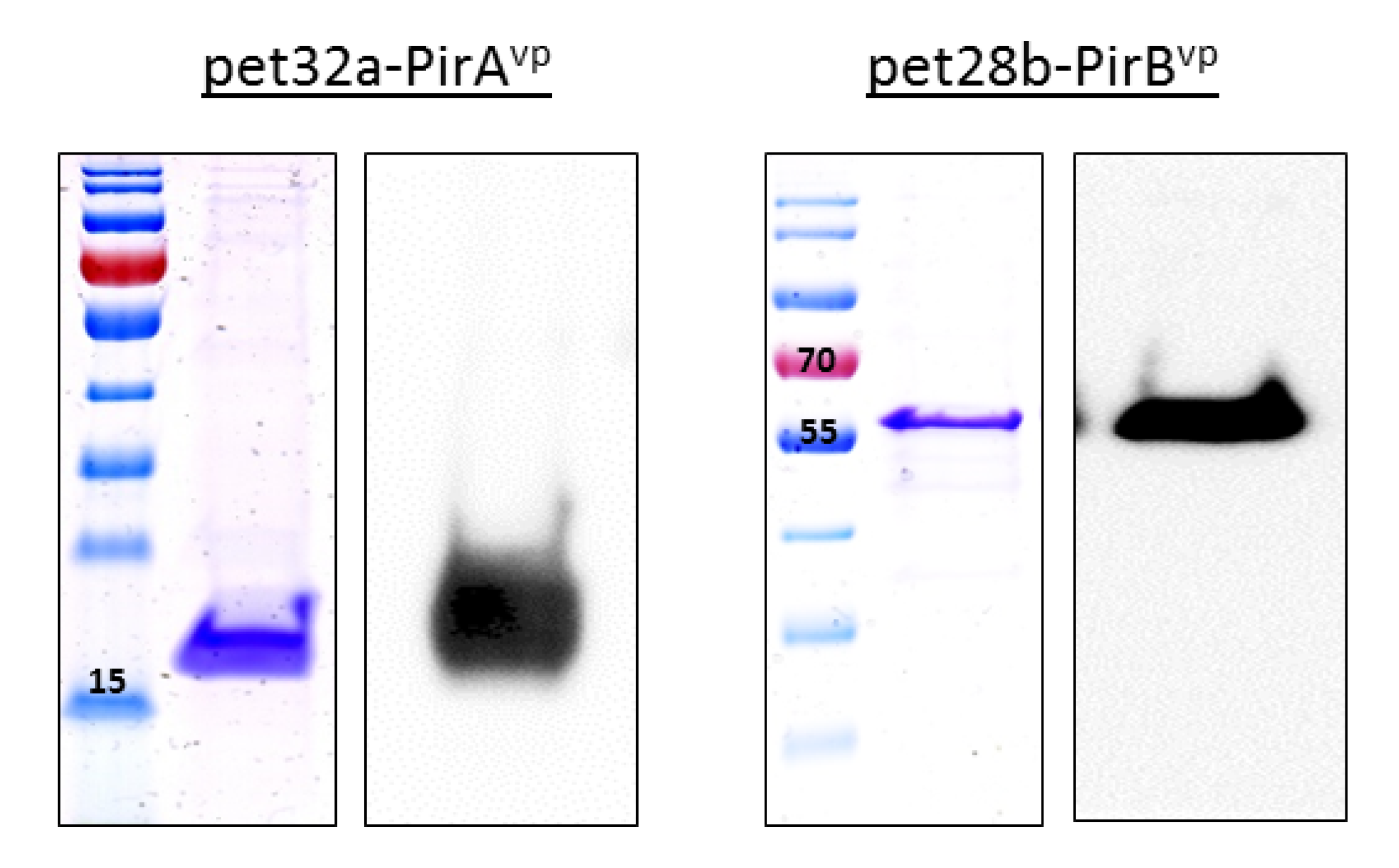
3.1. Expression of Recombinant Toxins
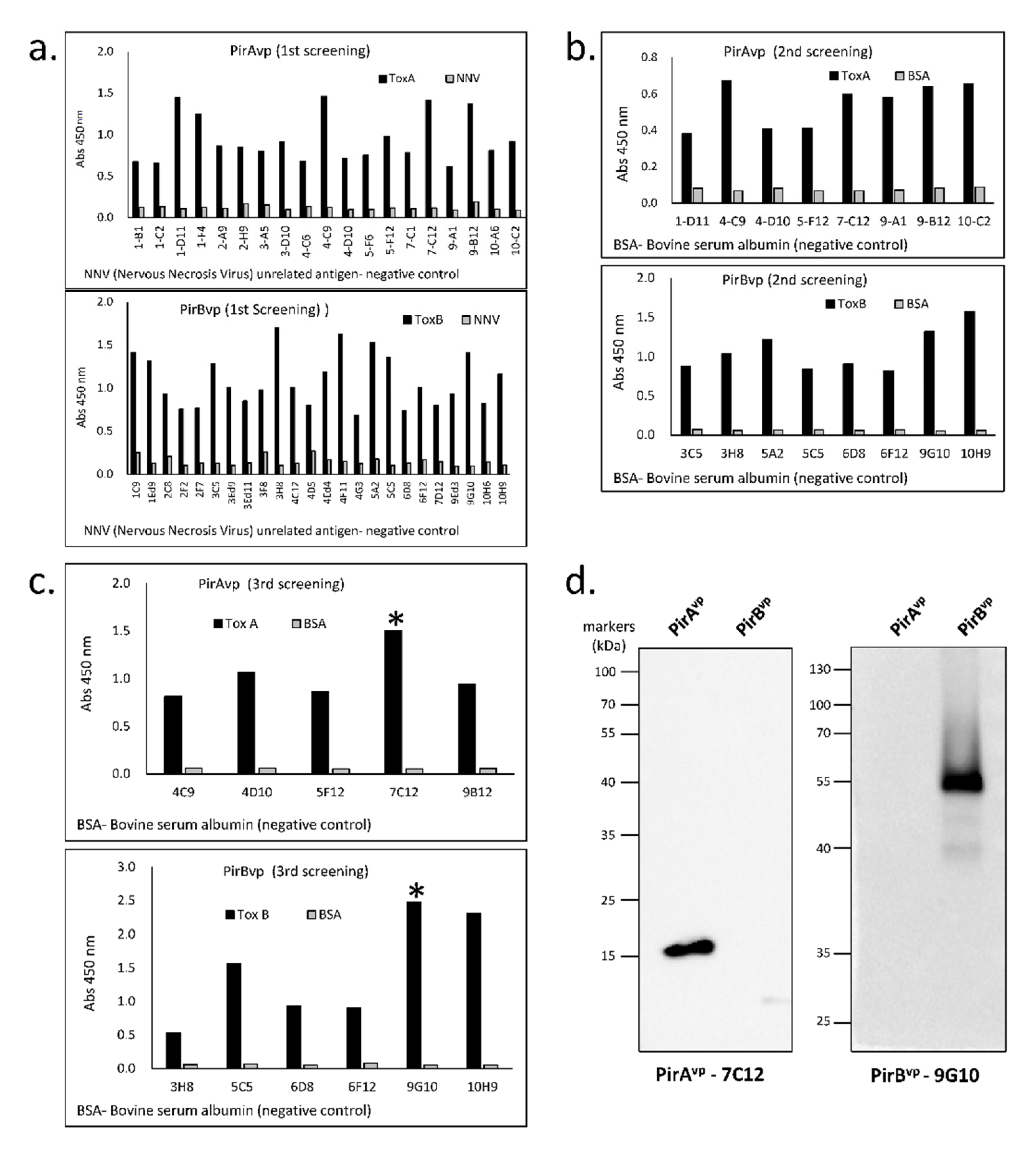
3.2. Specificity of Established Toxin-Specific VLRBs
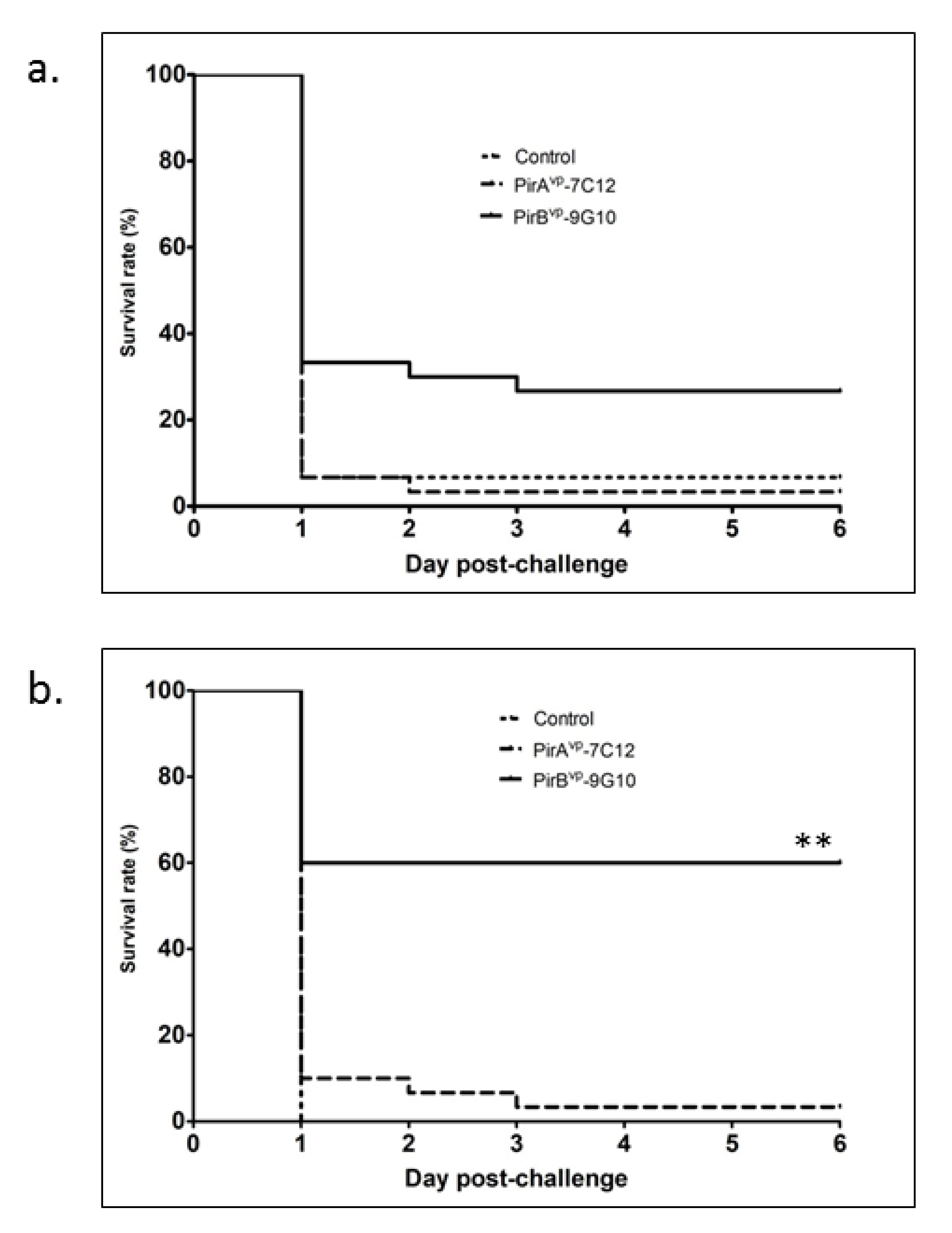
3.3. Protective Efficiency of Toxin-Specific VLRBs in Shrimp Infected with V. parahaemolyticus
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Qiu, L.M.; Song, L.S.; Zhang, H.; Zhao, J.M.; Wang, L.L.; Yu, Y.D.; Li, C.H.; Li, F.M.; Xing, K.Z.; et al. Cloning and characterization of a novel C-type lectin gene from shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2009, 26, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gil, B.; Soto-Rodriguez, S.; Lozano, R.; Betancourt-Lozano, M. Draft genome sequence of Vibrio parahaemolyticus strain M0605, which causes severe mortalities of shrimps in Mexico. Genome Announc. 2014, 2, e00055-14. [Google Scholar] [CrossRef] [PubMed]
- Nunan, L.; Lightner, D.; Pantoja, C.; Gomez-Jimenez, S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis. Aquat. Org. 2014, 111, 81–86. [Google Scholar] [CrossRef]
- De la Peña, L.D.; Cabillon, N.A.; Catedral, D.D.; Amar, E.C.; Usero, R.C.; Monotilla, W.D.; Calpe, A.T.; Fernandez, D.D.; Saloma, C.P. Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeus vannamei and P. monodon cultured in the Philippines. Dis. Aquat. Org. 2015, 116, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, L.; Bayot, B.; Betancourt, I.; Pinzon, A. Draft genome sequence of pathogenic bacteria Vibrio parahaemolyticus strain Ba94C2, associated with acute hepatopancreatic necrosis disease isolate from South America. Genom. Data 2016, 9, 143–144. [Google Scholar] [CrossRef]
- NACA. Report of the Asia Pacific Emergency Regional Consultation on the Emerging Shrimp Disease: Early Mortality Syndrome (EMS)/Acute Hepatopancreatic Necrosis Syndrome (AHPNS); Network of Aquaculture Centres in Asia-Pacific: Bangkok, Thailand, 9–10 August 2012. [Google Scholar]
- De Schryver, P.; Defoirdt, T.; Sorgeloos, P. Early mortality syndrome outbreaks: A microbial management issue in shrimp farming. PLoS Pathog. 2014, 10, e1003919. [Google Scholar] [CrossRef]
- Lai, H.C.; Ng, T.H.; Ando, M.; Lee, C.T.; Chen, I.T.; Chuang, J.C.; Mavichak, R.; Chang, S.H.; Yeh, M.D.; Chiang, Y.A.; et al. Pathogenesis of acute hepatopancreatic necrosis disease (AHPND) in shrimp. Fish Shellfish Immunol. 2015, 47, 1006–1014. [Google Scholar] [CrossRef]
- Broberg, C.A.; Calder, T.J.; Orth, K. Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect. 2011, 13, 992–1001. [Google Scholar] [CrossRef]
- Zhang, L.; Orth, K. Virulence determinants for Vibrio parahaemolyticus infection. Curr. Opin. Microbiol. 2013, 16, 70–77. [Google Scholar] [CrossRef]
- Lee, C.T.; Chen, I.T.; Yang, Y.T.; Ko, T.P.; Huang, Y.T.; Huang, J.Y.; Huang, M.F.; Lin, S.J.; Chen, C.Y.; Lin, S.S. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef]
- Sirikharin, R.; Taengchaiyaphum, S.; Sanguanrut, P.; Chi, T.D.; Mavichak, R.; Proespraiwong, P.; Nuangsaeng, B.; Thitamadee, S.; Flegel, T.W.; Sritunyalucksana, K. Characterization and PCR detection of binary, Pir-like toxins from Vibrio parahaemolyticus isolates that cause acute hepatopancreatic necrosis disease (AHPND) in shrimp. PLoS ONE 2015, 10, e0126987. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.R.M.; Paganini, J.; Pontarotti, P. Convergent evolution of the adaptive immune response in jawed vertebrates and cyclostomes: An evolutionary biology approach based study. Dev. Comp. Immunol. 2017, 75, 120–126. [Google Scholar] [CrossRef]
- Alder, M.N.; Rogozin, I.B.; Iyer, L.M.; Glazko, G.V.; Cooper, M.D.; Pancer, Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science 2005, 310, 1970–1973. [Google Scholar] [CrossRef]
- Herrin, B.R.; Alder, M.N.; Roux, K.H.; Sina, C.; Ehrhardt, G.R.A.; Boydston, J.A.; Turnbough, C.L., Jr.; Cooper, M.D. Structure and specificity of lamprey monoclonal antibodies. Proc. Natl. Acad. Sci. USA 2008, 105, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Das, S.; Herrin, B.R.; Hirano, M.; Cooper, M.D. Definition of a third VLR gene in hagfish. Proc. Natl. Acad. Sci. USA 2013, 110, 15013–15018. [Google Scholar] [CrossRef]
- Fujii, T.; Nakagawa, H.; Murakawa, S. Immunity in lamprey. I. Production of haemolytic and haemagglutinating antibody to sheep red blood cells in Japanese lampreys. Dev. Comp. Immunol. 1979, 3, 441–451. [Google Scholar] [CrossRef]
- Alder, M.N.; Herrin, B.R.; Sadlonova, A.; Stockard, C.R.; Grizzle, W.E.; Gartland, L.A.; Gartland, G.L.; Boydston, J.A.; Turnbough, C.L., Jr.; Cooper, M.D. Antibody responses of variable lymphocyte receptors in the lamprey. Nat. Immunol. 2008, 9, 319–327. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.; Im, S.P.; Kim, S.W.; Lazarte, J.M.; Jung, J.W.; Gong, T.W.; Kim, Y.R.; Lee, J.H.; Kim, H.J.; et al. Generation and characterization of hagfish variable lymphocyte receptor B against glycoprotein of viral hemorrhagic septicemia virus (VHSV). Mol. Immunol. 2018, 99, 30–38. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, J.S.; Kim, J.; Im, S.P.; Kim, S.W.; Lazarte, J.; Kim, Y.R.; Chun, J.H.; Ha, M.W.; Kim, H.S.; et al. Characterization of Hagfish (Eptatretus burgeri) Variable Lymphocyte Receptor-Based Antibody and Its Potential Role in the Neutralization of Nervous Necrosis Virus. J. Immunol. 2020, 204, 718–725. [Google Scholar] [CrossRef]
- Available online: www.fao.org/in-action/globefish/market-reports/resource-detail/en/c/1261310 (accessed on 5 November 2020).
- Gottstein, B.; Hemmeler, E. Egg yolk immunoglobulin Y as an alternative antibody in the serology of echinococcosis. Z. Parasitenkd. 1985, 71, 273–278. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Lin, L.; Yao, D.; Sun, J.; Du, X.; Zhang, Y. Passive Immune-Protection of Litopenaeus vannamei against Vibrio harveyi and Vibrio parahaemolyticus Infections with Anti-Vibrio Egg Yolk (IgY)-Encapsulated Feed. Int. J. Mol. Sci. 2016, 17, 723. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, T.; Thirumalaikumar, E.; Lelin, C.; Palanikumar, P.; Michaelbabu, M.; Citarasu, T. Physicochemical properties of anti Vibrio harveyi egg yolk antibody (IgY) and its immunological influence in Indian white shrimp Fenneropenaeus indicus. Fish Shellfish Immunol. 2018, 74, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, X.; Guo, E.; Zhou, P.; Xu, D.; Qi, Z.; Deng, L.J. The preparation and antibacterial effect of egg yolk immunoglobulin (IgY) against the outer membrane proteins of Vibrio parahaemolyticus. Sci. Food Agric. 2019, 99, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Pedrosa-Gerasmio, I.R.; Alenton, R.R.R.; Nozaki, R.; Kondo, H.; Hirono, I. Anti-PirA-like toxin immunoglobulin (IgY) in feeds passively immunizes shrimp against acute hepatopancreatic necrosis disease. J. Fish Dis. 2019, 42, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Waterfield, N.; Kamita, S.G.; Hammock, B.D.; Ffrench-Constant, R. The Photorhabdus Pir toxins are similar to a developmentally regulated insect protein but show no juvenile hormone esterase activity. FEMS Microbiol. Lett. 2005, 245, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Hsu, K.C.; Wang, H.C. Structural Insights into the Cytotoxic Mechanism of Vibrio parahaemolyticus PirAvp and PirBvp Toxins. Mar. Drugs. 2017, 15, 373. [Google Scholar] [CrossRef]
- Peraro, M.D.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2015, 14, 77–92. [Google Scholar] [CrossRef]
- Kitami, M.; Kadotani, T.; Nakanishi, K.; Atsumi, S.; Higurashi, S.; Ishizaka, T.; Watanabe, A.; Sato, R. Bacillus thuringiensis Cry Toxins Bound Specifically to Various Proteins via Domain III, Which Had a Galactose-Binding Domain-Like Fold. Biosci. Biotechnol. Biochem. 2011, 75, 305–312. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef]
- Adang, M.; Crickmore, N.; Jurat-Fuentes, J. Diversity of Bacillus thuringiensis Crystal Toxins and Mechanism of Action. Adv. Insect Physiol. 2014, 47, 39–87. [Google Scholar]
- Bravo, A.; Gómez, I.; Porta, H.; García-Gómez, B.I.; Rodriguez-Almazan, C.; Pardo, L.; Soberón, M. Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microb. Biotechnol. 2012, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Grochulski, P.; Masson, L.; Borisova, S.; Pusztai-Carey, M.; Schwartz, J.L.; Brousseau, R.; Cygler, M. Bacillus thuringiensis CryIA(a) insecticidal toxin: Crystal structure and channel formation. J. Mol. Biol. 1995, 254, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Soberón, M.; Pardo, L.; Muñóz-Garay, C.; Sánchez, J.; Gómez, I.; Porta, H.; Bravo, A. Pore formation by Cry toxins. Adv. Exp. Med. Biol. 2010, 677, 127–142. [Google Scholar]
- Pigott, C.R.; Ellar, D.J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef]
- Im, S.P.; Kim, J.; Lee, J.S.; Kim, S.W.; Jung, J.W.; Lazarte, J.M.S.; Kim, J.Y.; Kim, Y.R.; Lee, J.H.; Chong, R.S.M.; et al. Potential use of genetically engineered variable lymphocyte receptor B specific to avian influenza virus H9N2. J. Immunol. 2018, 201, 3119–3128. [Google Scholar] [CrossRef]
- Loc Tran, L.; Nunan, L.; Redman, R.; Mohney, L.; Pantoja, C.; Fitzsimmons, K.; Lightner, D. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar] [CrossRef]
- Joshi, J.; Srisala, J.; Truong, V.T.; Chen, I.-T.; Nuangsaeng, N.; Suthienkul, O.; Lo, C.L.; Flegel, T.W.; Sritunyalucksana, K.; Thitamadee, S. Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND). Aquaculture 2014, 428, 297–302. [Google Scholar] [CrossRef]
- Kim, J.; Im, S.P.; Lee, J.S.; Lazarte, J.; Kim, S.W.; Jung, J.W.; Kim, J.Y.; Kim, Y.R.; Lee, S.; Kim, G.J.; et al. Globular-shaped variable lymphocyte receptors B antibody multimerized by a hydrophobic clustering in hagfish. Sci. Rep. 2018, 8, 10801. [Google Scholar] [CrossRef]
- Tinwongger, S.; Nochiri, Y.; Thawonsuwan, J.; Nozaki, R.; Kondo, H.; Awasthi, S.; Hinenoya, A.; Yamasaki, S.; Hirono, I. Virulence of acute hepatopancreatic necrosis disease PirAB-like relies on secreted proteins not on gene copy number. J. Appl. Microbiol. 2016, 121, 1755–1765. [Google Scholar] [CrossRef]
| Name | Sequence (5′-3′) | Reference/Accession No. |
|---|---|---|
| For PirABvp amplification | ||
| p32-ToxA/Nco1-Fwd | TATACCATGGAGTAACAATATAAAACATG | AB972427.1 |
| p32-ToxA/Sac1-Rev | GATGAGCTCTTAGTGGTAATAGATTGTAC | AB972427.1 |
| p2b-ToxB/Nco1-Fwd: | ATACCATGGGCATGACTAACGAATACGTTGTAACAATGTC | AB972427.1 |
| p2b-ToxB-TStrep/STOP/Sac1/Rev | AGCGAGCTCTCACTTTTCAAACTGCGGATGGCTCCACGCGCTGCCACCGCTACCGCCACCGCTACCGCCACCTTTCTCAAACTGTGGATGGCT | AB972427.1 |
| For cell line | ||
| LRRNT Sfi I forward | AGGCCACCGGGGCCTGTCCTTCACGGTGTTCCTG | [19] |
| Stalk Sfi I reverse | TGGCCCAGAGGCCCGCGTTCATGACACGGCCGA | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarte, J.M.S.; Kim, Y.R.; Lee, J.S.; Chun, J.H.; Kim, S.W.; Jung, J.W.; Kim, J.; Kayansamruaj, P.; Thompson, K.D.; Kim, H.; et al. Passive Immunization with Recombinant Antibody VLRB-PirAvp/PirBvp—Enriched Feeds against Vibrio parahaemolyticus Infection in Litopenaeus vannamei Shrimp. Vaccines 2021, 9, 55. https://doi.org/10.3390/vaccines9010055
Lazarte JMS, Kim YR, Lee JS, Chun JH, Kim SW, Jung JW, Kim J, Kayansamruaj P, Thompson KD, Kim H, et al. Passive Immunization with Recombinant Antibody VLRB-PirAvp/PirBvp—Enriched Feeds against Vibrio parahaemolyticus Infection in Litopenaeus vannamei Shrimp. Vaccines. 2021; 9(1):55. https://doi.org/10.3390/vaccines9010055
Chicago/Turabian StyleLazarte, Jassy Mary S., Young Rim Kim, Jung Seok Lee, Jin Hong Chun, Si Won Kim, Jae Wook Jung, Jaesung Kim, Pattanapon Kayansamruaj, Kim D. Thompson, Hyeongsu Kim, and et al. 2021. "Passive Immunization with Recombinant Antibody VLRB-PirAvp/PirBvp—Enriched Feeds against Vibrio parahaemolyticus Infection in Litopenaeus vannamei Shrimp" Vaccines 9, no. 1: 55. https://doi.org/10.3390/vaccines9010055
APA StyleLazarte, J. M. S., Kim, Y. R., Lee, J. S., Chun, J. H., Kim, S. W., Jung, J. W., Kim, J., Kayansamruaj, P., Thompson, K. D., Kim, H., & Jung, T. S. (2021). Passive Immunization with Recombinant Antibody VLRB-PirAvp/PirBvp—Enriched Feeds against Vibrio parahaemolyticus Infection in Litopenaeus vannamei Shrimp. Vaccines, 9(1), 55. https://doi.org/10.3390/vaccines9010055






