Post-Vaccination Yellow Fever Antiserum Reduces Zika Virus in Embryoid Bodies When Placental Cells are Present
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Virus Propagation
2.2. Embryoid Body Formation and Imaging
2.3. Monolayer Infection and Imaging
2.4. ELISA
Cross-Reactivity Assay
2.5. Embryoid Body Antibody Assay
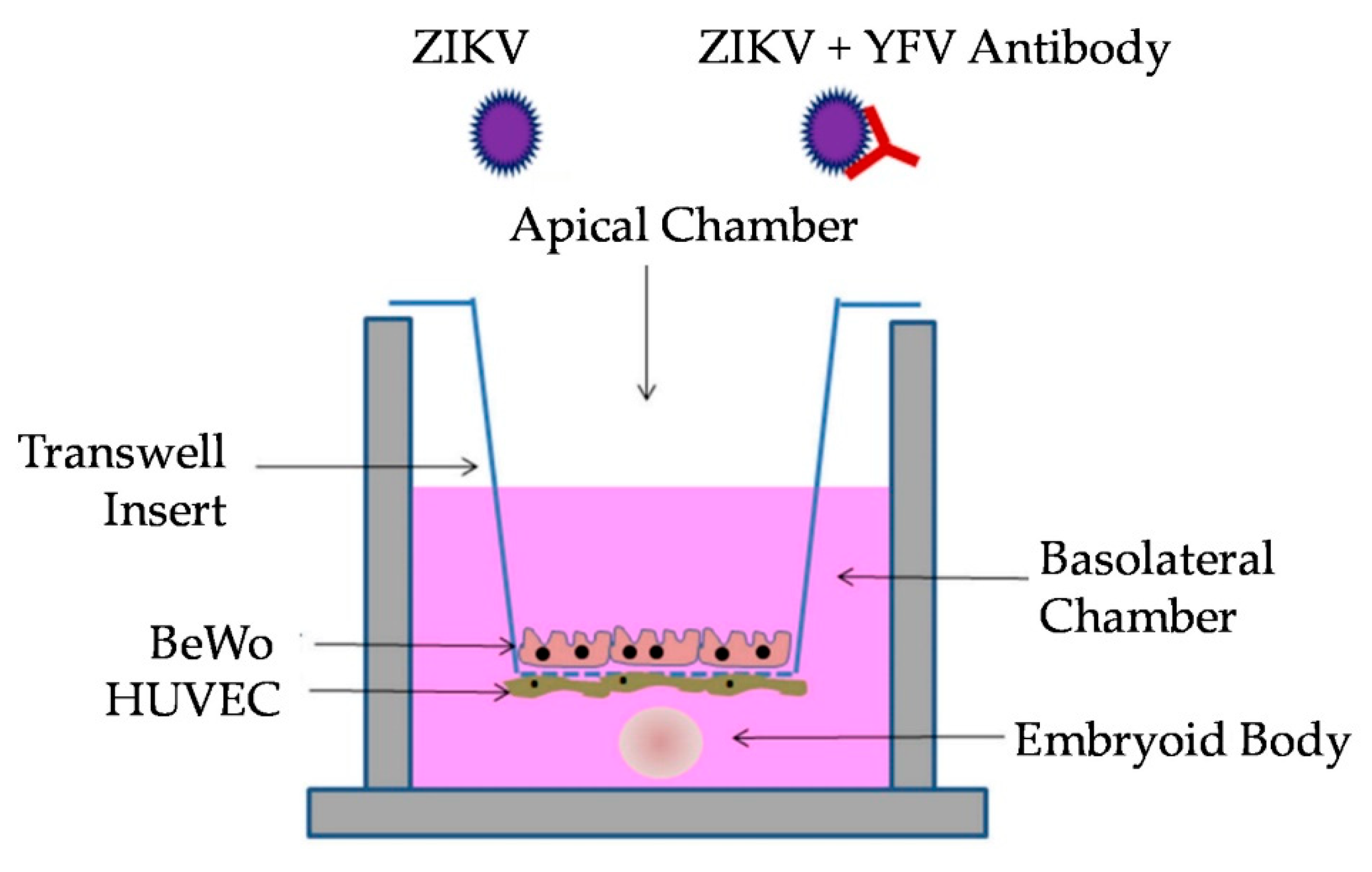
2.6. Transwell Co-Culture
2.7. Transwell Antibody Assay
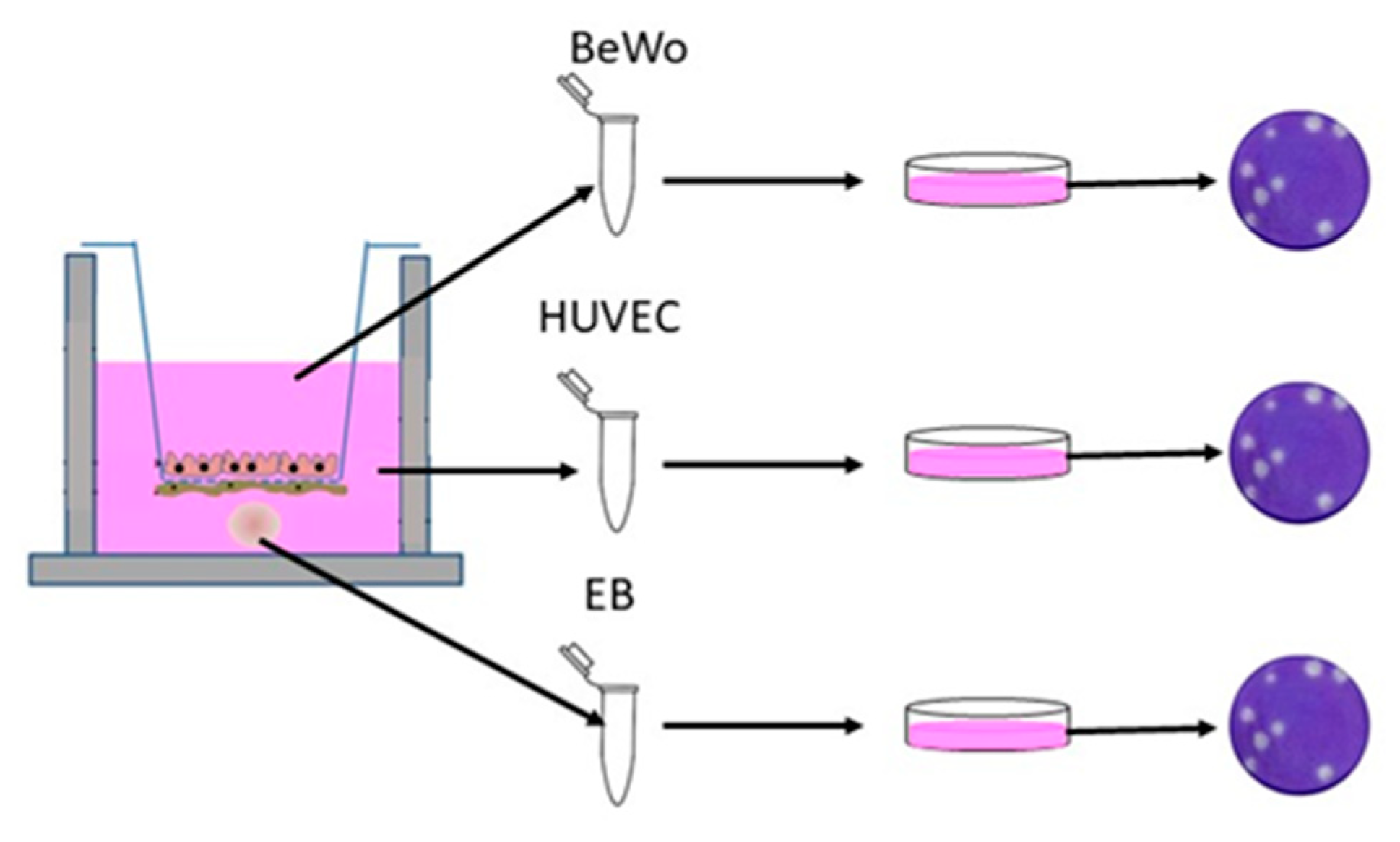
2.8. Viral Quantification
3. Results
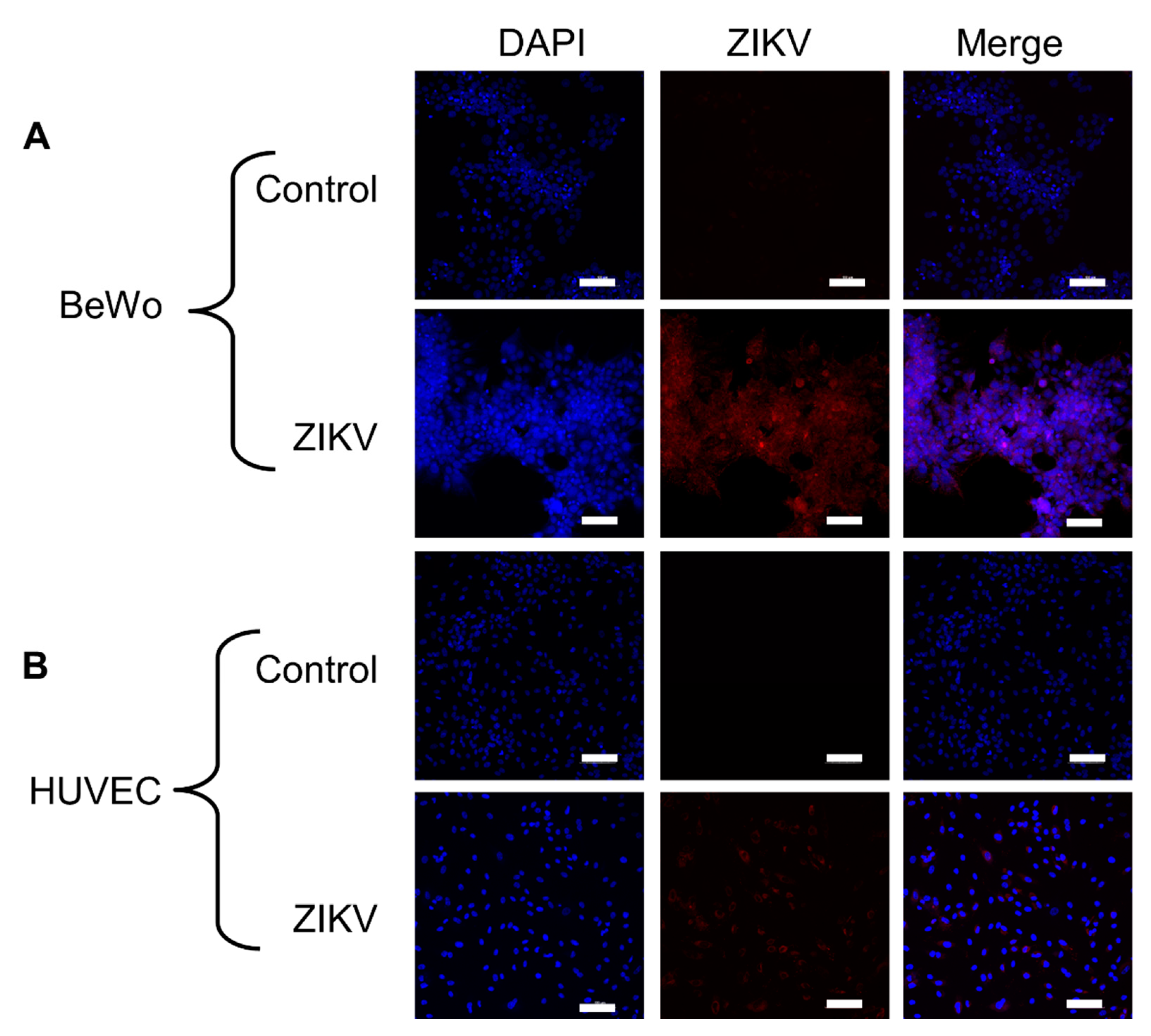
3.1. Placental Cells Are Permissive to ZIKV Infection
3.2. YFV Post-Vaccination Antiserum Is Cross-Reactive with ZIKV Antigen
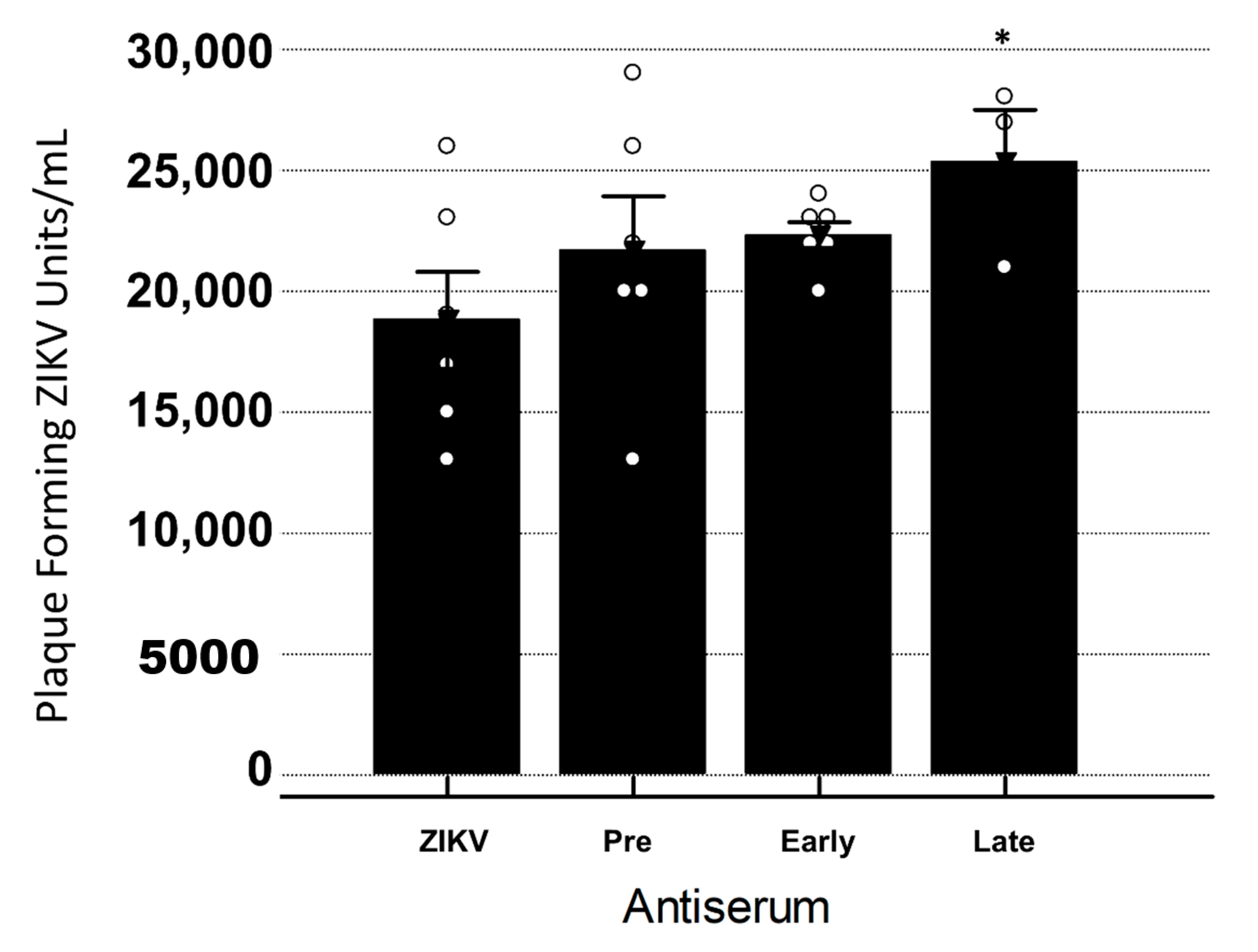
3.3. Late YFV Post-Vaccination Antiserum Enhances ZIKV In Vero Cells
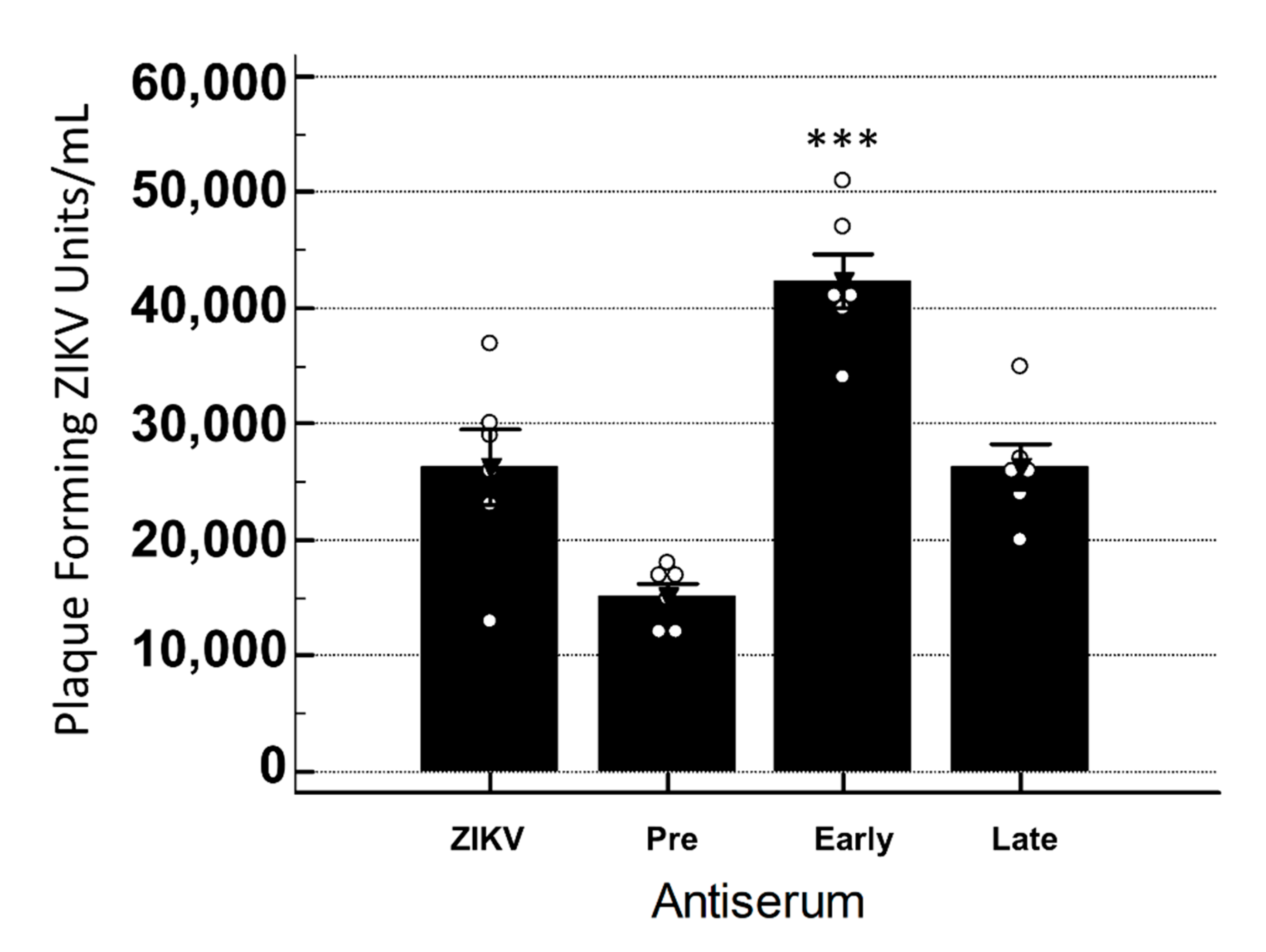
3.4. YFV Early Antiserum Enhances ZIKV in Embryoid Bodies
3.5. YFV Late Antiserum Reduces ZIKV Infection of EBs in Co-Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Charlier, C.; Beaudoin, M.-C.; Couderc, T.; Lortholary, O.; Lecuit, M. Arboviruses and pregnancy: Maternal, fetal, and neonatal effects. Lancet Child Adolesc. Health 2017, 1, 134–146. [Google Scholar] [CrossRef]
- Haddow, A.J.; Williams, M.C.; Woodall, J.P.; Simpson, D.I.; Goma, L.K. Twelve Isolations of Zika Virus From Aedes (Stegomyia) Africanus (Theobald) Taken in and Above a Uganda Forest. Bull. World Health Organ. 1964, 31, 57–69. [Google Scholar] [PubMed]
- Zanluca, C.; Melo, V.C.A.d.; Mosimann, A.L.P.; Santos, G.I.V.d.; Santos, C.N.D.d.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Memórias Do Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Douam, F.; Ploss, A. Yellow Fever Virus: Knowledge Gaps Impeding the Fight Against an Old Foe. Trends Microbiol. 2018, 26, 913–928. [Google Scholar] [CrossRef]
- Barrett, A.D.T. Yellow fever live attenuated vaccine: A very successful live attenuated vaccine but still we have problems controlling the disease. Vaccine 2017, 35, 5951–5955. [Google Scholar] [CrossRef]
- WHO. WHO Vaccine-preventable Diseases: Monitoring System. Available online: https://apps.who.int/immunization_monitoring/globalsummary/diseases?dc%5Bt%5D=m&dc%5Bgivenfrom%5D=0&dc%5Bgivento%5D=791&dc%5Br%5D%5B%5D=AMRO&dc%5Bd%5D%5B%5D=Yellow+fever&commit=Ok+with+the+selection (accessed on 7 April 2020).
- WHO. Yellow Fever- Brazil. Available online: https://www.who.int/csr/don/18-april-2019-yellow-fever-brazil/en/ (accessed on 20 May 2020).
- Netto, E.M.; Moreira-Soto, A.; Pedroso, C.; Höser, C.; Funk, S.; Kucharski, A.J.; Rockstroh, A.; Kümmerer, B.M.; Sampaio, G.S.; Luz, E.; et al. High Zika Virus Seroprevalence in Salvador, Northeastern Brazil Limits the Potential for Further Outbreaks. mBio 2017, 8, e01390-17. [Google Scholar] [CrossRef]
- Wen, J.; Shresta, S. Antigenic cross-reactivity between Zika and dengue viruses: Is it time to develop a universal vaccine? Curr. Opin. Immunol. 2019, 59, 1–8. [Google Scholar] [CrossRef]
- Willis, E.; Hensley, S.E. Characterization of Zika virus binding and enhancement potential of a large panel of flavivirus murine monoclonal antibodies. Virology 2017, 508, 1–6. [Google Scholar] [CrossRef]
- Hermanns, K.; Göhner, C.; Kopp, A.; Schmidt, A.; Merz, W.M.; Markert, U.R.; Junglen, S.; Drosten, C. Zika virus infection in human placental tissue explants is enhanced in the presence of dengue virus antibodies in-vitro. Emerg. Microbes Infect. 2018, 7, 198. [Google Scholar] [CrossRef]
- Souza, N.C.S.e.; Félix, A.C.; de Paula, A.V.; Levi, J.E.; Pannuti, C.S.; Romano, C.M. Evaluation of serological cross-reactivity between yellow fever and other flaviviruses. Int. J. Infect. Dis. 2019, 81, 4–5. [Google Scholar] [CrossRef]
- CDC. Zika MAC-ELISA: Instructions for Use; CDC: Atlanta, GA, USA, 2018. [Google Scholar]
- WHO. Global Measles and Rubella Update May 2020. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_monthlydata/en/ (accessed on 21 May 2020).
- Pierson, T.C.; Diamond, M.S. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev. Mol. Med. 2008, 10, e12. [Google Scholar] [CrossRef]
- Throsby, M.; Geuijen, C.; Goudsmit, J.; Bakker, A.Q.; Korimbocus, J.; Kramer, R.A.; Clijsters-van der Horst, M.; de Jong, M.; Jongeneelen, M.; Thijsse, S.; et al. Isolation and Characterization of Human Monoclonal Antibodies from Individuals Infected with West Nile Virus. J. Virol. 2006, 80, 6982. [Google Scholar] [CrossRef]
- Schlesinger, J.J.; Chapman, S. Neutralizing F(ab’)2 fragments of protective monoclonal antibodies to yellow fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J. Gen. Virol. 1995, 76 Pt 1, 217–220. [Google Scholar] [CrossRef]
- Oliphant, T.; Nybakken, G.E.; Engle, M.; Xu, Q.; Nelson, C.A.; Sukupolvi-Petty, S.; Marri, A.; Lachmi, B.E.; Olshevsky, U.; Fremont, D.H.; et al. Antibody recognition and neutralization determinants on domains I and II of West Nile virus envelope protein. J. Virol. 2006, 80, 12149–12159. [Google Scholar] [CrossRef]
- Staples, J.E.; Bocchini, J.A.; Rubin, L.; Fischer, M. Yellow Fever Vaccine Booster Doses: Recommendations of the Advisory Committee on Immunization Practices. 2015. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6423a5.htm (accessed on 20 May 2020).
- Thomas, R.E.; Lorenzetti, D.L.; Spragins, W.; Jackson, D.; Williamson, T. The safety of yellow fever vaccine 17D or 17DD in children, pregnant women, HIV+ individuals, and older persons: Systematic review. Am. J. Trop. Med. Hyg. 2012, 86, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Gotuzzo, E.; Yactayo, S.; Córdova, E. Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013, 89, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.D.; Pierson, T.C.; McAllister, D.; Hanna, S.L.; Puffer, B.A.; Valentine, L.E.; Murtadha, M.M.; Hoxie, J.A.; Doms, R.W. Characterization of neutralizing antibodies to West Nile virus. Virology 2005, 336, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Foo, S.S.; Bruzone, R.; Dihn, L.V.; King, N.J.; Mahalingam, S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015, 268, 24. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Kawaoka, Y. Antibody-dependent enhancement of viral infection: Molecular mechanisms and in vivo implications. Rev. Med Virol. 2003, 13, 387–398. [Google Scholar] [CrossRef]
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe 2017, 21, 561–567. [Google Scholar] [CrossRef]
- Schmidt, A.; Morales-Prieto, D.M.; Pastuschek, J.; Fröhlich, K.; Markert, U.R. Only humans have human placentas: Molecular differences between mice and humans. J. Reprod. Immunol. 2015, 108, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.S.; Rytting, E.; Mose, T.; Knudsen, L.E. Modeling placental transport: Correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion. Toxicol. Vitr. 2009, 23, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.R.; Pozor, M.A.; Pu, R.; Barr, K.L.; Beachboard, S.E.; MacLachlan, N.J.; Prakoso, D.; Long, M.T. Experimental Infection of Pregnant Female Sheep with Zika Virus During Early Gestation. Viruses 2019, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, R.J.; Haese, N.N.; Smith, J.L.; Colgin, L.M.A.; MacAllister, R.P.; Greene, J.M.; Parkins, C.J.; Kempton, J.B.; Porsov, E.; Wang, X.; et al. A neonatal nonhuman primate model of gestational Zika virus infection with evidence of microencephaly, seizures and cardiomyopathy. PLoS ONE 2020, 15, e0227676. [Google Scholar] [CrossRef] [PubMed]
- Appelt-Menzel, A.; Cubukova, A.; Günther, K.; Edenhofer, F.; Piontek, J.; Krause, G.; Stüber, T.; Walles, H.; Neuhaus, W.; Metzger, M. Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells. Stem Cell Rep. 2017, 8, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Aquino, G.V.; Dabi, A.; Bruce, E.D. Assessing the translocation of silver nanoparticles using an in vitro co-culture model of human airway barrier. Toxicol. Vitr. 2019, 56, 1–9. [Google Scholar] [CrossRef]
- Dekali, S.; Gamez, C.; Kortulewski, T.; Blazy, K.; Rat, P.; Lacroix, G. Assessment of an in vitro model of pulmonary barrier to study the translocation of nanoparticles. Toxicol. Rep. 2014, 1, 157–171. [Google Scholar] [CrossRef]
- Garcia, C.M.; Darland, D.C.; Massingham, L.J.; D’Amore, P.A. Endothelial cell–astrocyte interactions and TGFβ are required for induction of blood–neural barrier properties. Dev. Brain Res. 2004, 152, 25–38. [Google Scholar] [CrossRef]
- Cartwright, L.; Poulsen, M.S.; Nielsen, H.M.; Pojana, G.; Knudsen, L.E.; Saunders, M.; Rytting, E. In vitro placental model optimization for nanoparticle transport studies. Int. J. Nanomed. 2012, 7, 497–510. [Google Scholar] [CrossRef]
- Aengenheister, L.; Keevend, K.; Muoth, C.; Schonenberger, R.; Diener, L.; Wick, P.; Buerki-Thurnherr, T. An advanced human in vitro co-culture model for translocation studies across the placental barrier. Sci. Rep. 2018, 8, 5388. [Google Scholar] [CrossRef]
- Myllynen, P.; Vähäkangas, K. Placental transfer and metabolism: An overview of the experimental models utilizing human placental tissue. Toxicol. In Vitro. 2013, 27, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, L.; Lacconi, V.; Massimiani, M.; Magrini, A.; Pietroiusti, A. In vitro experimental models to study the efficiency of the placental barrier for environmental toxicants: Tumor cell lines versus trophoblast primary cells. Biomed. Prev. 2018, 1. [Google Scholar] [CrossRef]
- Orendi, K.; Kivity, V.; Sammar, M.; Grimpel, Y.; Gonen, R.; Meiri, H.; Lubzens, E.; Huppertz, B. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta 2011, 32, S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Evseenko, D.A.; Paxton, J.W.; Keelan, J.A. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1357–R1365. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S. Cell Types of the Placenta. In Vascular Biology of the Placenta; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Heaton, S.J.; Eady, J.J.; Parker, M.L.; Gotts, K.L.; Dainty, J.R.; Fairweather-Tait, S.J.; McArdle, H.J.; Srai, K.S.; Elliott, R.M. The use of BeWo cells as an in vitro model for placental iron transport. Am. J. Physiol. Cell Physiol. 2008, 295, C1445–C1453. [Google Scholar] [CrossRef]
- Li, H.; Ravenzwaay, B.v.; Rietjens, I.M.C.M.; Louisse, J. Assessment of an in vitro transport model using BeWo b30 cells to predict placental transfer of compounds. Arch. Toxicol. 2013, 87, 1661–1669. [Google Scholar] [CrossRef]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000, 6, 88–95. [Google Scholar] [CrossRef]
- Guo, N.-N.; Liu, L.-P.; Zhang, Y.-X.; Cai, Y.-T.; Guo, Y.; Zheng, Y.-W.; Li, Y.-M. Early prediction of the differentiation potential during the formation of human iPSC-derived embryoid bodies. Biochem. Biophys. Res. Commun. 2019, 516, 673–679. [Google Scholar] [CrossRef]
- Sheridan, S.D.; Surampudi, V.; Rao, R.R. Analysis of embryoid bodies derived from human induced pluripotent stem cells as a means to assess pluripotency. Stem Cells Int. 2012, 2012, 738910. [Google Scholar] [CrossRef]
- LaBeaud, A.D.; Muchiri, E.M.; Ndzovu, M.; Mwanje, M.T.; Muiruri, S.; Peters, C.J.; King, C.H. Interepidemic Rift Valley fever virus seropositivity, northeastern Kenya. Emerg. Infect. Dis. 2008, 14, 1240–1246. [Google Scholar] [CrossRef]
- Schultz, E.M.; Jones, T.J.; Barr, K.L. Antibodies for Venezuelan Equine Encephalitis Virus Protect Embryoid Bodies from Chikungunya Virus. Viruses 2020, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Barr, K.L.; Schwarz, E.R.; Prakoso, D.; Imtiaz, K.; Pu, R.; Morris, J.G., Jr.; Khan, E.; Long, M.T. Strain-Dependent Activity of Zika Virus and Exposure History in Serological Diagnostics. Trop. Med. Infect. Dis. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Steiner, S.; Simeone, R.; Simon, E.; Bhatnagar, J.; Oduyebo, T.; Free, R.; Denison, A.M.; Rabeneck, D.B.; Ellington, S.; Petersen, E.; et al. Evaluation of Placental and Fetal Tissue Specimens for Zika Virus Infection—50 States and District of Columbia, January-December, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 636–643. [Google Scholar] [CrossRef]
- Martines, R.B.; Bhatnagar, J.; de Oliveira Ramos, A.M.; Davi, H.P.; Iglezias, S.D.; Kanamura, C.T.; Keating, M.K.; Hale, G.; Silva-Flannery, L.; Muehlenbachs, A.; et al. Pathology of congenital Zika syndrome in Brazil: A case series. Lancet 2016, 388, 898–904. [Google Scholar] [CrossRef]
- Peng, H.; Liu, B.; Yves, T.D.; He, Y.; Wang, S.; Tang, H.; Ren, H.; Zhao, P.; Qi, Z.; Qin, Z. Zika Virus Induces Autophagy in Human Umbilical Vein Endothelial Cells. Viruses 2018, 10, 259. [Google Scholar] [CrossRef]
- Anfasa, F.; Goeijenbier, M.; Widagdo, W.; Siegers, J.Y.; Mumtaz, N.; Okba, N.; van Riel, D.; Rockx, B.; Koopmans, M.P.G.; Meijers, J.C.M.; et al. Zika Virus Infection Induces Elevation of Tissue Factor Production and Apoptosis on Human Umbilical Vein Endothelial Cells. Front. Microbiol. 2019, 10, 817. [Google Scholar] [CrossRef]
- Zani, A.; Zhang, L.; McMichael, T.M.; Kenney, A.D.; Chemudupati, M.; Kwiek, J.J.; Liu, S.L.; Yount, J.S. Interferon-induced transmembrane proteins inhibit cell fusion mediated by trophoblast syncytins. J. Biol. Chem. 2019, 294, 19844–19851. [Google Scholar] [CrossRef]
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Bramley, J.C.; Morosky, S.; Marques, E.T.; Cherry, S.; Sadovsky, Y.; Coyne, C.B. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe 2016, 19, 705–712. [Google Scholar] [CrossRef]
- Bei, R. Product Information Sheet for NR-50355; Resources, B., Ed.; National Institute of Allergy and Infectious Diseases: Washington, DC, USA, 2016. [Google Scholar]
- Loe, M.W.C.; Lee, R.C.H.; Chu, J.J.H. Antiviral activity of the FDA-approved drug candesartan cilexetil against Zika virus infection. Antivir. Res. 2019, 172, 104637. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chen, M.-C.; Liu, S.; Callahan, V.M.; Bracci, N.R.; Lehman, C.W.; Dahal, B.; de la Fuente, C.L.; Lin, C.-C.; Wang, T.T.; et al. Phloretin inhibits Zika virus infection by interfering with cellular glucose utilisation. Int. J. Antimicrob. Agents 2019, 54, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef] [PubMed]
- STEMdiff Cerebral Organoid Kit, 1_2_0 ed.; Technologies, S., Ed.; StemCell Technologies: Vancouver, BC, USA, 2020; Volume DX21849. [Google Scholar]
- Sheridan, M.A.; Balaraman, V.; Schust, D.J.; Ezashi, T.; Roberts, R.M.; Franz, A.W.E. African and Asian strains of Zika virus differ in their ability to infect and lyse primitive human placental trophoblast. PLoS ONE 2018, 13, e0200086. [Google Scholar] [CrossRef]
- Aldo, P.; You, Y.; Szigeti, K.; Horvath, T.L.; Lindenbach, B.; Mor, G. HSV-2 enhances ZIKV infection of the placenta and induces apoptosis in first-trimester trophoblast cells. Am. J. Reprod. Immunol. 2016, 76, 348–357. [Google Scholar] [CrossRef]
- Zare Mehrjardi, M.; Shobeirian, F. The role of the placenta in prenatally acquired Zika virus infection. VirusDisease 2017, 28, 247–249. [Google Scholar] [CrossRef]
- Vianna, R.A.d.O.; Rua, E.C.; Fernandes, A.R.; dos Santos, T.C.S.; Dalcastel, L.A.B.; dos Santos, M.L.B.; de Paula, P.d.S.; de Carvalho, F.R.; Pache de Faria, A.d.O.; Almeida, P.L.; et al. Experience in diagnosing congenital Zika syndrome in Brazilian children born to asymptomatic mothers. Acta Trop. 2020, 206, 105438. [Google Scholar] [CrossRef] [PubMed]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.M.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.R.; Oliveira, L.J.; Bonfante, F.; Pu, R.; Pozor, M.A.; Maclachlan, N.J.; Beachboard, S.; Barr, K.L.; Long, M.T. Experimental Infection of Mid-Gestation Pregnant Female and Intact Male Sheep with Zika Virus. Viruses 2020, 12, 291. [Google Scholar] [CrossRef] [PubMed]
- Lenglet, Y.; Barau, G.; Robillard, P.Y.; Randrianaivo, H.; Michault, A.; Bouveret, A.; Gérardin, P.; Boumahni, B.; Touret, Y.; Kauffmann, E.; et al. Chikungunya infection in pregnancy: Evidence for intrauterine infection in pregnant women and vertical transmission in the parturient. Survey of the Reunion Island outbreak. J. De Gynecol. Obstet. Et Biol. De La Reprod. 2006, 35, 578–583. [Google Scholar] [CrossRef]
- Touret, Y.; Randrianaivo, H.; Michault, A.; Schuffenecker, I.; Kauffmann, E.; Lenglet, Y.; Barau, G.; Fourmaintraux, A. Early maternal-fetal transmission of the Chikungunya virus. Presse Med 2006, 35, 1656–1658. [Google Scholar] [CrossRef]
- Chen, C.I.; Clark, D.C.; Pesavento, P.; Lerche, N.W.; Luciw, P.A.; Reisen, W.K.; Brault, A.C. Comparative pathogenesis of epidemic and enzootic Chikungunya viruses in a pregnant Rhesus macaque model. Am. J. Trop. Med. Hyg. 2010, 83, 1249–1258. [Google Scholar] [CrossRef]
- Watanaveeradej, V.; Endy, T.P.; Simasathien, S.; Kerdpanich, A.; Polprasert, N.; Aree, C.; Vaughn, D.W.; Nisalak, A. The study transplacental chikungunya virus antibody kinetics, Thailand. Emerg. Infect. Dis. J. 2006, 12, 1770–1772. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.B.; Cedillo-Barron, L.; Gonzalez, Y.A.M.E.; Garcia-Cordero, J.; Campos, F.D.; Namorado-Tonix, K.; Perez, F. Serological tests reveal significant cross-reactive human antibody responses to Zika and Dengue viruses in the Mexican population. Acta Trop. 2020, 201, 105201. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.; de Oliveira-Filho, E.F.; Fernandes, A.I.; Brito, C.A.; Marques, E.T.; Tenorio, M.C.; Gil, L.H. Previous dengue or Zika virus exposure can drive to infection enhancement or neutralisation of other flaviviruses. Mem. Do Inst. Oswaldo Cruz 2019, 114, e190098. [Google Scholar] [CrossRef] [PubMed]
- McCracken, M.K.; Gromowski, G.D.; Friberg, H.L.; Lin, X.; Abbink, P.; De La Barrera, R.; Eckles, K.H.; Garver, L.S.; Boyd, M.; Jetton, D.; et al. Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog 2017, 13, e1006487. [Google Scholar] [CrossRef]
- Jia, Q.; Jia, C.; Liu, Y.; Yang, Y.; Qi, J.; Tong, L.; Chen, H.; Zhang, M.; Che, J.; Li, B.; et al. Clinical evidence for the immunogenicity and immune persistence of vaccination with yellow fever virus strain 17D in Chinese peacekeepers deployed to Africa. Antivir. Res. 2019, 162, 1–4. [Google Scholar] [CrossRef]
- Staples, J.E.; Monath, T.P.; Gershman, M.D.; Barrett, A.D.T. 63—Yellow Fever Vaccines. In Plotkin’s Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1181–1265.e1120. [Google Scholar] [CrossRef]
- Pape, K.A.; Maul, R.W.; Dileepan, T.; Paustian, A.S.; Gearhart, P.J.; Jenkins, M.K. Naive B Cells with High-Avidity Germline-Encoded Antigen Receptors Produce Persistent IgM(+) and Transient IgG(+) Memory B Cells. Immunity 2018, 48, 1135–1143.e1134. [Google Scholar] [CrossRef]
- Wec, A.Z.; Haslwanter, D.; Abdiche, Y.N.; Shehata, L.; Pedreño-Lopez, N.; Moyer, C.L.; Bornholdt, Z.A.; Lilov, A.; Nett, J.H.; Jangra, R.K.; et al. Longitudinal dynamics of the human B cell response to the yellow fever 17D vaccine. Proc. Natl. Acad. Sci. USA 2020, 117, 6675–6685. [Google Scholar] [CrossRef]
- Inoue, T.; Moran, I.; Shinnakasu, R.; Phan, T.G.; Kurosaki, T. Generation of memory B cells and their reactivation. Immunol. Rev. 2018, 283, 138–149. [Google Scholar] [CrossRef]
- Lyski, Z.L.; Messer, W.B. Approaches to Interrogating the Human Memory B-Cell and Memory-Derived Antibody Repertoire Following Dengue Virus Infection. Front. Immunol. 2019, 10, 1276. [Google Scholar] [CrossRef]
- Kiskova, T.; Mytsko, Y.; Schepelmann, M.; Helmer, H.; Fuchs, R.; Miedl, H.; Wadsack, C.; Ellinger, I. Expression of the neonatal Fc-receptor in placental-fetal endothelium and in cells of the placental immune system. Placenta 2019, 78, 36–43. [Google Scholar] [CrossRef]
- Simister, N.E.; Story, C.M. Human placental Fc receptors and the transmission of antibodies from mother to fetus. J. Reprod. Immunol. 1997, 37, 1–23. [Google Scholar] [CrossRef]
- Ellinger, I.; Schwab, M.; Stefanescu, A.; Hunziker, W.; Fuchs, R. IgG transport across trophoblast-derived BeWo cells: A model system to study IgG transport in the placenta. Eur. J. Immunol. 1999, 29, 733–744. [Google Scholar] [CrossRef]
- Zimmerman, M.G.; Quicke, K.M.; O’Neal, J.T.; Arora, N.; Machiah, D.; Priyamvada, L.; Kauffman, R.C.; Register, E.; Adekunle, O.; Swieboda, D.; et al. Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 2018, 24, 731–742.e736. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Singh, G.; Acklin, J.A.; Lee, S.; Duehr, J.E.; Chokola, A.N.; Frere, J.J.; Hoffman, K.W.; Foster, G.A.; Krysztof, D.; et al. Dengue Virus Immunity Increases Zika Virus-Induced Damage during Pregnancy. Immunity 2019, 50, 751–762.e755. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Urbina, A.N.; Wu, C.-C.; Lin, C.-Y.; Thitithanyanont, A.; Assavalapsakul, W.; Lu, P.-L.; Chen, Y.-H.; Wang, S.-F. An epidemiological survey of the current status of Zika and the immune interaction between dengue and Zika infection in Southern Taiwan. Int. J. Infect. Dis. 2020, 93, 151–159. [Google Scholar] [CrossRef]
- Pierson, T.C. Modeling antibody-enhanced dengue virus infection and disease in mice: Protection or pathogenesis? Cell Host Microbe 2010, 7, 85–86. [Google Scholar] [CrossRef]
- Barr, K.L.; Anderson, B.D. Dengue viruses exhibit strain-specific infectivity and entry requirements in vitro. Virus Adapt. Treat. 2013, 2013, 9. [Google Scholar] [CrossRef][Green Version]
- Petitt, M.; Tabata, T.; Puerta-Guardo, H.; Harris, E.; Pereira, L. Zika virus infection of first-trimester human placentas: Utility of an explant model of replication to evaluate correlates of immune protection ex vivo. Curr. Opin. Virol. 2017, 27, 48–56. [Google Scholar] [CrossRef]
- Evans-Gilbert, T. Vertically transmitted chikungunya, Zika and dengue virus infections: The pathogenesis from mother to fetus and the implications of co-infections and vaccine development. Int. J. Pediatrics Adolesc. Med. 2019. [Google Scholar] [CrossRef]
- Reyes, L.; Golos, T.G. Hofbauer Cells: Their Role in Healthy and Complicated Pregnancy. Front. Immunol. 2018, 9, 2628. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.P.; Choudhury, R.H.; Aplin, J.D. Functional changes in Hofbauer cell glycobiology during human pregnancy. Placenta 2015, 36, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Seval, Y.; Korgun, E.T.; Demir, R. Hofbauer Cells in Early Human Placenta: Possible Implications in Vasculogenesis and Angiogenesis. Placenta 2007, 28, 841–845. [Google Scholar] [CrossRef] [PubMed]
- King, N.J.C.; Teixeira, M.M.; Mahalingam, S. Zika Virus: Mechanisms of Infection During Pregnancy. Trends Microbiol. 2017, 25, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Maeki, T.; Tajima, S.; Ikeda, M.; Kato, F.; Taniguchi, S.; Nakayama, E.; Takasaki, T.; Lim, C.-K.; Saijo, M. Analysis of cross-reactivity between flaviviruses with sera of patients with Japanese encephalitis showed the importance of neutralization tests for the diagnosis of Japanese encephalitis. J. Infect. Chemother. 2019, 25, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, S.; Pierson, T.C. Cross-Reactive Flavivirus Antibody: Friend and Foe? Cell Host Microbe 2018, 24, 622–624. [Google Scholar] [CrossRef] [PubMed]


| ELISA | YFV Antigen | ZIKV Antigen | ||||
|---|---|---|---|---|---|---|
| Pre | Early | Late | Pre | Early | Late | |
| IgA, IgM, IgG | - | - | + | - | - | - |
| IgG | - | - | + | - | - | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultz, E.M.; Jones, T.J.; Hopkins, H.K.; Zeng, J.; Barr, K.L. Post-Vaccination Yellow Fever Antiserum Reduces Zika Virus in Embryoid Bodies When Placental Cells are Present. Vaccines 2020, 8, 752. https://doi.org/10.3390/vaccines8040752
Schultz EM, Jones TJ, Hopkins HK, Zeng J, Barr KL. Post-Vaccination Yellow Fever Antiserum Reduces Zika Virus in Embryoid Bodies When Placental Cells are Present. Vaccines. 2020; 8(4):752. https://doi.org/10.3390/vaccines8040752
Chicago/Turabian StyleSchultz, Emily M., TyAnthony J. Jones, Hannah K. Hopkins, Jingmei Zeng, and Kelli L. Barr. 2020. "Post-Vaccination Yellow Fever Antiserum Reduces Zika Virus in Embryoid Bodies When Placental Cells are Present" Vaccines 8, no. 4: 752. https://doi.org/10.3390/vaccines8040752
APA StyleSchultz, E. M., Jones, T. J., Hopkins, H. K., Zeng, J., & Barr, K. L. (2020). Post-Vaccination Yellow Fever Antiserum Reduces Zika Virus in Embryoid Bodies When Placental Cells are Present. Vaccines, 8(4), 752. https://doi.org/10.3390/vaccines8040752






