Specific Antibodies and Arachidonic Acid Mediate the Protection Induced by the Schistosoma mansoni Cysteine Peptidase-Based Vaccine in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Parasites and Animals
2.3. Cysteine Peptidases
2.4. Experimental Design
2.5. Serum and Organ Collection
2.6. Parasitological Parameters
2.7. Immunologic Assays
2.8. Serum Uric Acid and Lipid Assays
2.9. Immunohistochemistry
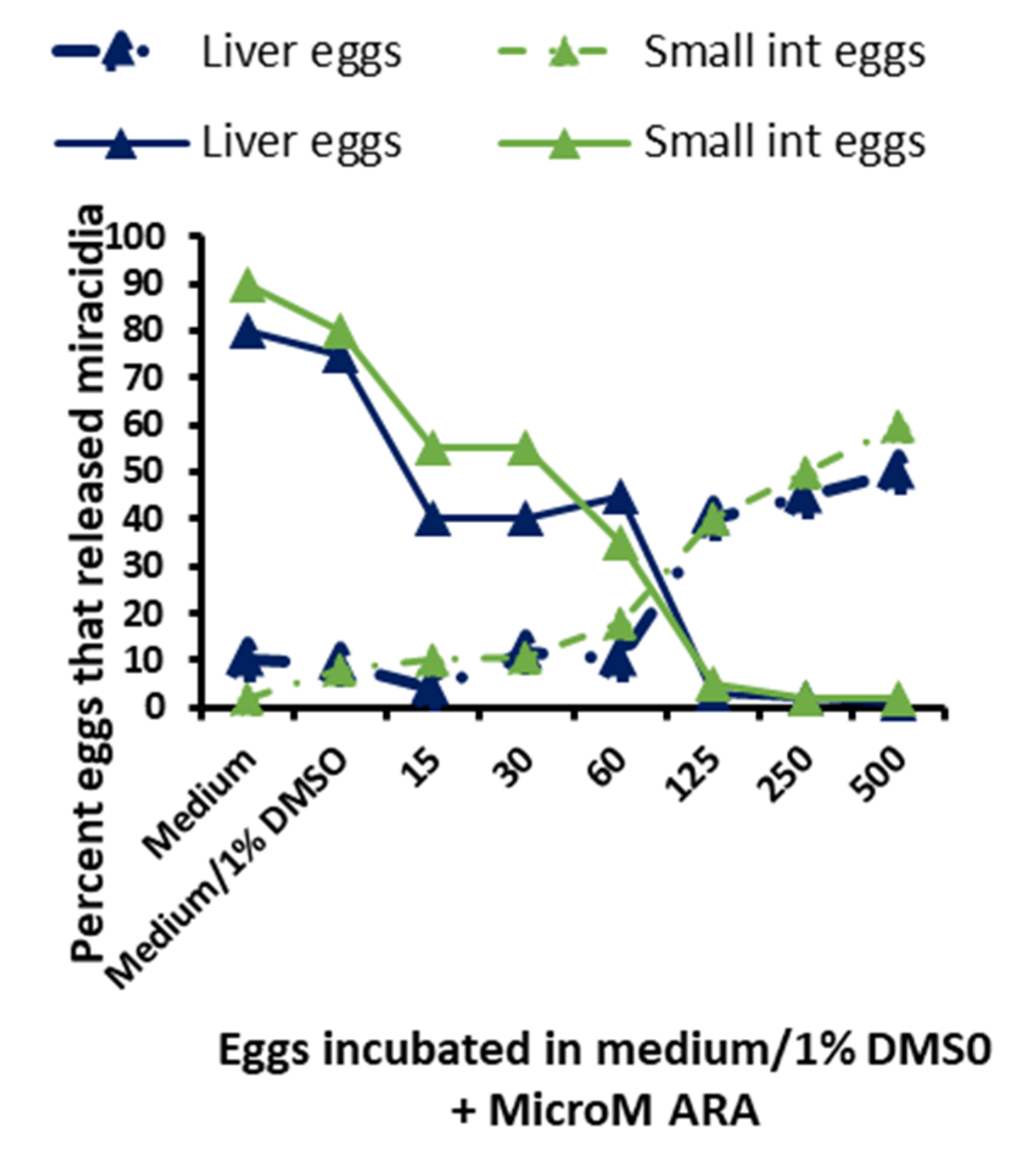
2.10. Arachidonic Acid In Vitro Ovocidal Potential
2.11. Statistical Analysis
3. Results
3.1. Parasitological Data
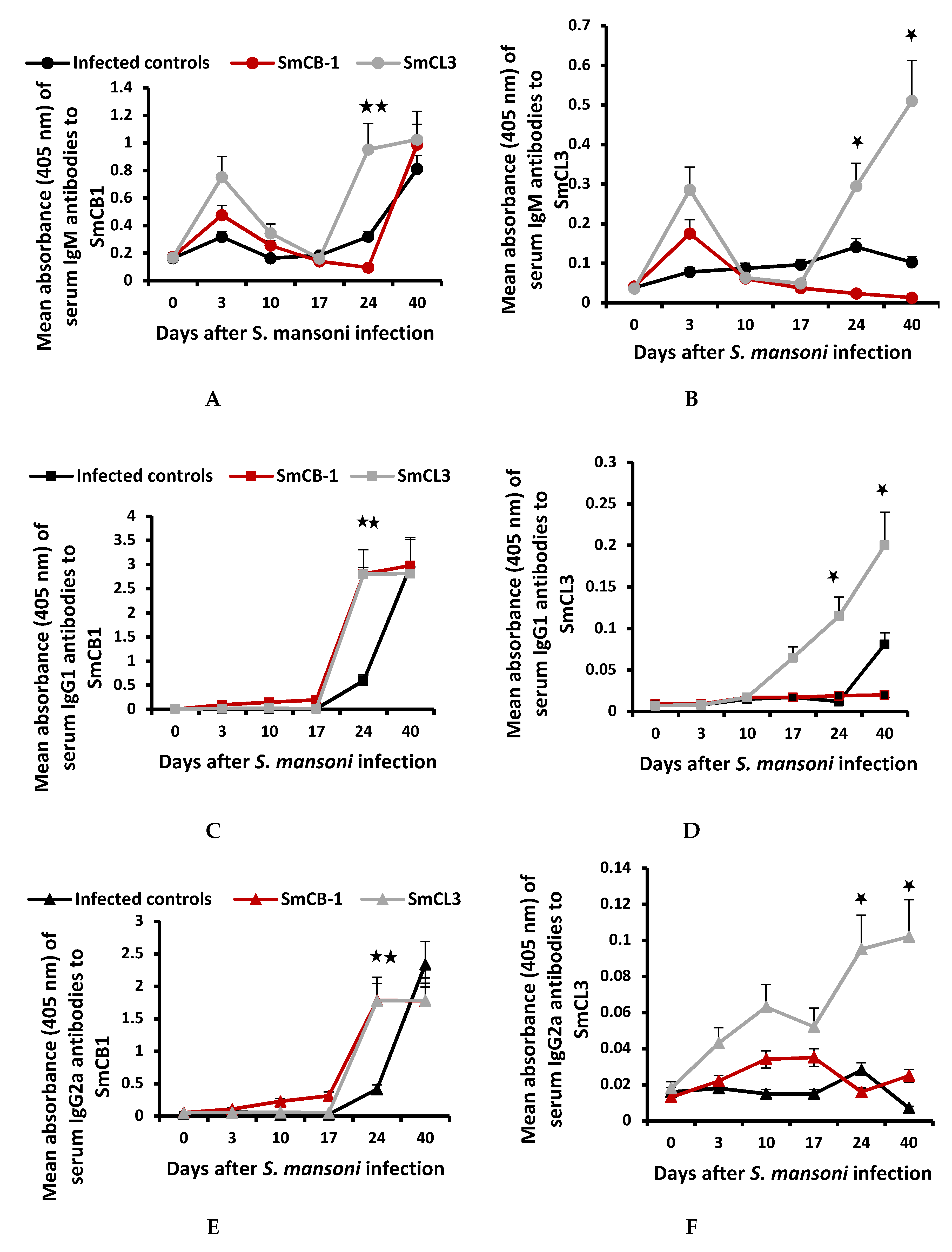
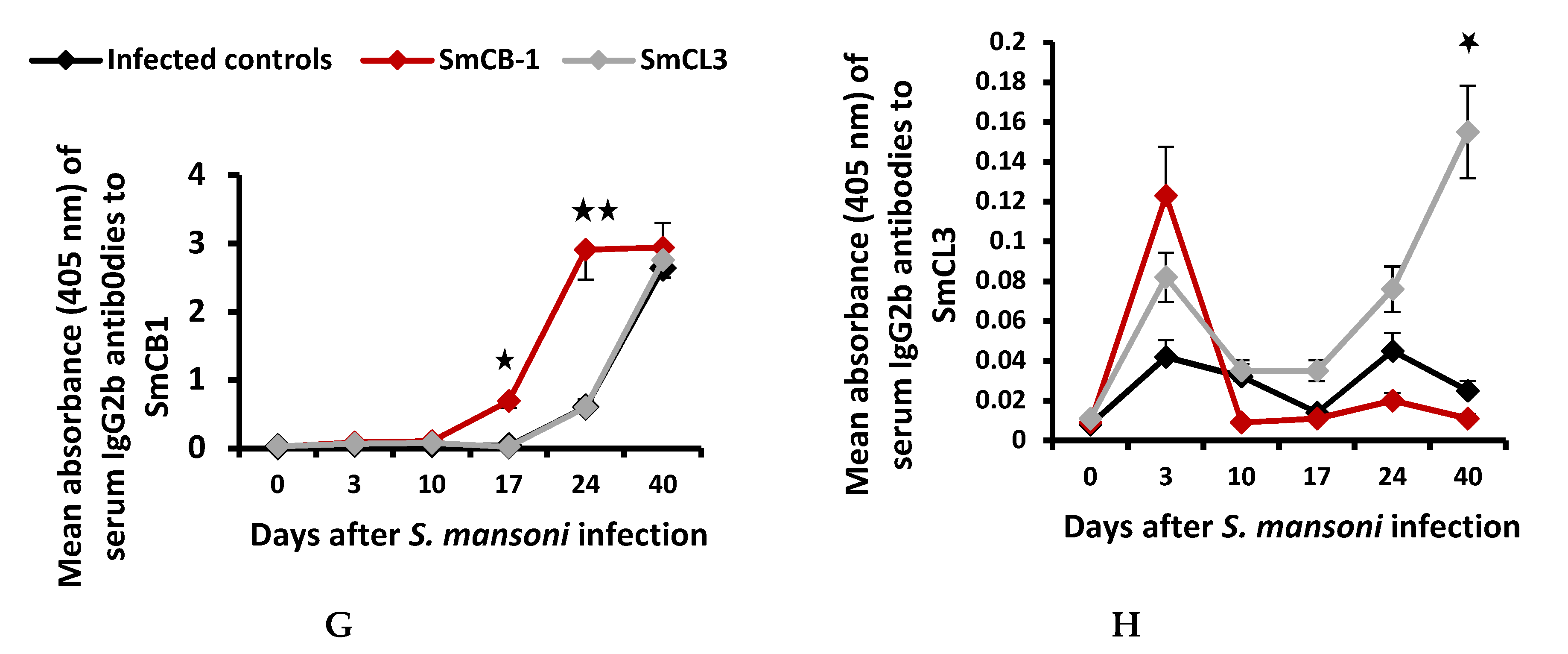
3.2. Serum Immunological Data
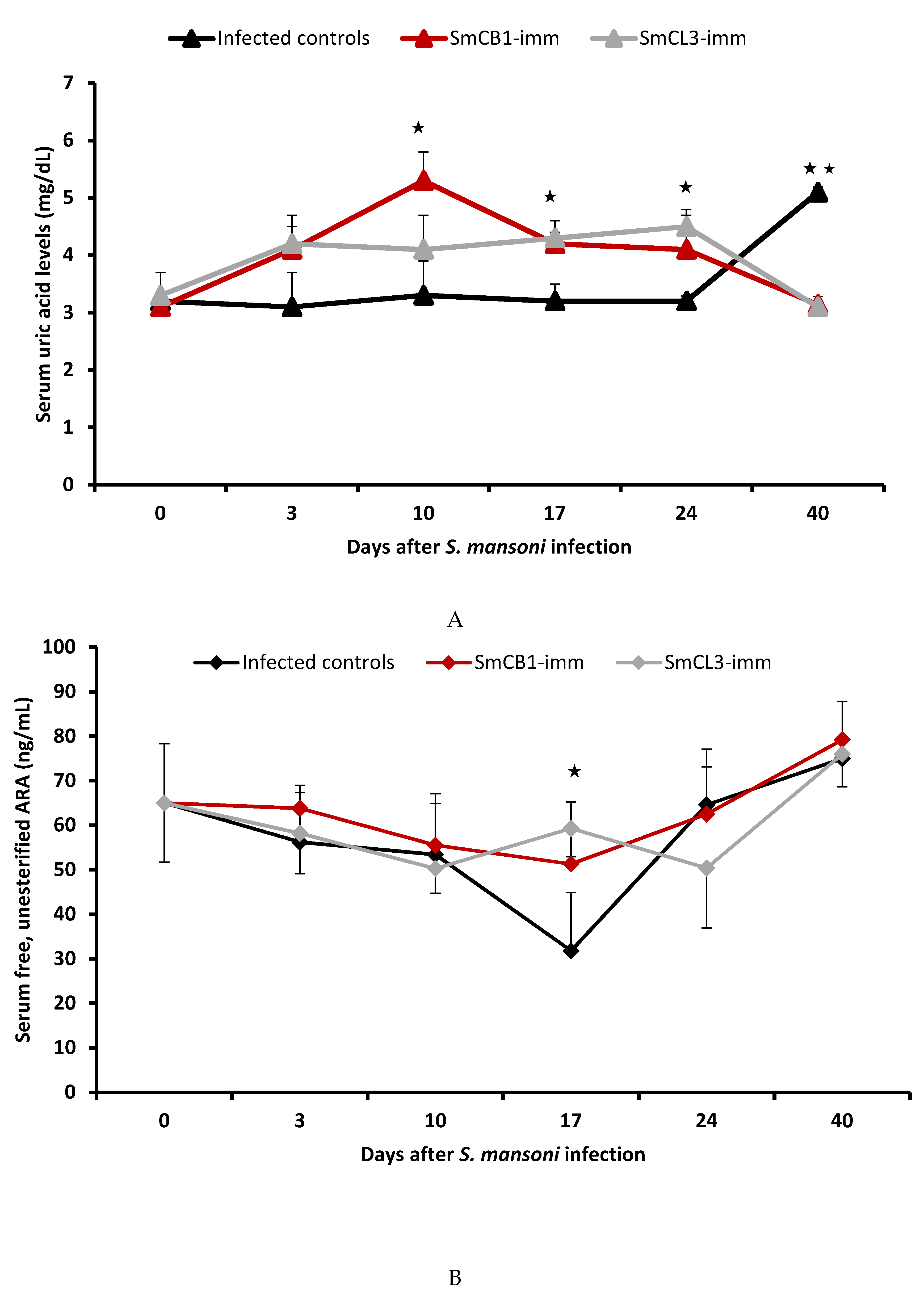
3.3. Serum Uric Acid Levels
3.4. Serum Lipid Analyses
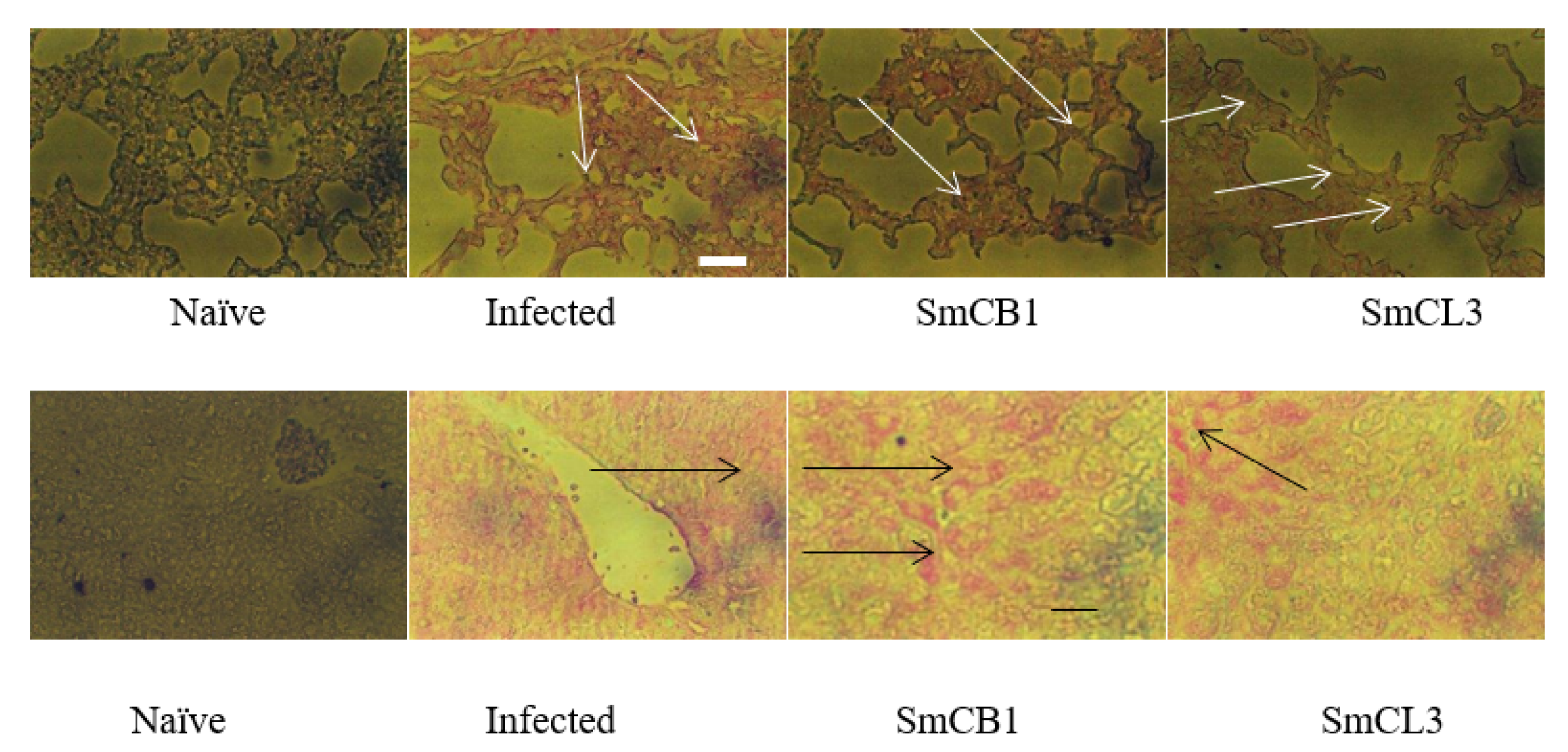
3.5. Lung and Liver Immunohistochemistry
3.6. In Vitro Schistosomicidal and Ovocidal Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Ridi, R.; Tallima, H. Vaccine-induced protection against murine schistosomiasis mansoni with larval excretory-secretory antigens and papain or type-2 cytokines. J. Parasitol. 2013, 99, 194–202. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H.; Selim, S.; Donnelly, S.; Cotton, S.; Gonzales Santana, B.; Dalton, J.P. Cysteine peptidases as schistosomiasis vaccines with inbuilt adjuvanticity. PLoS ONE 2014, 9, e85401. [Google Scholar] [CrossRef] [PubMed]
- El Ridi, R.; Tallima, H.; Dalton, J.P.; Donnelly, S. Induction of protective immune responses against schistosomiasis using functionally active cysteine peptidases. Front. Genet. 2014, 5, 119. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; Dalton, J.P.; El Ridi, R. Induction of protective immune responses against schistosomiasis haematobium in hamsters and mice using cysteine peptidase-based vaccine. Front. Immunol. 2015, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, N.; Tallima, H.; Hafez, E.A.; El Ridi, R. Papain-based vaccination modulates Schistosoma mansoni infection-induced cytokine signals. Scand. J. Immunol. 2016, 83, 128–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tallima, H.; Dvořák, J.; Kareem, S.; Abou El Dahab, M.; Abdel Aziz, N.; Dalton, J.P.; El Ridi, R. Protective immune responses against Schistosoma mansoni infection by immunization with functionally active gut-derived cysteine peptidases alone and in combination with glyceraldehyde 3-phosphate dehydrogenase. PLoS Negl. Trop. Dis. 2017, 11, e0005443. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; Abou El Dahab, M.; Kareem, S.; Dalton, J.P.; El Ridi, R. Protection against Schistosoma haematobium infection in hamsters by immunization with Schistosoma mansoni gut-derived cysteine peptidases, SmCB1 and SmCL3. Vaccine 2017, 35, 6977–6983. [Google Scholar] [CrossRef]
- Abdel Aziz, N.; Tallima, H.; Abou El Dahab, M.; El Ridi, R. Immunogenicity and protective capacity of Schistosoma haematobium recombinant cathepsin L against infection of hamsters with S. haematobium. Vaccine Res. 2019, 6, 1–8. Available online: http://vacres.pasteur.ac.ir/article-1-164-en.pdf (accessed on 5 November 2020). [CrossRef]
- Tallima, H.; Abou El Dahab, M.; El Ridi, R. Role of T lymphocytes and papain enzymatic activity in the protection induced by the cysteine protease against Schistosoma mansoni in mice. J. Adv. Res. 2019, 17, 73–84. [Google Scholar] [CrossRef]
- Bergquist, N.R.; Colley, D.G. Schistosomiasis vaccine: Research to development. Parasitol. Today 1998, 14, 99–104. [Google Scholar] [CrossRef]
- Wan, H.; Winton, H.L.; Soeller, C.; Tovey, E.R.; Gruenert, D.C.; Thompson, P.J.; Stewart, G.A.; Taylor, G.W.; Garrod, D.R.; Cannell, M.B.; et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Investig. 1999, 104, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Asokananthan, N.; Graham, P.T.; Stewart, D.J.; Bakker, A.J.; Eidne, K.A.; Thompson, P.J.; Stewart, G.A. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: The cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J. Immunol. 2002, 169, 4572–4578. [Google Scholar] [CrossRef]
- Baker, S.F.; Yin, Y.; Runswick, S.K.; Stewart, G.A.; Thompson, P.J.; Garrod, D.R.; Robinson, C. Peptidase allergen Der p 1 initiates apoptosis of epithelial cells independently of tight junction proteolysis. Mol. Membr. Biol. 2003, 20, 71–81. [Google Scholar] [CrossRef]
- Collins, P.R.; Stack, C.M.; O’Neill, S.M.; Doyle, S.; Ryan, T.; Brennan, G.P.; Mousley, A.; Stewart, M.; Maule, A.G.; Dalton, J.P.; et al. Cathepsin L1, the major protease involved in liver fluke (Fasciola hepatica) virulence: Propetide cleavage sites and autoactivation of the zymogen secreted from gastrodermal cells. J. Biol. Chem. 2004, 279, 17038–17046. [Google Scholar] [CrossRef]
- Saenz, S.A.; Taylor, B.C.; Artis, D. Welcome to the neighborhood: Epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 2008, 226, 172–190. [Google Scholar] [CrossRef]
- Tang, H.; Cao, W.; Kasturi, S.P.; Ravindran, R.; Nakaya, H.I.; Kundu, K.; Murthy, N.; Kepler, T.B.; Malissen, B.; Pulendran, B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat. Immunol. 2010, 11, 608–617. [Google Scholar] [CrossRef]
- Donnelly, S.; Dalton, J.P.; Robinson, M.W. How pathogen-derived cysteine proteases modulate host immune responses. Adv. Exp. Med. Biol. 2011, 712, 192–207. [Google Scholar] [CrossRef]
- Halim, T.Y.; Krauss, R.H.; Sun, A.C.; Takei, F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Iijima, K.; Elias, M.K.; Seno, S.; Tojima, I.; Kobayashi, T.; Kephart, G.M.; Kurabayashi, M.; Kita, H. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J. Immunol. 2014, 192, 4032–4042. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier epithelial cells and the control of type 2 immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef]
- Deckers, J.; De Bosscher, K.; Lambrecht, B.N.; Hammad, H. Interplay between barrier epithelial cells and dendritic cells in allergic sensitization through the lung and the skin. Immunol. Rev. 2017, 278, 131–144. [Google Scholar] [CrossRef]
- Shi, Y.; Evans, J.E.; Rock, K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003, 425, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Soullié, T.; van Nimwegen, M.; Willart, M.A.; Muskens, F.; Jung, S.; Hoogsteden, H.C.; Hammad, H.; Lambrecht, B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008, 205, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Willart, M.A.; van Nimwegen, M.; Bergen, I.; Pouliot, P.; Virchow, J.C.; Rogers, N.; Osorio, F.; Reis e Sousa, C.; Hammad, H.; et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 2011, 34, 527–540. [Google Scholar] [CrossRef]
- Kono, H.; Chen, C.J.; Ontiveros, F.; Rock, K.L. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Investig. 2010, 120, 1939–1949. [Google Scholar] [CrossRef]
- Ghaemi-Oskouie, F.; Shi, Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr. Rheumatol. Rep. 2011, 13, 160–166. [Google Scholar] [CrossRef]
- Steiger, S.; Harper, J.L. Mechanisms of spontaneous resolution of acute gouty inflammation. Curr. Rheumatol. Rep. 2014, 16, 392. [Google Scholar] [CrossRef]
- Gold, M.J.; Hiebert, P.R.; Park, H.Y.; Stefanowicz, D.; Le, A.; Starkey, M.R.; Deane, A.; Brown, A.C.; Liu, G.; Horvat, J.C.; et al. Mucosal production of uric acid by airway epithelial cells contributes to particulate matter-induced allergic sensitization. Mucosal Immunol. 2016, 9, 809–820. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Araya, J.; Rodrigo, R.; Videla, L.A.; Thielemann, L.; Orellana, M.; Pettinelli, P.; Poniachik, J. Increase in long-chain polyunsaturated fatty acid n−6/n−3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. 2004, 106, 635–643. [Google Scholar] [CrossRef]
- Choi, Y.J.; Shin, H.S.; Choi, H.S.; Park, J.W.; Jo, I.; Oh, E.S.; Lee, K.Y.; Lee, B.H.; Johnson, R.J.; Kang, D.H. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Investig. 2014, 94, 1114–1125. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits. A Review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Margalit, A.; Duffin, K.L.; Shaffer, A.F.; Gregory, S.A.; Isakson, P.C. Altered arachidonic acid metabolism in urate crystal induced inflammation. Inflammation 1997, 21, 205–222. [Google Scholar] [CrossRef]
- El Ridi, R.; Aboueldahab, M.; Tallima, H.; Salah, M.; Mahana, N.; Fawzi, S.; Mohamed, S.H.; Fahmy, O.M. In vitro and in vivo activities of arachidonic acid against Schistosoma mansoni and Schistosoma haematobium. Antimicrob. Agents Chemother. 2010, 54, 3383–3389. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H.; Salah, M.; Aboueldahab, M.; Fahmy, O.M.; Al-Halbosiy, M.F.; Mahmoud, S.S. Efficacy and mechanism of action of arachidonic acid in the treatment of hamsters infected with Schistosoma mansoni or Schistosoma haematobium. Int. J. Antimicrob. Agents 2012, 39, 232–239. [Google Scholar] [CrossRef]
- Selim, S.; El Sagheer, O.; El Amir, A.; Barakat, R.; Hadley, K.; Bruins, M.J.; El Ridi, R. Efficacy and safety of arachidonic acid for treatment of Schistosoma mansoni-infected children in Menoufiya, Egypt. Am. J. Trop. Med. Hyg. 2014, 91, 973–981. [Google Scholar] [CrossRef]
- Barakat, R.; Abou El-Ela, N.E.; Sharaf, S.; El Sagheer, O.; Selim, S.; Tallima, H.; Bruins, M.J.; Hadley, K.B.; El Ridi, R. Efficacy and safety of arachidonic acid for treatment of school-age children in Schistosoma mansoni high-endemicity regions. Am. J. Trop. Med. Hyg. 2015, 92, 797–804. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H.; Migliardo, F. Biochemical and biophysical methodologies open the road for effective schistosomiasis therapy and vaccination. Biochim. Biophys. Acta 2016, 1861, 3613–3620. [Google Scholar] [CrossRef]
- Amaral, K.B.; Silva, T.P.; Malta, K.K.; Carmo, L.A.S.; Dias, F.F.; Almeida, M.R.; Andrade, G.F.; Martins, J.S.; Pinho, R.R.; Costa-Neto, S.F.; et al. Natural Schistosoma mansoni infection in the wild reservoir Nectomys squamipes leads to excessive lipid droplet accumulation in hepatocytes in the absence of liver functional impairment. PLoS ONE 2016, 11, e0166979. [Google Scholar] [CrossRef]
- Hanna, V.S.; Gawish, A.; Abou El Dahab, M.; Tallima, H.; El Ridi, R. Is arachidonic acid and endoschistosomicide? J. Adv. Res. 2018, 11, 81–89. [Google Scholar] [CrossRef]
- Crusco, A.; Whiteland, H.; Baptista, R.; Forde-Thomas, J.E.; Beckmann, M.; Mur, L.A.J.; Nash, R.J.; Westwell, A.D.; Hoffmann, K.F. Antischistosomal properties of sclareol and its heck-coupled derivatives: Design, synthesis, biological evaluation, and untargeted metabolomics. ACS Infect. Dis. 2019, 5, 1188–1199. [Google Scholar] [CrossRef]
- Liu, R.; Ye, F.; Zhong, Q.P.; Wang, S.H.; Chai, T.; Dong, H.F.; Ming, Z. Comparative serum metabolomics between SCID mice and BALB/c mice with or without Schistosoma japonicum infection: Clues to the abnormal growth and development of schistosome in SCID mice. Acta Trop. 2019, 200, 105186. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.S.; Karunaratne, L.B.; Lewis, F.A.; Freitas, T.C.; Liang, Y.S. Schistosomiasis. Curr. Protoc. Immunol. 2013, 103, 19.1.1–19.1.58. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.S.; McLaren, D.J. Schistosoma mansoni: Evidence that site-dependent host responses determine when and where vaccine immunity is expressed in different rodent species. Parasitology 1990, 100, 57–63. [Google Scholar] [CrossRef]
- Dean, D.A.; Mangold, B.L. Autoradiographic analysis of resistance to reinfection with Schistosoma mansoni in mice. Evidence that the liver is a major site of worm elimination. Am. J. Trop. Med. Hyg. 1984, 33, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Noon, J.B.; Schwarz, E.M.; Ostroff, G.R.; Aroian, R.V. A highly expressed intestinal cysteine protease of Ancylostoma ceylanicum protects vaccinated hamsters from hookworm infection. PLoS Negl. Trop. Dis. 2019, 13, e0007345. [Google Scholar] [CrossRef]
- Dalton, J.P.; Smith, A.M.; Clough, K.A.; Brindley, P.J. Digestion of haemoglobin by schistosomes: 35 years on. Parasitol. Today 1995, 11, 299–303. [Google Scholar] [CrossRef]
- Dvorák, J.; Mashiyama, S.T.; Sajid, M.; Braschi, S.; Delcroix, M.; Schneider, E.L.; McKerrow, W.H.; Bahgat, M.; Hansell, E.; Babbitt, P.C.; et al. SmCL3, a gastrodermal cysteine protease of the human blood fluke Schistosoma mansoni. PLoS. Negl. Trop. Dis. 2009, 3, e449. [Google Scholar] [CrossRef]
- Klein, J.; Horejsi, V. Immunology, 2nd ed.; Blackwell Science: Oxford, UK, 1997. [Google Scholar]
- Rumjanek, F.D.; Simpson, A.J. The incorporation and utilization of radiolabelled lipids by adult Schistosoma mansoni in vitro. Mol. Biochem. Parasitol. 1980, 1, 31–44. [Google Scholar] [CrossRef]
- Brash, A.R. Arachidonic acid as a bioactive molecule. J. Clin. Investig. 2001, 107, 1339–1345. [Google Scholar] [CrossRef]
- Wynn, T.A. Immune deviation as a strategy for schistosomiasis vaccines designed to prevent infection and egg-induced immunopathology. Microbes Infect. 1999, 1, 525–534. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, D.; Sun, W.; Zhang, Q.; Zhang, J.; Xue, X.; Shen, L.; Pan, W. Schistosoma japonicum chimeric protein with a novel adjuvant induced a polarized Th1 immune response and protection against liver egg burdens. BMC Infect. Dis. 2009, 9, 54. [Google Scholar] [CrossRef]
- Doenhoff, M.J.; Stanley, R.G.; Griffiths, K.; Jackson, C.L. An anti-atherogenic effect of Schistosoma mansoni infections in mice associated with a parasite-induced lowering of blood total cholesterol. Parasitology 2002, 125, 415–421. [Google Scholar] [CrossRef]
- Sanya, R.E.; Webb, E.L.; Zziwa, C.; Kizindo, R.; Sewankambo, M.; Tumusiime, J.; Nakazibwe, E.; Oduru, G.; Niwagaba, E.; Nakawungu, P.K.; et al. The effect of helminth infections and their treatment on metabolic outcomes: Results of a cluster-randomized trial. Clin. Infect. Dis. 2020, 71, 601–613. [Google Scholar] [CrossRef]
- Tallima, H.; Hanna, V.S.; El Ridi, R. Arachidonic acid is a safe and efficacious schistosomicide, and an endoschistosomicide in natural and experimental infections, and cysteine peptidase vaccinated hosts. Front. Immunol. 2020, in press. [Google Scholar]
- Shiose, A.; Sumimoto, H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J. Biol. Chem. 2000, 275, 13793–13801. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Queiroz, C.; Nyakundi, R.; Ogongo, P.; Rikoi, H.; Egilmez, N.K.; Farah, I.O.; Kariuki, T.M.; LoVerde, P.T. Protective potential of antioxidant enzymes as vaccines for schistosomiasis in a non-human primate model. Front. Immunol. 2015, 6, 273. [Google Scholar] [CrossRef]
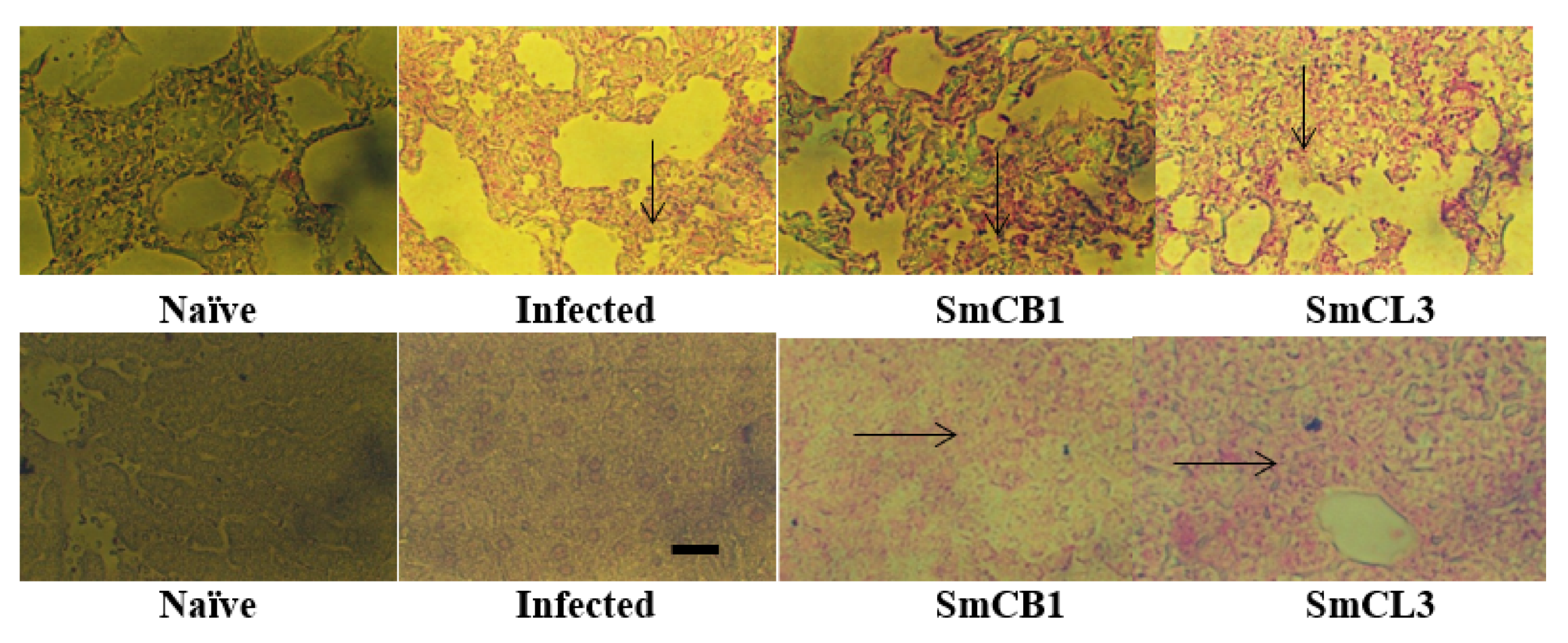
| VACCINE | |||
|---|---|---|---|
| Parameter | Infected Controls | SmCB1-Immunized | SmCL3-Immunized |
| Total worm burden | |||
| Mean ± SD | 82.4 ± 3.5 | 36.5 ± 5.6 | 42.1 ± 5.8 |
| p value | <0.005 | <0.005 | |
| Reduction (%) * | 55.7 | 48.9 | |
| Male worm burden | |||
| Mean ± SD | 42.8 ± 4.6 | 19.0 ± 3.8 | 22.1 ± 3.4 |
| p value | <0.005 | <0.005 | |
| Reduction (%) | 55.6 | 48.6 | |
| Female worm burden | |||
| Mean ± SD | 39.2 ± 3.1 | 17.5 ± 2.0 | 20.1 ± 4.8 |
| p value | <0.005 | <0.005 | |
| Reduction (%) | 55.3 | 48.7 | |
| Liver egg counts | |||
| ean ± SD | 35,800 ± 13198 | 37,500 ± 6442 | 40,666 ± 12193 |
| p value Reduction (%) | NS | NS | |
| Intestine egg counts | |||
| Mean ± SD | 29,200 ± 6610 | 30,500 ± 7259 | 29,500 ± 9354 |
| p value Reduction (%) | NS | NS | |
| % Immature ova ** | |||
| ean ± SD | 41.4 ± 8.8 | 33.2 ± 7.7 | 26.2 ± 5.6 |
| p value Reduction (%) | NS | 0.0087 36.7 | |
| % Mature ova | |||
| Mean ± SD | 48.2 ± 13.5 | 26.1 ± 9.0 | 41.6 ± 9.0 |
| p value Reduction (%) | 0.012 45.8 | NS | |
| % Dead ova | |||
| Mean ± SD | 10.3 ± 5.4 | 40.6 ± 8.1 | 32.1 ± 6.5 |
| p value Increase (%) | 0.0043 74.6 | 0.0087 67.9 | |
| Granuloma number *** | |||
| Mean ± SD | 11.3 ± 0.8 | 7.5 ± 0.9 | 7.8 ± 1.1 |
| p value Reduction (%) | 0.0079 33.6 | 0.0079 31.0 | |
| Granuloma diameter | |||
| Mean ± SD | 393.0 ± 39.0 | 299.5 ± 55.0 | 279.5 ± 52.2 |
| p value Reduction (%) | <0.0001 23.7 | <0.0001 28.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallima, H.; Abou El Dahab, M.; El Ridi, R. Specific Antibodies and Arachidonic Acid Mediate the Protection Induced by the Schistosoma mansoni Cysteine Peptidase-Based Vaccine in Mice. Vaccines 2020, 8, 682. https://doi.org/10.3390/vaccines8040682
Tallima H, Abou El Dahab M, El Ridi R. Specific Antibodies and Arachidonic Acid Mediate the Protection Induced by the Schistosoma mansoni Cysteine Peptidase-Based Vaccine in Mice. Vaccines. 2020; 8(4):682. https://doi.org/10.3390/vaccines8040682
Chicago/Turabian StyleTallima, Hatem, Marwa Abou El Dahab, and Rashika El Ridi. 2020. "Specific Antibodies and Arachidonic Acid Mediate the Protection Induced by the Schistosoma mansoni Cysteine Peptidase-Based Vaccine in Mice" Vaccines 8, no. 4: 682. https://doi.org/10.3390/vaccines8040682
APA StyleTallima, H., Abou El Dahab, M., & El Ridi, R. (2020). Specific Antibodies and Arachidonic Acid Mediate the Protection Induced by the Schistosoma mansoni Cysteine Peptidase-Based Vaccine in Mice. Vaccines, 8(4), 682. https://doi.org/10.3390/vaccines8040682





