A Novel Approach for the Treatment of T Cell Malignancies: Targeting T Cell Receptor Vβ Families
Abstract
1. Introduction
2. Materials and Methods
2.1. CD64 Immune Receptor Construction (CD64 IR)
2.2. Recombinant Lentivirus Production
2.3. Generation of Vβ12 TCR Transduced SupT1 Cell Line (SupT1-Vβ12)
2.4. Primary T Cell Activation, Transduction, and Expansion
2.5. Cytotoxicity Assays
2.6. Vβ-Family-Specific Antibodies and Specificity Determination
2.7. In Vivo Studies
2.8. Subjects and TISSUES
2.9. TCR Library Sequencing
2.10. Raw Sequence Analysis
3. Results
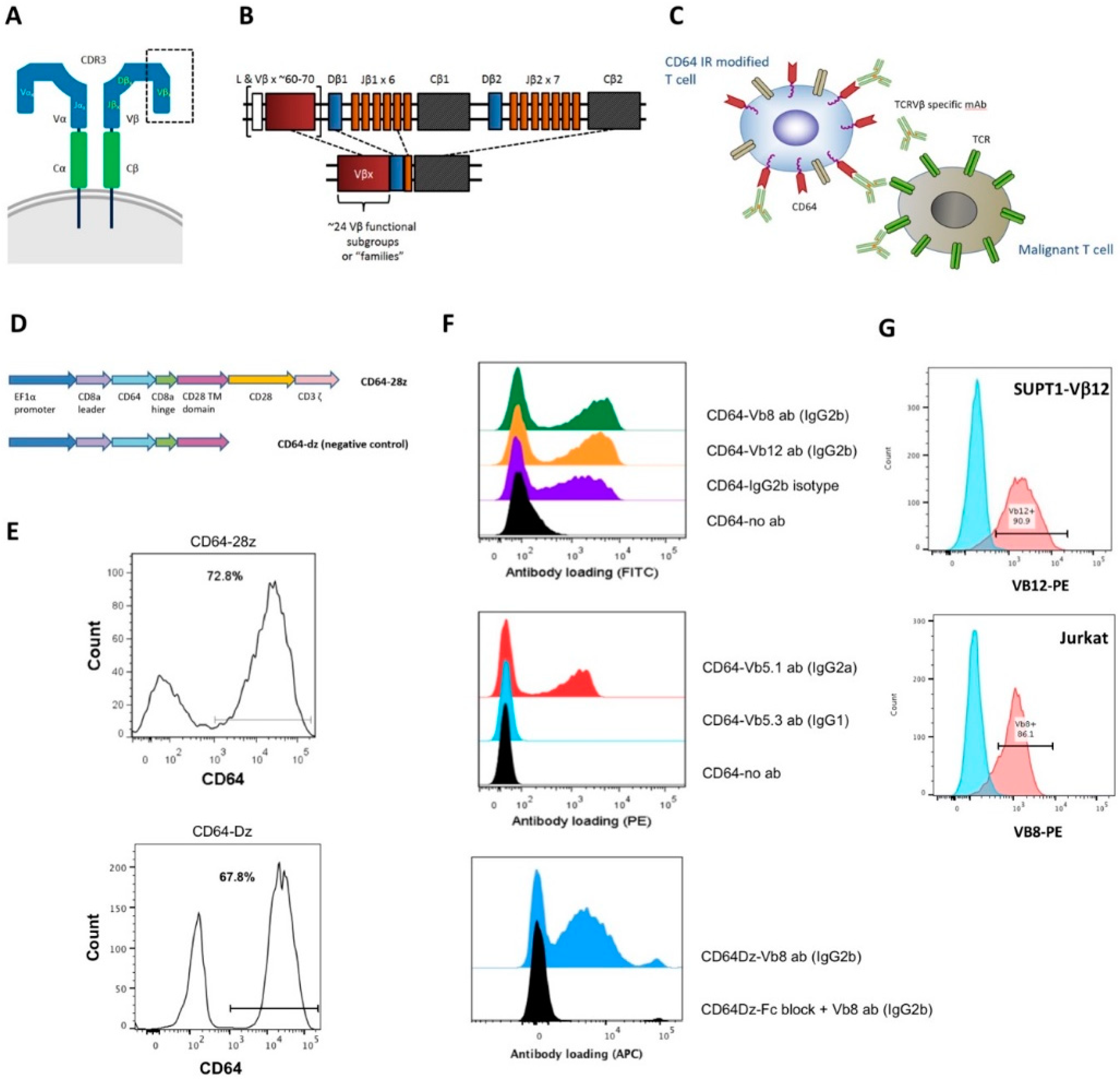
3.1. Constructing a Flexible Platform for TCR Vβ Family Targeting
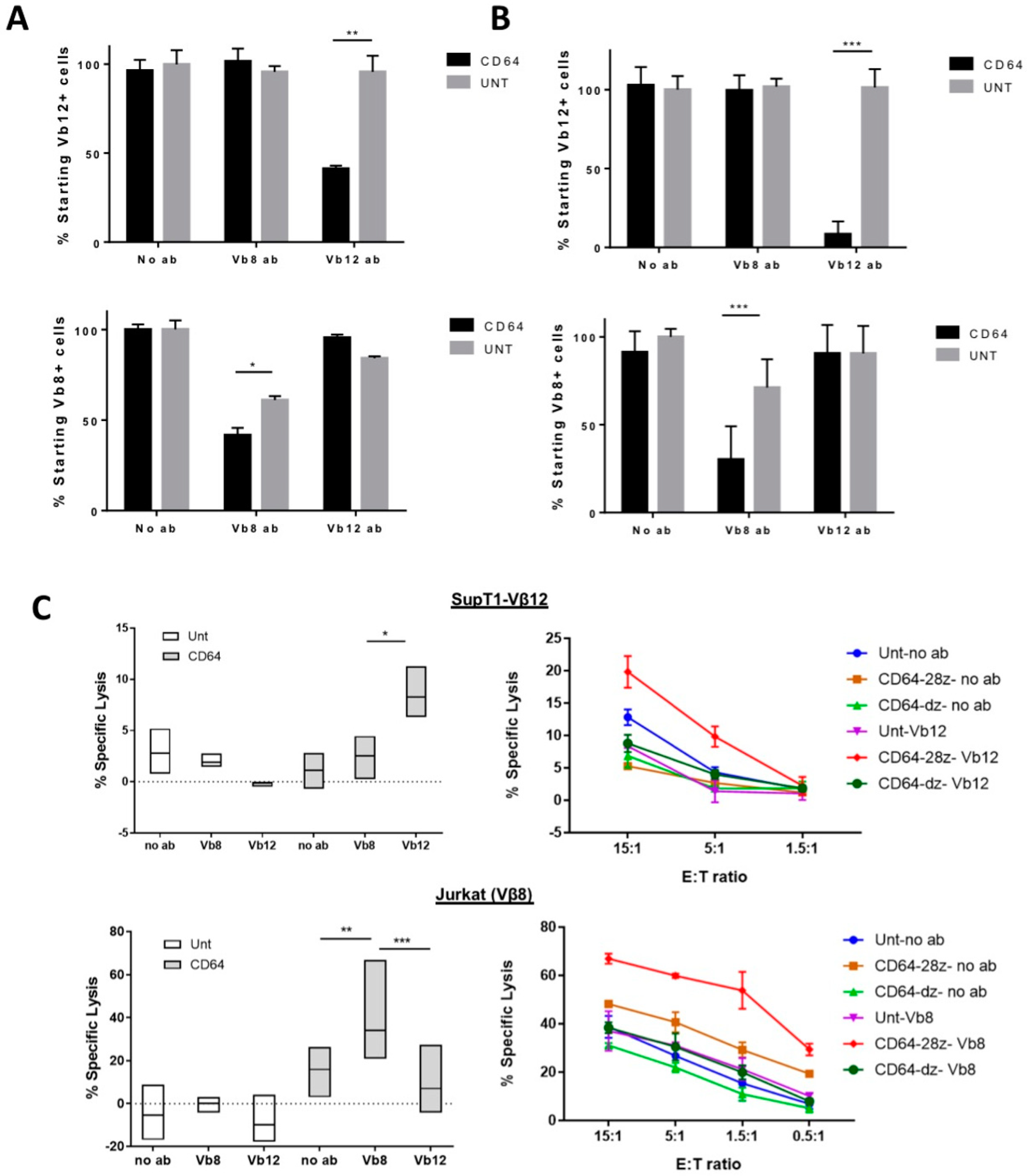
3.2. CD64 IR-Modified T Cells Display Specific Cytolytic Function against TCR Vβ Families
3.3. CD64 IR T Cells Lyse Target T Cells by CD64 IR TCR Activation
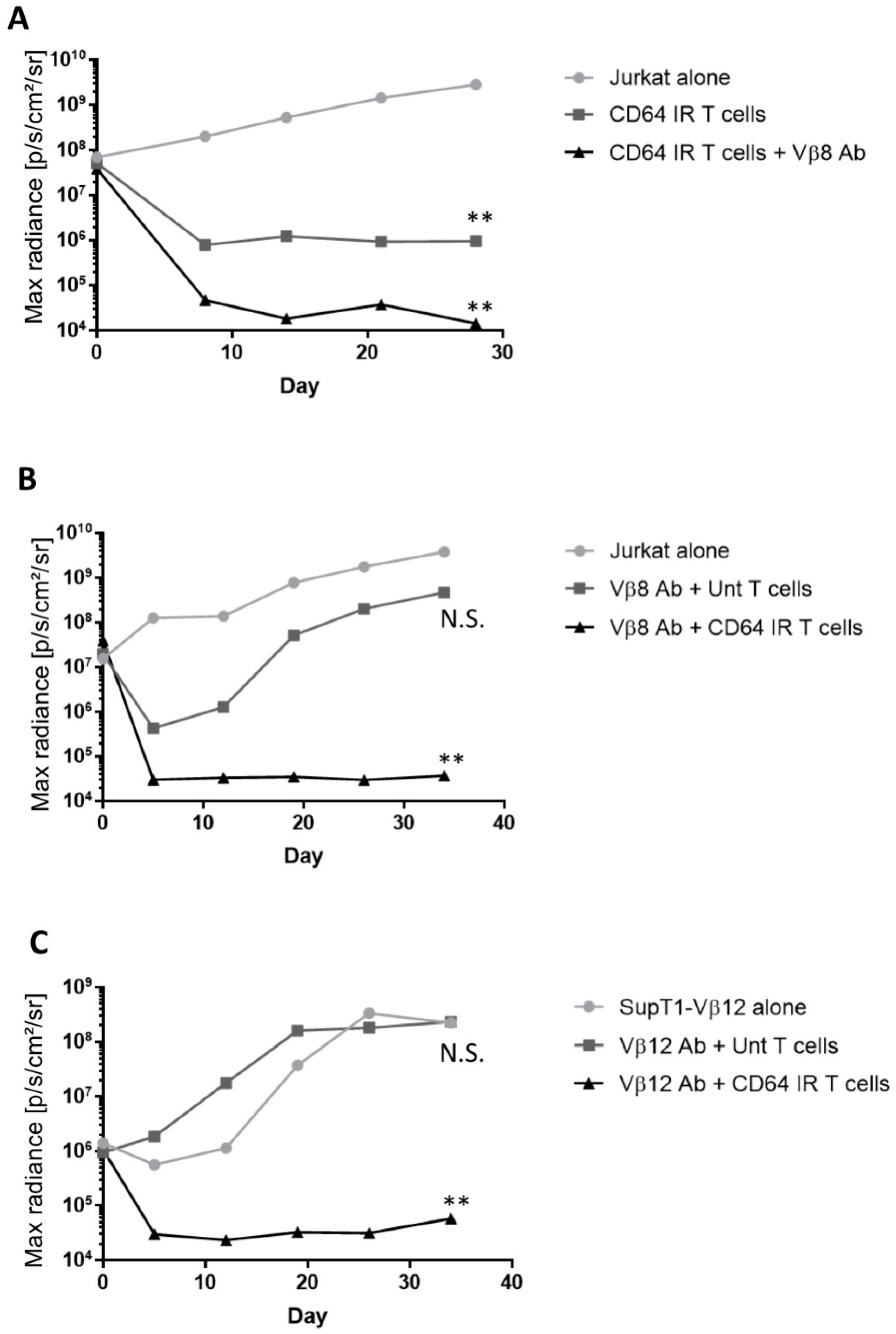
3.4. Vβ Antibody in Conjunction with CD64 IR T Cells Prevents T Cell Malignancy Outgrowth In Vivo
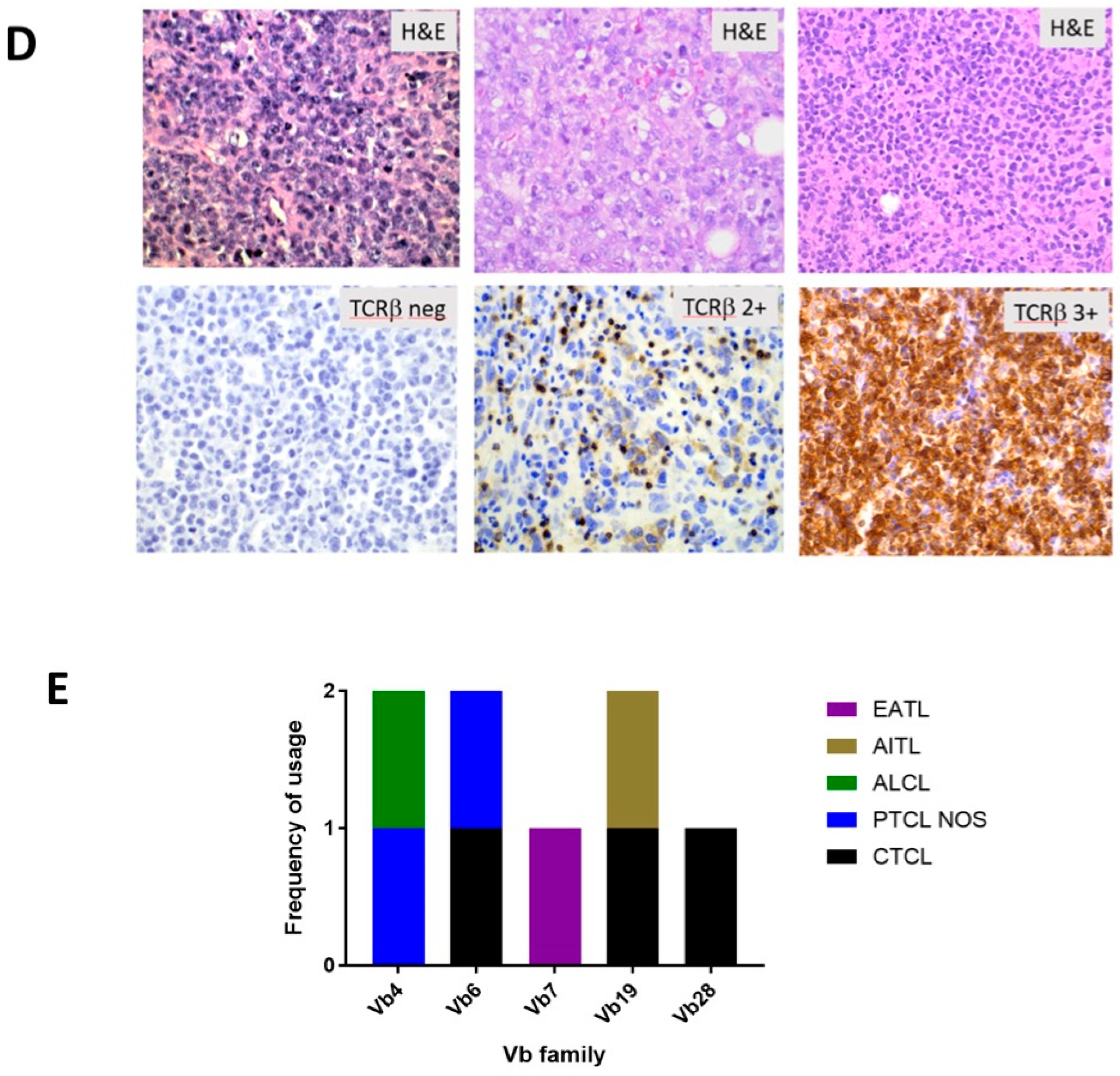
3.5. A Subset of Primary PTCL Samples Show αβ TCR Expression with Varied Vβ Family Usage
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coiffier, B.; Brousse, N.; Peuchmaur, M.; Berger, F.; Gisselbrecht, C.; Bryon, P.A.; Diebold, J. Peripheral T-cell lymphomas have a worse prognosis than B-cell lymphomas: A prospective study of 361 immunophenotyped patients treated with the LNH-84 regimen. The GELA (Groupe d’Etude des Lymphomes Agressives). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1990, 1, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Vose, J. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study: Pathology Findings and Clinical Outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [PubMed]
- Lee, H.-Z.; Kwitkowski, V.E.; Del Valle, P.L.; Ricci, M.S.; Saber, H.; Habtemariam, B.A.; Bullock, J.; Bloomquist, E.; Li Shen, Y.; Chen, X.-H.; et al. FDA Approval: Belinostat for the Treatment of Patients with Relapsed or Refractory Peripheral T-cell Lymphoma. Clin. Cancer Res. 2015, 21, 2666–2670. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Advani, R.H.; Bartlett, N.L.; Jacobsen, E.D.; Sharman, J.P.; O’Connor, O.A.; Siddiqi, T.; Kennedy, D.A.; Oki, Y. Objective responses in relapsed T-cell lymphomas with single agent brentuximab vedotin. Blood 2014, 123, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Thieblemont, C.; Van Den Neste, E.; Plantier, I.; Castaigne, S.; Lefort, S.; Macro, M.; Sebban, C.; Belhadj, K.; Bordessoule, D. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010, 116, 1–8. [Google Scholar] [CrossRef]
- Sehn, L.H.; Chua, N.; Mayer, J.; Dueck, G.; Trněný, M.; Bouabdallah, K.; Fowler, N.; Delwail, V.; Press, O.; Salles, G.; et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): A randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016, 17, 1081–1093. [Google Scholar] [CrossRef]
- Cheson, B.D. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. J. Clin. Oncol. 2010, 28, 3525–3530. [Google Scholar] [CrossRef]
- Mamonkin, M.; Rouce, R.H.; Tashiro, H.; Brenner, M.K. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood 2015, 126, 983–992. [Google Scholar] [CrossRef]
- Gomes-Silva, D.; Srinivasan, M.; Sharma, S.; Lee, C.M.; Wagner, D.L.; Davis, T.H.; Rouce, R.H.; Bao, G.; Brenner, M.K.; Mamonkin, M. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 2017, 130, 285–296. [Google Scholar] [CrossRef]
- Chen, K.H.; Wada, M.; Firor, A.E.; Pinz, K.G.; Jares, A.; Liu, H.; Salman, H.; Golightly, M.; Lan, F.; Jiang, X.; et al. Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget 2016, 7, 56219–56232. [Google Scholar] [CrossRef]
- Maciocia, P.M.; Wawrzyniecka, P.A.; Philip, B.; Ricciardelli, I.; Akarca, A.U.; Onuoha, S.C.; Legut, M.; Cole, D.K.; Sewell, A.K.; Gritti, G.; et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat. Med. 2017, 23, 1416. [Google Scholar] [CrossRef]
- Huang, J.; Alexey, S.; Li, J.; Jones, T.; Grande, G.; Douthit, L.; Xie, J.; Chen, D.; Wu, X.; Michael, M.; et al. Unique CDR3 epitope targeting by CAR-T cells is a viable approach for treating T-cell malignancies. Leukemia 2019, 33, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.; Riley, J.; Carroll, R.; Vonlaer, D.; June, C. Suppression of HIV-1 infection in primary CD4 T cells transduced with a self-inactivating lentiviral vector encoding a membrane expressed gp41-derived fusion inhibitor. Clin. Immunol. 2005, 115, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Song, D.-G.; Ye, Q.; Carpenito, C.; Poussin, M.; Wang, L.-P.; Ji, C.; Figini, M.; June, C.H.; Coukos, G.; Powell, D.J. In Vivo Persistence, Tumor Localization, and Antitumor Activity of CAR-Engineered T Cells Is Enhanced by Costimulatory Signaling through CD137 (4-1BB). Cancer Res. 2011, 71, 4617–4627. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Dudley, M.E.; Wunderlich, J.R.; Hughes, M.S.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Topalian, S.L.; Kammula, U.S.; Restifo, N.P.; et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006, 314, 126–129. [Google Scholar] [CrossRef]
- Folch, G.; Lefranc, M.-P. The Human T cell Receptor Beta Variable (TRBV) Genes. Exp. Clin. Immunogenet. 2000, 17, 42–54. [Google Scholar] [CrossRef]
- Van Dongen, J.J.M.; Langerak, A.W.; Brüggemann, M.; Evans, P.A.S.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; García-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar] [CrossRef]
- Meng, W.; Zhang, B.; Schwartz, G.W.; Rosenfeld, A.M.; Ren, D.; Thome, J.J.C.; Carpenter, D.J.; Matsuoka, N.; Lerner, H.; Friedman, A.L.; et al. An atlas of B-cell clonal distribution in the human body. Nat. Biotechnol. 2017, 35, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.S.; Yu, Y.Y.L.; Dudley, M.E.; Zheng, Z.; Robbins, P.F.; Li, Y.; Wunderlich, J.; Hawley, R.G.; Moayeri, M.; Rosenberg, S.A.; et al. Transfer of a TCR Gene Derived from a Patient with a Marked Antitumor Response Conveys Highly Active T-Cell Effector Functions. Hum. Gene Ther. 2005, 16, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Haas, M. Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol. Cell. Biol. 1990, 10, 5502–5509. [Google Scholar] [CrossRef]
- Schneider, U.; Schwenk, H.-U.; Bornkamm, G. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int. J. Cancer 1977, 19, 621–626. [Google Scholar] [CrossRef]
- Robinson, M.A. The human T cell receptor beta-chain gene complex contains at least 57 variable gene segments. Identification of six V beta genes in four new gene families. J. Immunol. 1991, 146, 4392–4397. [Google Scholar]
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.; Liu, H.; Grilley, B.; et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor—redirected lymphocytes. J. Clin. Investig. 2017, 127, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.K.; Wrightham, M.; Glennie, M.J.; Jones, D.B.; Cattan, A.R.; Feizi, T.; Hamblin, T.J.; Stevenson, G.T. Antibodies to Shared Idiotypes as Agents for Analysis and Therapy for Human B Cell Tumors. Blood 1986, 68, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Neelapu, S.S.; Gause, B.L.; Janik, J.E.; Muggia, F.M.; Gockerman, J.P.; Winter, J.N.; Flowers, C.R.; Nikcevich, D.A.; Sotomayor, E.M.; et al. Vaccination With Patient-Specific Tumor-Derived Antigen in First Remission Improves Disease-Free Survival in Follicular Lymphoma. J. Clin. Oncol. 2011, 29, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, T.J.; Cattan, A.R.; Glennie, M.J.; Mackenzie, M.R.; Stevenson, F.K.; Watts, H.F.; Stevenson, G.T. Initial Experience in Treating Human Lymphoma With a Chimeric Univalent Derivative of Monoclonal Anti-idiotype Antibody. Blood 1987, 69, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Barrett, D.; Teachey, D.T.; Grupp, S.A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Urbanska, K.; Sharma, P.; Nejati, R.; Shaw, L.; Lim, M.S.; Schuster, S.J.; Jr., D.J.P. A Novel Approach for the Treatment of T Cell Malignancies: Targeting T Cell Receptor Vβ Families. Vaccines 2020, 8, 631. https://doi.org/10.3390/vaccines8040631
Wang J, Urbanska K, Sharma P, Nejati R, Shaw L, Lim MS, Schuster SJ, Jr. DJP. A Novel Approach for the Treatment of T Cell Malignancies: Targeting T Cell Receptor Vβ Families. Vaccines. 2020; 8(4):631. https://doi.org/10.3390/vaccines8040631
Chicago/Turabian StyleWang, Jie, Katarzyna Urbanska, Prannda Sharma, Reza Nejati, Lauren Shaw, Megan S. Lim, Stephen J. Schuster, and Daniel J. Powell Jr. 2020. "A Novel Approach for the Treatment of T Cell Malignancies: Targeting T Cell Receptor Vβ Families" Vaccines 8, no. 4: 631. https://doi.org/10.3390/vaccines8040631
APA StyleWang, J., Urbanska, K., Sharma, P., Nejati, R., Shaw, L., Lim, M. S., Schuster, S. J., & Jr., D. J. P. (2020). A Novel Approach for the Treatment of T Cell Malignancies: Targeting T Cell Receptor Vβ Families. Vaccines, 8(4), 631. https://doi.org/10.3390/vaccines8040631




